Abstract
Ureteral stents play a fundamental role in the relief of several symptoms associated with common urinary diseases in the modern society, such as strictures, obstruction or promotion of ureteral healing. Even though ureteral stents have been used for more than 40 years and their performance had a huge development over time, they are still related with complications that include stent encrustation and urinary tract infections. Therefore, efforts from the research community still continue to better meet the clinical needs. Ureteral stent’s materials have a great influence on their efficacy, mostly in terms of mechanical and physicochemical properties. Thus, understanding the stent material’s properties is fundamental to address problems of encrustation, bacterial adhesion, patient discomfort and the troubles during insertion, by working on the softness, flexibility and surface properties of the device.
Considerable progress has been done on ureteral stent’s properties with the aim to meet the clinical problems encountered. Even though this progress does not end up with an ureteral stent without associated complications, it allows to understand the behaviour of different materials and designs in the urologic environment. Indeed, the vast amount of work done and respective outputs have been proven that the different materials can complement each other’s disadvantages, for example, the metals can bear with the high compression that polymeric stents cannot. The goal is to combine the advantages of each material without their associated complications. Therefore, the use of biodegradable materials and combination of different raw materials, together with design adjustments appears to be the future of ureteral stents design.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ureteral stents play a fundamental role in the relief of several symptoms associated with common urinary diseases in the modern society, such as strictures, obstruction or promotion of ureteral healing [1, 2]. Even though ureteral stents have been used for more than 40 years and their performance had a huge development over time, they are still related with complications that include stent encrustation and urinary tract infections [1, 2]. Therefore, efforts from the research community still continue to better meet the clinical needs. Ureteral stent’s materials have a great influence on their efficacy, mostly in terms of mechanical and physicochemical properties [3]. Thus, understanding the stent material’s properties is fundamental to address problems of encrustation, bacterial adhesion, patient discomfort and the troubles during insertion, by working on the softness, flexibility and surface properties of the device [3].
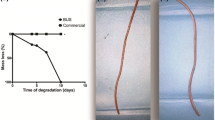
Ureteral stents were described for the first time by Herdman back in 1949 [4]. Among the various biologically and chemically inert polymers that were popular at that time, polyethylene was used owing to its considerable tensile strength, flexibility, biocompatibility and hydrophobic properties. However, during the first animal studies tube blockages and hydronephrosis were detected as the main drawbacks [4]. Another suitable polymer that was at the time used for the manufacture of ureteral stents was silicone, which can withstand high temperatures, facilitating the sterilization process that, in turn, prevent infections [5, 6]. Silicone based stents were less likely to promote encrustations and infections while still being effective in different urological conditions. Nonetheless, due to the low radial strength, silicone-based stents were inefficient in bearing with high external compression [5, 6]. Thereby, the research efforts have turned the tide to merge the flexibility and elasticity of silicone with the rigidity of polyethylene, which resulted on the development of polyurethane as raw material for ureteral stents. Indeed, polyurethane mechanical properties were promissory, but this polymer also demonstrated higher predisposition for encrustation than silicone-based materials [7]. Metals and biodegradable materials have been also used for ureteral stents manufacturing due to their remarkable properties. Metallic ureteral stents are very efficient in situations of high compression forces and when long term treatments are required [1]. A recurrent disadvantage with metallic stents is tissue hyperplasia and increased propensity to develop encrustation due to longer indwelling time periods [3]. On the other side, biodegradable ureteral stents (BUS) provide the uniqueness of self-degradation but obtaining a controlled and homogeneous is still the main obstacle for development of BUS (Fig. 1). On the next sections of this chapter, the three main classes of materials used for ureteral stents manufacture will be individually addressed and most recent findings will be discussed in order to shed the light on the advances and future perspectives in this field.
