Abstract
Earlier chapters of this work have described procedures and protocols that are applicable to most chambers, this chapter has a slightly different focus; we predominantly consider multiphase processes where the applications are on phase transfer of chemical species rather than chemical reactions and the processes are generally occurring in highly specialized chambers. Three areas are described. Firstly, cloud formation processes; here, precise control of physical and thermodynamic properties is required to generate reproducible results. The second area examined is the air/sea interface, looking at the formation of aerosols from nonanoic acid as a surfactant with humic acid as a photosensitizer. The final apparatus described is the Roland von Glasow sea-ice chamber where a detailed protocol for the reproducible formation of sea-ice is given along with an outlook of future work. The systems studied in all three sections are characterized by difficulties in making detailed in situ observations in the real world, either due to the transitory nature of systems or the practical difficulties in accessing the systems. While these specialized simulation chambers may not perfectly reproduce conditions in the real world, the chambers do provide more facile opportunities for making extended and reproducible measurements to investigate fundamental physical and chemical processes, at significantly lower costs.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Chapters 2–7 have described procedures and protocols that are applicable to most chambers, although there will be variations dependent on the chemical application of the chamber (e.g., whether the primary focus is gas- or aerosol-phase chemistry). This chapter has a slightly different focus; we predominantly consider non-chemical applications in a few more specialized chambers. Section 8.1 describes the study of cloud processes focusing particularly on the AIDA (Karlsruhe) and LACIS (Leipzig) chambers. Sections 8.2 and 8.3 consider air–ocean and air–sea-ice interactions. The systems studied in all three sections are characterized by difficulties in making detailed in situ observations in the real world, either due to the transitory nature of systems (e.g., clouds or the continual breaking and reformation of the sea surface layer) or the practical difficulties in accessing the systems (e.g., use of aircraft for cloud systems or the logistics of polar expeditions). While these specialized simulation chambers may not perfectly reproduce conditions in the real world, the chambers do provide more facile opportunities for making extended and reproducible measurements, at significantly lower costs.
The chapter also gives a wider flavor of the roles that simulation chambers can play in atmospheric science. While the main focus is on physical aspects, chemical measurements in the gas, liquid, aerosol, and solid phases can also be made. Providing some background in non-conventional uses of simulation chambers may give practitioners in more conventional chambers some ideas about how their work can be extended or made more realistic by considering additional interactions or processes. Many chambers run access programs, either through ACTRIS or their institutions, and so it may be possible to access these chambers to further develop your ideas.
8.1 Application of Simulation Chambers to the Study of Cloud Processes
8.1.1 Introduction
Clouds are the source of precipitation, contribute significantly to the Earth’s radiation budget, and are therefore an important player for both the weather and the climate. In the last few decades, comprehensive research activities have been conducted to understand cloud processes and the associated interactions (Mason and Ludlam 1951; Hobbs 1991; Kreidenweis et al. 2019) which lead to an increase of the quantitative knowledge of these systems. Nevertheless, not all processes and their influence on weather and climate are yet sufficiently well understood and quantified (Quaas et al. 2009; Seinfeld et al. 2016; Kreidenweis et al. 2019).
A reason for our limited understanding is that atmospheric clouds are highly complex systems. Clouds are transient and usually occur in places that are not easily accessible, making an extensive characterization of clouds very difficult. Furthermore, the observation of a large number of clouds is required as no cloud is like another. The study of atmospheric clouds is therefore very ambitious, expensive, and sometimes even impossible (Stratmann et al. 2009). In consequence, laboratory investigations under well-defined and reproducible conditions are needed in addition to atmospheric observations in order to better understand and quantify cloud processes and related interactions (List et al. 1986; Stratmann et al. 2009; Kreidenweis et al. 2019).
Over the last 40 years, a number of laboratory facilities such as expansion cloud chambers, continuous-flow systems, and wind tunnels have been developed and applied to aerosol and cloud research under controlled, reproducible, and atmospherically relevant conditions (see Chang et al. (2016, 2017) and Cziczo et al. (2017) for details about specific laboratory facilities). The results obtained with these laboratory facilities have already filled in gaps in the puzzle of understanding aerosol–cloud interactions (Chang et al. 2016).
Cloud simulation facilities have been developed and used during the previous few decades to investigate a wide range of processes of relevance for the formation and life cycles of atmospheric clouds. Experiments at these facilities can be conducted over a wide range of simulated and well-controlled thermodynamic, physical, and chemical conditions of relevance for large variety of atmospheric cloud types and climatic regions. Among the addressed research topics are aerosol-cloud condensation nuclei (CCN) processes, ice-nucleation (IN) processes, ice crystal processes, and turbulence effects in cloud microphysics.
The advantage to using a cloud chamber for the study of ice nucleation is that it is a close analogy for how ice nucleation occurs in the atmosphere. The AIDA chamber has been used for experiments on the ice-nucleation activity of a variety of aerosols such as mineral dust (Möhler et al. 2006), ammonium sulfate (Abbatt et al. 2006), bacteria (Möhler et al. 2008), or soil dust (Steinke et al. 2016). A new ice-nucleation active surface site (INAS) density was developed as a result of these cloud chamber experiments (Connolly et al. 2009; Niemand et al. 2012) and was used in models to predict the atmospheric abundance of INPs (Ullrich et al. 2017). The Manchester Ice Cloud Chamber (MICC) has been used to quantify ice nucleation on mineral particles and secondary organic aerosol using the INAS metric to quantify ice-nucleation efficiency (see Emersic et al. 2015; Frey et al. 2018). In general, chamber expansion techniques agree with other techniques to quantify ice nucleation, such as drop-freezing cold stages, at temperatures lower than −25 °C; however, at temperatures higher than −20 °C, we tend to see higher INAS values when using the chamber method (Hiranuma et al. 2015; DeMott et al. 2018; Emersic et al. 2015). The reasons for this are unknown at present. A further advantage to using a cloud chamber is that competition effects between aerosol external mixtures can be measured and understood, therefore improving models (e.g., Simpson et al. 2018).
The Leipzig Aerosol Cloud Interaction Simulator (LACIS) (Stratmann et al. 2004; Hartmann et al. 2011)—which is a laminar flow tube at TROPOS (Leipzig, Germany)—has been used to study aerosol–cloud interaction processes under controllable and reproducible conditions. With LACIS, both the hygroscopic growth and droplet activation of various inorganic (Wex et al. 2005; Niedermeier et al. 2008) and organic materials such as HULIS (humic-like substances; Wex et al. 2007; Ziese et al. 2008), soot (Henning et al. 2010; Stratmann et al. 2010), and secondary organic aerosol particles (Wex et al. 2009; Petters et al. 2009) could be consistently described. Furthermore, LACIS has been comprehensively applied for the investigation and quantification of the immersion freezing behavior of biological (Augustin et al. 2013; Hartmann et al. 2013), mineral dust (Niedermeier et al. 2010; Augustin-Bauditz et al. 2014; Hartmann et al. 2016), and ash particles (Grawe et al. 2016, 2018).
The fall-speed of ice particles within atmospheric clouds has a strong impact on climate feedback (Mitchell et al. 2011). Ice particle fall-speed is governed by the size and shape of the particles (Heymsfield and Westbrook 2010). In the atmosphere, a fundamental process in the generation of large precipitating particles is the coming together and subsequent aggregation of two or more ice crystals. Many in situ observations have confirmed that ice crystal aggregation is important over a large range of ambient temperatures between 0 °C and −60 °C (e.g., Connolly et al. 2005; Gallagher et al. 2012); however, the rates that aggregation occurs at different ambient conditions are very uncertain. Laboratory experiments including cloud chambers are able to study and quantify the ice crystal aggregation process and other secondary ice crystal processes under a range of simulated conditions (e.g., Connolly et al. 2012). In such experiments, tall chambers, such as the 10-m-high Manchester Ice Cloud Chamber, are desirable to enable sufficient time for the ice crystals to sediment and interact with each other.