2 Materials for Ureteral Stents
2.1 Polymeric Materials
Polymers are attractive base materials for biomedical applications due to their inert nature, and constitute the first materials explored for ureteral stent development [8]. Currently, polymeric ureteral stents are the most common in the market, known by being inexpensive and well-tolerated by patients [3]. Certainly, the extensive research on polymers lead to a widespread understanding of their properties, the companies developed proprietary blends and high-quality polymeric ureteral stents are now commercially available [9]. The aim of the current studies on polymeric ureteral stents are focused on improving the biocompatibility, the indwelling time without significant encrustations and infections, and the ease of insertion and retrieval, maintaining the appropriate mechanical properties and radiopaque nature [3, 8]. Polyethylene was the first material employed on the design of ureteral stents, that is not used anymore due to the substantial drawbacks associated to it, namely the easy fragmentation caused by the brittleness of the material and the high rates of encrustation and infection [3, 10]. Currently, silicone and polyurethane are the most used polymers for ureteral stents manufacture [3, 8] (Fig. 1). Silicone has been extensively used, since the earlier beginning of ureteral stents production. Zimskind and colleagues, in 1967, studied for the first time the suitability of silicone for ureteral stents, describing the application of a piece of silicone tube with open ends and side holes to promote long term ureteral drainage of compromised ureters [6]. Nowadays, silicone is considered as a gold standard due to its unique properties, such as the less propensity of encrustation and bacteria contamination, non-toxicity and the improved comfort due to its softness and high lubricity [3, 11, 12]. Besides the aforementioned features, silicone is also easy to shape and process, facilitating the production phase. However, the high flexibility and elasticity is also a disadvantage during the placement on tight and tortuous ureters or when high compression (e.g. tumours) is present [3]. Additionally, difficulties in manoeuvring it with the guidewire were also reported [10]. The use of polyurethane in the urologic field is popular since the earlier beginning due to the suitable mechanical properties, however, as a stiff material, causes discomfort and pain to the patients, being also reported epithelial erosion and ulceration when compared to other materials [2]. The problems encountered in ureteral stents also instigated companies to develop optimized polyurethane-based proprietary formulations, like Sof-Flex®, Tecoflex®, Hydrothane® and ChronoFlex® [13]. Nowadays, polyurethane’s chemical characteristics can be tuned, such as the surface wettability and surface energy, which allows the control of other properties like encrustation and bacterial adhesion propensity [14]. Other polymers were also developed, such as the polyester copolymer, Silitek®, a proprietary polymer that becomes soft and flexible at body temperature, with a reported excellent biocompatibility, Perculfex®, polyethylene-vinyl acetate and styrene/ethylenebutylene/styrene block copolymers, F-Flex®, and poly(methyl methacrylate)/poly(hydroxyethyl methacrylate) (PMMA/pHEMA) with improved mechanical properties than silicone [15]. Albeit all the reported polymer’s formulations, the available ureteral stents are not devoid of clinical complications, thereby, investigations aiming to modify the base polymers are still on going. A recent work presented by Rebl et al. addressed the influence of physical properties of different polymers’ samples on their propensity to develop encrustation [14]. The data revealed that the encrustation degree is correlated with the surface charge and hydrophobicity of the polymer samples, a lower encrustation propensity was observed for polymers with strong negative surface charge and good hydrophilicity [14]. This behaviour is justified by the fact that the most common components of the infectious urinary stones are negatively charged, and, consequently, can be repelled by strongly negative charged polymers’ samples [14]. Rosman et al. also explored the bacterial resistance and anti-biofilm properties of a polyacrylonitrile based ureteral stent (pAguaMedicina™, Pediatric Ureteral Stent, Q Urological) where a considerable reduction on bacterial colonization and biofilm formation in Broth (Trypticase Soy Agar broth), Broth with human urine, and Broth with swine blood was observed when compared with a commonly used commercial ureteral stent (Boston Scientific, USA) [16]. An interesting approach is a combinatorial approach of different materials, taking advantage of the properties of the individual counterparts. For example, Silhouette® ureteral stent consist on a nitinol wire covered with a synthetic polymer, thus this stent present an improved resistance due to the presence of metal on its structure and a good biocompatibility provided by the polymeric revetment [3, 17]. Table 1 presents examples of the polymeric commercial ureteral stents available on the market.
2.2 Metallic Materials
Metallic based ureteral stents were developed to treat ureteral obstruction caused by a malignant external compression, usually a tumour, and for patients needing chronical indwelling ureteral stents [18, 19]. In this context, polymeric ureteral stents are ineffective due to the inadequate drainage and requirement of replacement in a short time period, causing discomfort and extra hospital costs [19, 20] (Fig. 1). A metallic ureteral stent has an improved radial strength that provides long-lasting ureteral patency—12 months to 2 years—tackling the problem of low compression strength and shorter indwelling time—usually 3 months—of polymeric stents [21, 22]. The success rate of a treatment with a metallic stent is between 37 and 100% [19, 22, 23]. Current metallic ureteral stents could be double-J shaped as the traditional polymeric ones (Resonance®), self-expandable (Wallstent™, Allium), balloon expandable (Uventa™), thermo-expandable (Memokath 051) and/or covered with a polymer (Uventa™) [1, 24]. Resonance® has a double-J shape with an occluded lumen and, even though this exclusive design makes the stent insertion and retrieval more difficult, it assures ureteral patency and urine flow under high external compression [25]. Blaschko et al. have reported a significant higher flow rate for Resonance® when compared with a 6F standard stent under high extrinsic compression, 5.15 mL/min and 0.64 mL/min respectively [26]. In another exciting study, Christman et al. compared the radial compression resistance of different ureteral stents—Silhouette®, Sof-Curl™, Resonance®, Polaris™ Ultra, and Percuflex®. The data indicated that Resonance® had a significant higher resistance to compression, followed by Silhouette®, which could be justified by the nitinol wire coil present on Silhouette® [17]. Resonance® is currently seen as a reference for malignant ureteral obstructions owing to the numerous advantages already reported, such as good biocompatibility, suitability for magnetic resonance imaging examination, inhibition of endogenous tissue growth and high flexibility due to the tightly coiled wire of the spiral shaped design [3, 27, 28]. Additionally, Resonance® is soft and, more importantly, has an indwelling time of more than 12 months, during which it retains its suitable features [27]. Chen et al. conducted a study where they compared the performance of Resonance® with an ordinary polymeric stent on patients with malignant ureteral obstruction [22]. The authors confirmed that after 1 year of stent placement, the stents patency decreased 60% in the polymeric stent group and only 9.3% metallic stent group, indicating that metallic stents with good drainage effect for a long period of time are superior to the traditional polymeric stents for patients who require long term stenting [22]. Up to now, different metallic ureteral stents were developed and accessible on ureteral stents market. Memokath 051 is a thermo-expandable nickel titanium alloy with a very tight coil design [20]. Memokath 051 deploys in warm saline and shrinks in cool saline, which is an attractive benefit for placing and retrieving them from the body [3]. Complications such as stent migration and encrustation were reported, together with tissue ingrowth and stent occlusion [15, 29]. Uventa is another commercially available metallic ureteral stent composed of a double layer of nickel and titanium alloys with polytetrafluoroethylene (PTFE) layer between them, designed to prevent migration and tissue adhesion [30]. The success rates of Uventa for malignant ureteral obstructions are between 64.8–81.7% and the associated complications include tumour progression beyond the stent, tissue ingrowth and pain [31]. Another metallic stent model is Wallstent, a self-expanding stent composed of cobalt-based microfilaments woven in crisscross pattern [32]. Unfortunately, Wallstent is also associated with pain, stent migration and tissue ingrowth [30]. Allium Ureteral Stent is made of nitinol and covered with a copolymer, with the purpose to prevent encrustation and tissue growth [33]. The major advantage of Allium Ureteral Stent is the easy removal owing to its particular design [33]. Passage™ is a coil-based metallic ureteral stent with improved flexibility and comfort and higher resistance to radial compression when compared with Resonance® and Silhouette® [1, 34]. Nitinol is a biocompatible material, composed of titanium oxide and nickel with a better corrosion resistance than stainless steel—a material that was previously seen as a reference for stents—possessing also memory shape, i.e. it can be manipulated as needed for stent insertion and afterwards recovers its original shape [21]. Most of the currently available metallic ureteral stents are made of nitinol. Table 2 presents metallic ureteral stents currently available on the market and their composition.