Ice clouds make a major contribution to radiative forcing in the atmosphere, both trapping IR radiation and reflecting solar radiation. The balance between these processes determines whether clouds have a net warming or cooling, and depends on the macro-physical and optical properties of the cloud, in particular, the ice water content and the cloud optical depth. Furthermore, accurate retrieval of ice cloud properties using remote sensing platforms requires knowledge of the light scattering properties of ice crystals (such as their backscatter and volume extinction). In contrast to liquid clouds, there is a gap in knowledge about the backscatter and volume extinction properties of atmospheric ice clouds. Moreover, irregularities on the ice crystal surfaces can affect their general light scattering properties (e.g., Liu et al. 2013) and there is increasing evidence that ice particles within cirrus clouds are dominated by ice particles that have substantially roughened surfaces (e.g., Ulanowski et al. 2014). Cloud chambers can play a vital role in narrowing the gap in our knowledge by providing direct measurements of the light scattering properties of ice crystals under a range of conditions. These measurements aid in the development of parameterizations and provide data with which to test advanced scattering models (e.g., Smith et al. 2015, 2016).
Atmospheric clouds are often inhomogeneous, nonstationary, and intermittent. They cover a huge range of spatial and temporal scales with cross-scale interactions between turbulent fluid dynamics and microphysical processes that influence the development and the behavior of clouds (Bodenschatz et al. 2010). Turbulence drives mixing and entrainment in clouds leading to strong fluctuations in temperature, water vapor, and consequently (super-)saturation as well as aerosol particle concentration affecting cloud droplet/ice crystal formation and growth/decay (Siebert et al. 2006; Chandrakar et al. 2016; Siebert and Shaw 2017). On the other hand, the phase transformation processes can introduce bulk-buoyancy effects and influence cloud dynamics (Stevens 2005; Malinowski et al. 2008; Bodenschatz et al. 2010).
To date, there are very few laboratory facilities for the study of aerosol–cloud–turbulence interaction processes because of the high requirements for accuracy and reproducibility of experimental parameters. One example is the turbulent moist-air wind tunnel LACIS-T (turbulent Leipzig Aerosol Cloud Interaction Simulator, Niedermeier et al. 2020) at TROPOS. LACIS-T can specifically be used to study the influence of turbulence on cloud microphysical processes, such as droplet and ice crystal formation. The investigations take place under controlled and reproducible flow and thermodynamic conditions. The temperature range of warm, mixed-phase, and cold clouds (i.e., −40 °C < T < 25 °C) can be covered. The continuous-flow design of LACIS-T allows for the investigation of processes occurring on small spatial (micrometer to decimeter scale) and temporal scales (up to a few seconds), with a Lagrangian perspective. A specific benefit of LACIS-T is the well-defined location of aerosol particle injection directly into the turbulent mixing zone as well as the precise control of the respective initial and boundary flow velocity and thermodynamic conditions.
8.1.2 Design of Expansion-Type Cloud Chambers to Study Cloud Microphysical Processes
Expansion-type cloud chambers are capable of simulating processes occurring in air parcels that undergo steady cooling, e.g., in updrafting air parcels related to convective or lee wave cloud formation. Cooling in such chambers is induced by active pumping to the cloud chamber. The rate of pressure reduction is related to a well-defined adiabatic cooling rate, and thus to an increase of relative humidity. The operation of an expansion-type cloud simulation facility requires a clean and vacuum-tight cloud chamber with precise temperature and pressure control. Two such facilities are operated in Europe, the AIDA cloud chamber (Aerosol Interactions and Dynamics in the Atmosphere) at the Karlsruhe Institute of Technology (KIT) (https://www.imk-aaf.kit.edu/AIDA_facilities.php), and Manchester Ice Cloud Chamber (MICC, http://www.cas.manchester.ac.uk/restools/cloudchamber/) at the University of Manchester. In both facilities, the cloud chamber is located inside a cold room, cooled by air ventilation, and has rigid walls of high heat capacity, but without active wall cooling. This has the advantage of precise and homogeneous temperature control, but the disadvantage is that the wall temperatures remain almost constant, while the gas is cooled adiabatically during the expansion run of a cloud experiment. This results in an increasing temperature difference between walls and the gas volume inside the cloud chamber, thus to an increasing heat flux into the volume causing a steady reduction of the cooling rate at a constant pumping rate. By that, both the super-cooling and the duration of a single cloud run starting at a certain pressure and wall temperature are limited, and it is not possible to operate such cloud chambers for longer time periods at constant cooling rates. Therefore, the new dynamic cloud chamber AIDAd was developed at KIT and came into operation in early 2020. This new cloud simulation chamber has active wall cooling and can therefore be operated with isothermal gas and wall temperature distributions in a wide range of cooling rates and a wide temperature range. The setup, instrumentation, and operation parameters of the three expansion-type chambers AIDA, AIDAd, and MICC will briefly be described in the following sections.