2.3 Biodegradable Materials
Biodegradable ureteral stents are an appealing alternative since its use eliminates the need of a second surgery for the stent removal, avoid additional ureter damage, pain and discomfort, and diminishes the treatment costs [1, 3, 21], Table 3. These exceptional features and decreased propensity for bacterial adherence and encrustation motivated the investigations on biodegradable materials for ureteral stents development [1, 21] (Fig. 1). A crucial concern when producing a BUS is that the degradation profile of ureteral stents should occur in a controllable and adequate form, i.e. efficient mechanical properties must be assured during the treatment time and the degradation has to occur in an homogeneous way, avoiding additional ureteral obstruction [9, 21, 35]. In fact, these are very challenging features to obtain and constitute a critical point during the development process [3, 35]. BUS have been fabricated from synthetic polymers, naturally origin polymers, biodegradable metals or a combination of biodegradable polymers and metals [3, 35]. The concept of biodegradable material applied for ureteral stents date back to 1997, in which Schlick and Planz evaluated the degree of dissolution in acidic and alkaline artificial urine of two polymers (G100X-15LB and G100X-20LB) [36]. With these raw materials, they aimed at producing an ureteral stent with controlled degradation by alkalinizing the urine through medication. However, in clinical practice this concept is risky as a basic urine pH can lead to extra complications, such as precipitation of urine salts and also the development of a suitable environment for the growth of uropathogens growth [1, 21]. Olweny et al. in 2002 introduced the use of poly-l-lactide-co-glycolide (PLGA) as BUS material in a porcine model [37]. Other studies followed this direction and BUS were developed using PLGA, Poly-l,d-lactide (PLA), poly-l-lactic acid (PLLA), polycaprolactone (PCL) and poly-dl-lactic acid (PDLLA), nonetheless problems of inadequate degradation and toxicity were frequently found, with the exception of some promising results obtained in dogs with poly-l,d-lactide (SR-PLA96) where reduced inflammation and good biocompatibility was obtained [1, 21, 38,39,40]. Some concerns affecting the stent degradation are the size and shape, the molecular weight of the polymer, the presence of other ingredients and the respective proportions, among others, and improvements of BUS’s characteristics are made by optimizing these features [1, 35]. Yang et al. proposed the use of PLGA for ureteral stents with a particular stent design that is different from the ones usually employed for BUS-braided and spiralled. The data suggested an homogeneous and controllable degradation and better radial compression strength when compared with a commercial stent [41]. This design is based on a multilayer immersion method using PGLA, zein-a natural protein- and barium sulfate [41]. Later on, Zhang et al. reported the use of a novel biodegradable polymer, methoxypoly(ethylene glycol)-block-poly(l-lactide-ran-Ɛ-caprolactone) (mPEG-PLACL), that present less propensity for encrustation and superior biocompatibility [42]. Soria et al. scrutinised the performance of an innovative anti-reflux BUS, BraidStent, in 24 female pigs where only part of the ureter was intubated [43]. The stent degraded in 3–6 weeks without obstructive fragments and favourable anti-reflux properties [43]. Uriprene™ is a radiopaque glycolic acid-based stent that start the degradation process after 3 weeks, while after 7 and 10 weeks 60% and 100% of the stent was degraded, respectively, in porcine models [44]. This stent was designed to degrade in a specific direction, from the bladder to the kidney end, thereby preventing also the obstruction-formation fragments [1]. Uriprene™ provides similar drainage capacity as ordinary stents with less ureteral dilatation and microbial contamination [44]. The reported problem associated with this stent is the difficulty of insertion [21]. An improved version was later developed with a shorter degradation time (i.e., 4 weeks) [45]. Lingman et al. conducted clinical trial studies using a BUS produced from a proprietary formulation based on the natural origin polymer alginate [21, 46, 47]. The stent was biocompatible and presented appropriate patency up to 48 h, after that time the stent starts to degrade. The main problem of these stents is the permanence of fragments inside the patients for long periods, which required surgical intervention for removal. Recently, Barros et al. successfully reported the use of gelatin and alginate to produce an hydrogel BUS using the supercritical carbon dioxide technology in the production process, which proved to be beneficial for the mechanical properties [48]. In the first studies encouraging results in terms of biocompatibility and low propensity for bacterial contamination and encrustation were reported [48]. This model then showed good performance in vivo, in pig models, with better biocompatibility than a commercial ureteral stent and an homogeneous degradation profile [49, 50]. These works resulted in a patented BUS and the development of HydrUStent™, a biodegradable hydrogel stent for temporary treatments. HydrUStent™ was already validated in porcine model and is being currently preparing to start clinical trials [51].
Biodegradable metals can be used for prolonged time treatments, given the slower degradation rate when compared with biodegradable polymers. The potential of biodegradable metals for ureteral stents was studied for the first time by Lock et al. that investigated the antibacterial activity of magnesium (Mg)–4%Yttrium(Y), the Mg alloy AZ31 and commercially pure Mg. A decrease in Escherichia coli viable colonies was observed for all the tested Mg alloys when compared with commercial polyurethane stents [52]. Zang et al. studied the alloy ZK60 and pure Mg in terms of corrosion, in artificial urine, and histocompatibility in rat’s bladder where they verified that ZK60 had a faster degradation both in vitro and in the animal’s bladder and both metals reveal to be biocompatible [53]. Recently, Tie et al. reported for the first time the use of a Mg based alloy, ZJ31, in a large animal model for ureteral stent application [54]. The data indicated an homogeneous corrosion rate, good biocompatibility and antibacterial activity, when compared with stainless steel. The studies conducted up to now using biodegradable metals for ureteral stents application are still very scarce but promising. Thereby, it is envisioned the clinical translation of a biodegradable metallic ureteral stent in a near future.
Another appealing approach to improve the mechanical properties and degradation time of BUS is the combination of biodegradable polymers with biodegradable metals. Jin et al. evaluated the performance of a BUS based on filaments of Mg alloys covered with biodegradable polyurethane and a coating composed of a biodegradable polymer and barium sulphate [55]. The stents started to degrade after 1 week implantation on pig’s ureter and degraded completely after 4 weeks. The degradation process is not explained but the authors highlight the better drainage ability of the developed stents [55].
3 Conclusions and Future Perspectives
Considerable progress has been done on ureteral stent’s properties with the aim to meet the clinical problems encountered. Even though this progress does not end up with an ureteral stent without associated complications, it allows to understand the behaviour of different materials and designs in the urologic environment. Indeed, the vast amount of work done and respective outputs have been proven that the different materials can complement each other’s disadvantages, for example, the metals can bear with the high compression that polymeric stents cannot. The goal is to combine the advantages of each material without their associated complications. Indeed, promising works have been validating the success of this approach, such as the combination of polymers and metals (Silhouette®) or biodegradable polymers and biodegradable metals. Biodegradable materials seem to be a superior alternative due to their undoubtedly outstanding advantages, the only concern that still needs to be optimized thorough is the degradation rate. However, it should be highlighted the outstanding progresses that have been made in the design of ureteral stents by tailoring their composition. Therefore, the use of biodegradable materials and combination of different raw materials and design adjustments appears to be the future of ureteral stents design.
References
Lange D, Bidnur S, Hoag N, Chew BH. Ureteral stent-associated complications—where we are and where we are going. Nat Rev Urol. 2015;12(1):17–25.
Ramachandra M, Mosayyebi A, Carugo D, Somani BK. Strategies to improve patient outcomes and QOL: current complications of the design and placements of ureteric stents. Res Rep Urol. 2020;12:303–14.
Zhang K, Cui H, Jiang H, Hao Y, Long R, Ma Q, et al. The current status and applications of ureteral stents. Int J Clin Exp Med. 2020;13(4):2122–33.
Herdman JP. Polythene tubing in the experimental surgery of the ureter. Br J Surg. 1949;37(145):105–6.
Williams KG, Blacker AJ, Kumar P. Ureteric stents: the past, present and future. J Clin Urol. 2018;11(4):280–4.
Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97(5):840–4.
Tunney M. Comparative assessment of ureteral stent biomaterial encrustation. Biomaterials. 1996;17(15):1541–6.
Khandwekar AP, Doble M. Physicochemical characterisation and biological evaluation of polyvinylpyrrolidone–iodine engineered polyurethane (Tecoflex®). J Mater Sci Mater Med. 2011;22(5):1231–46.
Forbes C, Scotland KB, Lange D, Chew BH. Innovations in ureteral stent technology. Urol Clin N Am. 2019;46(2):245–55.
Stoller ML, Meng MV, editors. Urinary stone disease the practical guide to medical and surgical management. New York: Springer Science & Business Media; 2007.
Gadzhiev N, Gorelov D, Malkhasyan V, Akopyan G, Harchelava R, Mazurenko D, et al. Comparison of silicone versus polyurethane ureteral stents: a prospective controlled study. BMC Urol. 2020;20(1):1–5.
Krasovskaya SM, Uzhinova LD, Andrianova MY, Prischenko AA, Livantsov MV, Lomonosov MV. Biochemical and physico-chemical aspects of biomaterials calcification. Biomaterials. 1991;12(9):817–20.
Chew BH, Denstedt JD. Technology Insight: novel ureteral stent materials and designs. Nat Clin Pract Urol. 2004;1(1):44–8.
Rebl H, Renner J, Kram W, Springer A, Fritsch N, Hansmann H, et al. Prevention of encrustation on ureteral stents: which surface parameters provide guidance for the development of novel stent materials? Polymers. 2020;12(3):558.
Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009;182(6):2613–8.