The cloud simulation chamber AIDA
The AIDA chamber was designed and engineered as an atmospheric simulation chamber for long-term aerosol and trace gas chemistry experiments. It came into operation in 1997 and, during the first years of operation, was mainly used for experiments on heterogeneous chemistry (Kamm et al. 1999) and aerosol optical properties (Schnaiter et al. 2005). After an intensive period of polar stratospheric cloud research (Wagner et al. 2005; Zink et al. 2002); Möhler et al. 2006), AIDA was predominantly converted into an expansion-type cloud chamber (Möhler et al. 2003, 2005) and also used for a series of experiments on secondary aerosol formation (Saathoff et al. 2009). More recently, the AIDA chamber was also equipped with an LED light source to simulate the shortwave solar spectrum in the troposphere for experiments on atmospheric photochemistry.
Here, we focus on describing the setup, instrumentation, and operation of AIDA as a cloud simulation chamber. Figure 8.1 shows a schematic view of the facility, with the cloud chamber located in the cold box and surrounded by four platforms with ample space for the operation of instruments that are permanently installed or contributed and operated by partners of specific measurement campaigns. The cloud chamber is made of aluminum, has a height of about 7 m, a diameter of about 4 m, and a volume of 84 m3. A mixing fan is located about 1.5 m above the chamber floor with a vertical rotational axis co-aligned to the vertical axis of the cylindrical cloud chamber. The fan induces an upward directed air flow and eddy turbulence inside the chamber, and by that provides chamber internal mixing and homogeneity of trace gas, aerosol, and cloud components with a mixing time scale of about 1 min. The mixing time scale and homogeneous distribution of components inside the chamber are critical for the interpretation of experimental results that are obtained with a large number of instruments measuring or sampling at locations. The whole cloud chamber volume can be considered as a large and uniform cloud element or air parcel that experiences, within uncertainty and fluctuation ranges, the same dynamic change of cloud formation variables and processes. Figure 8.2 summarizes the instruments that are coupled to the AIDA chamber.
Schematic representation of the AIDA cloud simulation chamber facility. The cloud chamber is located inside a cold box with precise control of the temperature in the range from +60 °C to −90 °C. Spatial and temporal temperature homogeneity within ± 0.3 °C can be achieved inside the cold box and the cloud chamber. © KIT
The new dynamic cloud chamber AIDAd
The main advantage of the new dynamic cloud chamber AIDAd compared to the existing AIDA aerosol and cloud chamber facility is that it will allow one to investigate aerosol-cloud processes at simulated cloud updraft conditions in a wide range of well-controlled cooling rates, moisture content, as well as aerosol and trace gas mixtures and compositions. AIDAd was designed, engineered, and constructed in close collaboration with Bilfinger Noell GmbH, Germany. The vacuum chamber, cooling system, and cloud chamber were installed during 2019. First successful cooling runs were performed in August 2019, and final test runs are conducted during November 2019.