Rosman BM, Barbosa JABA, Passerotti CP, Cendron M, Nguyen HT. Evaluation of a novel gel-based ureteral stent with biofilm-resistant characteristics. Int Urol Nephrol. 2014;46(6):1053–8.
Christman MS, L’Esperance JO, Choe CH, Stroup SP, Auge BK. Analysis of ureteral stent compression force and its role in malignant obstruction. J Urol. 2009;181(1):392–6.
Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005;174(6):2125–8.
Asakawa J, Iguchi T, Tamada S, Ninomiya N, Kato M, Yamasaki T, et al. Outcomes of indwelling metallic stents for malignant extrinsic ureteral obstruction. Int J Urol. 2018;25(3):258–62.
Elsamra SE, Leavitt DA, Motato HA, Friedlander JI, Siev M, Keheila M, et al. Stenting for malignant ureteral obstruction: tandem, metal or metal-mesh stents. Int J Urol. 2015;22(7):629–36.
Barros AA, Oliveira C, Lima E, Duarte ARC, Healy K, Reis RL. Ureteral stents technology: biodegradable and drug-eluting perspective. In: Ducheyne P, editor. Comprehensive biomaterials II. 7th ed. Amsterdam: Elsevier; 2017. p. 793–812.
Chen Y, Liu C, Zhang Z, Xu P, Chen D, Fan X, et al. Malignant ureteral obstruction: experience and comparative analysis of metallic versus ordinary polymer ureteral stents. World J Surg Oncol. 2019;17(74):1–10.
Modi AP, Ritch CR, Arend D, Walsh RM, Ordonez M, Landman J, et al. Multicenter experience with metallic ureteral stents for malignant and chronic benign ureteral obstruction. J Endourol. 2010;24(7):1189–93.
Buchholz N, Hakenberg O, Bach C, Masood J, editors. Handbook of urinary stents: basic science and clinical applications. 1st ed. JP Medical Ltd: New York; 2016.
Turk T, Rao M, Polcari A. Updates on the use of ureteral stents: focus on the Resonance® stent. Med Devices Evid Res. 2011;4:11–5.
Blaschko SD, Deane LA, Krebs A, Abdelshehid CS, Khan F, Borin J, et al. In-vivo evaluation of flow characteristics of novel metal ureteral stent. J Endourol. 2007;21(7):780–3.
Miyazaki J, Onozawa M, Takahashi S, Maekawa Y, Yasuda M, Wada K, et al. The resonance® metallic ureteral stent in the treatment of malignant ureteral obstruction: a prospective observational study. BMC Urol. 2019;137:1–19.
Pedro RN, Hendlin K, Kriedberg C, Monga M. Wire-based ureteral stents: impact on tensile strength and compression. Urology. 2007;70(6):1057–9.
Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: an 11-year follow-up. BJU Int. 2009;103(3):372–6.
Kallidonis P, Kotsiris D, Sanguedolce F, Ntasiotis P, Liatsikos E, Papatsoris A. The effectiveness of ureteric metal stents in malignant ureteric obstructions: a systematic review. Arab J Urol. 2017;15(4):280–8.
Chung KJ, Park BH, Park B, Lee JH, Kim WJ, Baek M, et al. Efficacy and safety of a novel, double-layered, coated, self-expandable metallic mesh stent (Uventa™) in malignant ureteral obstructions. J Endourol. 2013;27(7):930–5.
Pollak JS, Rosenblatt MM, Egglin TK, Dickey KW, Glickman M. Treatment of ureteral obstructions with the wallstent endoprosthesis: preliminary results. J Vasc Interv Radiol. 1995;6(3):417–25.
Bahouth Z, Moskovitz B, Halachmi S, Nativ O. Allium stents: a novel solution for the management of upper and lower urinary tract strictures. Rambam Maimonides Med J. 2017;8(4):e0043.
Hendlin K, Korman E, Monga M. New metallic ureteral stents: improved tensile strength and resistance to extrinsic compression. J Endourol. 2012;26(3):271–4.
Wang L, Yang G, Xie H, Chen F. Prospects for the research and application of biodegradable ureteral stents: from bench to bedside. J Biomater Sci Polym Ed. 2018;29(14):1657–66.
Schlick RW, Planz K. Potentially useful materials for biodegradable ureteric stents. BJU Int. 1997;80(6):908–10.
Olweny EO, Landman J, Andreoni C, Collyer W, Kerbl K, Onciu M, et al. Evaluation of the use of a biodegradable ureteral stent after retrograde endopyelotomy in a porcine model. J Urol. 2002;167(5):2198–202.