AIDAd has a double-chamber design (Figs. 8.3 and 8.4), similar to the design of the dynamic cloud chambers of the Colorado State University (Demott and Rogers 1990) and the Meteorological Research Institute in Tsukuba, Japan (Tajiri et al. 2013). The cloud chamber is located inside an outer vacuum chamber composed of thin-walled flow channels with rectangular cross section. It is mounted on the top plate of the outer vacuum chamber. The vertical tubes are part of the inner synthetic oil circuits for the temperature control of the five cloud chamber segments. The vacuum chamber is also constructed in five segments. The bottom part holds all the coolant supply tubes and sensor feedthroughs, and can therefore not be removed. The other four segments can be removed to provide access to the inner cloud chamber for maintenance or installation work. The upper plate of the cloud chamber can also be removed to provide access to the inner part of the cloud chamber.
Pre-cooled synthetic oil is pumped through the flow channels in five independent circuits: the bottom, three identical cylindrical sections, and the top. The five inner circuits are connected with the pre-cooled oil reservoir through an outer circuit. The inner wall temperature of each segment can be controlled by either adding colder oil from the reservoir (outer circuit) upon request of the cooling system or by electrical heating.
The cloud chamber can either be operated at uniform temperatures in all segments or with a temperature difference of up to ±10 °C between two neighboring segments. Furthermore, cooling rates of up to 10 K min−1 can be applied to any of the five segments. In case of wall cooling, the gas inside the cloud chamber can also actively be cooled by controlled pressure reduction inside the vacuum chamber. Connecting tubes between the cloud chamber volume and the vacuum chamber keep the pressure difference between both volumes below a few hPa.
By controlled pumping, the temperature inside the cloud chamber volume can be kept close to the wall temperature, and therefore heat exchange between the volume and the walls will be minimized. In this case, the cloud chamber volume can be considered to behave like an updrafting atmospheric air parcel with adiabatic cooling conditions. Therefore, the AIDAd cloud chamber will be capable of simulation cloud processes at simulated and well-controlled adiabatic cooling rates between about 0.1 K min−1 and 10 K min−1.
The Manchester Ice Cloud Chamber (MICC)
The Manchester Ice Cloud Chamber (MICC) is a 1 m diameter, 10 m tall chamber situated on three floors of the Centre for Atmospheric Science at the University of Manchester. There are three separate cold rooms enclosing the cloud chamber, capable of being cooled to −55 °C using independently controlled compressors and fans within each enclosure (see Fig. 8.5 for a schematic of the chamber). Thermocouples are used to measure the gas temperature inside the chamber throughout its length.
Technical components and some of the instrumentation at the MICC facility. The chamber is housed within three cold rooms that span three floors of the building. Each cold room can be cooled to −55 °C using individual compressors. Instrumentation is variable and can be fitted to ports within each of the three sections
Clouds are made by two methods. The first method is similar to that described above for AIDA where a quasi-adiabatic expansion of the air inside the chamber is utilized to lead to cloud formation on aerosol particles within the chamber. Aerosol particles can be introduced using a rotating brush generator (see Emersic et al. 2015) or can be generated using the Manchester Aerosol Chamber (MAC) facility (see Frey et al. 2018), which is also in the Centre for Atmospheric Science at the University of Manchester, and pumped into the MICC. Aerosol particles and cloud particle properties are measured during the experiments by sampling the air from the chamber using pumps to draw the air through particle sampling instruments (see Fig. 8.5). These instruments can be placed at portholes on each of the three floors. This method typically creates cloudy conditions for 5–10 min due to a heat flux from the chamber walls, which eventually warms the air to temperatures where the air becomes sub-saturated with respect to water vapor.
The second method of creating ice and mixed-phase clouds is to introduce humid air into the chamber at temperatures below 273.15 K prior to nucleating ice by periodically allowing compressed air to exit a solenoid valve near the top of the chamber. It is possible to create long-lived ice and mixed-phase clouds using method 2. Method 2 has been used to study ice crystal aggregation (Connolly et al. 2012) and the light scattering properties of ice crystals (Smith et al. 2015, 2016).