Lumiaho J, Heino A, Tunnien V, Ala-Opas M, Talja M, Välimaa T, et al. New bioabsorbable polylactide ureteral stent in the treatment of ureteral lesions: an experimental study. J Endourol. 1999;13(2):107–12.
Li G, Wang Z-X, Fu W-J, Hong B-F, Wang X-X, Cao L, et al. Introduction to biodegradable polylactic acid ureteral stent application for treatment of ureteral war injury. BJU Int. 2011;108(6):901–6.
Lumiaho J, Heino A, Pietiläinen T, Ala-Opas M, Talja M, Välimaa T, et al. The morphological, in situ effects of a self-reinforced bioabsorbable polylactide (SR-PLA 96) ureteric stent; an experimental study. J Urol. 2000;164(4):1360–3.
Yang G, Xie H, Huang Y, Lv Y, Zhang M, Shang Y, et al. Immersed multilayer biodegradable ureteral stent with reformed biodegradation: an in vitro experiment. J Biomater Appl. 2017;31(8):1235–44.
Zhang Y, He J, Chen H, Xiong C. A new hydrophilic biodegradable ureteral stent restrain encrustation both in vitro and in vivo. J Biomater Appl. 2021;35(6):720–31.
Soria F, de la Cruz JE, Budia A, Serrano A, Galan-Llopis JA, Sanchez-Margallo FM. Experimental assessment of new generation of ureteral stents: biodegradable and antireflux properties. J Endourol. 2020;34(3):359–65.
Hadaschik BA, Paterson RF, Fazli L, Clinkscales KW, Shalaby SW, Chew BH. Investigation of a novel degradable ureteral stent in a porcine model. J Urol. 2008;180(3):1161–6.
Chew BH, Paterson RF, Clinkscales KW, Levine BS, Shalaby SW, Lange D. In vivo evaluation of the third generation biodegradable stent: a novel approach to avoiding the forgotten stent syndrome. J Urol. 2013;189(2):719–25.
Lingeman JE, Preminger GM, Berger Y, Denstedt JD, Goldstone L, Segura JW, et al. Use of a temporary ureteral drainage stent after uncomplicated ureteroscopy: results from a phase II clinical trial. J Urol. 2003;169(5):1682–8.
Lingeman JE, Schulsinger DA, Kuo RL. Phase I trial of a temporary ureteral drainage stent. J Endourol. 2003;17(3):169–71.
Barros AA, Rita A, Duarte C, Pires RA, Sampaio-Marques B, Ludovico P, et al. Bioresorbable ureteral stents from natural origin polymers. J Biomed Mater Res Part B Appl Biomater. 2015;103(3):608–17.
Barros AA, Oliveira C, Lima E, Duarte ARC, Reis RL. Gelatin-based biodegradable ureteral stents with enhanced mechanical properties. Appl Mater Today. 2016;5:9–18.
Barros AA, Oliveira C, Ribeiro AJ, Autorino R, Reis RL, Duarte ARC, et al. In vivo assessment of a novel biodegradable ureteral stent. World J Urol. 2018;36(2):277–83.
Reis R, Duarte AR, Barros A, Lima E, Oliveira C, Pinto J. An ureteral stent, methods and uses thereof. WO2016181371A1, 2016.
Lock JY, Wyatt E, Upadhyayula S, Whall A, Nuñez V, Vullev VI, et al. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J Biomed Mater Res Part A. 2014;102(3):781–92.
Zhang S, Bi Y, Li J, Wang Z, Yan J, Song J, et al. Biodegradation behavior of magnesium and ZK60 alloy in artificial urine and rat models. Bioact Mater. 2017;2(2):53–62.
Tie D, Liu H, Guan R, Holt-Torres P, Liu Y, Wang Y, et al. In vivo assessment of biodegradable magnesium alloy ureteral stents in a pig model. Acta Biomater. 2020;116:415–25.
Jin L, Yao L, Yuan F, Dai G, Xue B. Evaluation of a novel biodegradable ureteral stent produced from polyurethane and magnesium alloys. J Biomed Mater Res Part B Appl Biomater. 2020;109(9):665–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Pacheco, M., Silva, J.M., Aroso, I.M., Lima, E., Barros, A.A., Reis, R.L. (2022). Biomaterials for Ureteral Stents: Advances and Future Perspectives. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)