8.1.3 Design of a Chamber to Study the Influence of Turbulence onto Cloud Microphysical Processes: LACIS-T
LACIS-T (Niedermeier et al. 2020) at TROPOS is a turbulent moist-air wind tunnel. It is a closed-loop system being designed to generate a locally homogeneous and isotropic turbulent airflow. The temperature and water vapor saturation of the airflow can be precisely controlled and aerosol particles—acting as cloud condensation nuclei (CCN) or ice-nucleating particles (INPs)—can be injected into it. Under suitable conditions, cloud droplet formation or heterogeneous ice formation and the subsequent growth can be observed within the turbulent flow.
A schematic of LACIS-T is shown in Fig. 8.6. The main components are radial blowers, particle filters, valves, flow meters, the humidification system, heat exchangers, turbulence grid, the measurement section, and the adsorption dehumidifying system. These components are applied in order to generate two particle-free airflows (approximately 5000 l min-1 each) each of which is conditioned to a certain temperature and dew-point temperature (the range is −40 °C < T, Td < 25 °C). These two conditioned particle-free airflows pass passive square-mesh grids (mesh length of 1.9 cm, rod diameter of 0.4 cm, and a blockage of 30%) which are situated 20 cm above the measurement section (see Fig. 8.7) in order to create nearly isotropic and, in transverse planes, homogeneous turbulence in the center region of the measurement section.
A schematic of LACIS-T showing the individual components (© by Ingenieurbüro Mathias Lippold, VDI; TROPOS). The red arrows indicate the flow direction. (Figure reused with permission from Niedermeier et al. 2020 Open access under a CC BY 4.0 license, https://creativecommons.org/licenses/by/4.0/)
A sketch of the measurement section is shown including its dimensions, the position of the turbulence grids, the cutting edge, and the aerosol inlet (© by Ingenieurbüro Mathias Lippold, VDI; TROPOS). The red box on the right-hand side marks the location where the particles are injected. The picture in the center shows a formed cloud which is illuminated by a green laser light sheet. (Figure reused with permission from Niedermeier et al. 2020 Open access under a CC BY 4.0 license, https://creativecommons.org/licenses/by/4.0/)
At the inlet of the measurement section, the two conditioned particle-free airflows are merged and turbulently mixed. A wedge-shaped “cutting edge” separates both airflows right above the inlet (see right picture in Fig. 8.7). In the center of this cutting edge, three rectangular feedthroughs (20 mm × 1 mm each, 1 mm separation between feedthroughs) are located which represent the aerosol inlet. Here, aerosol particles of known chemical composition, size, and number concentration—size selection is conducted via a Differential Mobility Analyzer (DMA, type “Vienna medium”) and particles are counted by means of a condensation particle counter CPC (TSI 3010, TSI Inc., USA)—are injected into the mixing zone, in which cloud droplet formation and/or freezing take place at ambient pressure. A super-saturated environment can be created through the process of isobaric mixing (Bohren and Albrecht 1998). The exact humidity within the turbulent region depends on the temperatures and dew-point temperatures within the two particle-free airflows, as well as the location within the turbulent mixing zone. The mean velocity inside the measurement section can be varied between 0.5 and 2 m s−1.
The measurement section itself is of cuboidal shape. It is 2.0 m long, 0.8 m wide, and 0.2 m deep. The design of aerosol inlet and measurement section reduces wall effects onto the processes of interest occurring in the mixing zone. Furthermore, the measurement section design ensures flexibility in terms of instrument mounting as panels with required access ports can be mounted as well as customized optical windows can be installed. Depending on the experiment, the measurement section can be equipped with different instruments to measure the prevailing turbulence, thermodynamic, and microphysical properties. These include the following:
-
A hot-wire anemometer to measure the mean flow velocity and velocity fluctuations as well as to obtain turbulence characteristics such as turbulence intensity and dissipation rate.
-
Several PT100 resistance thermometers and a cold-wire anemometer to obtain mean temperature and temperature fluctuations.
-
Two dew-point hygrometers to monitor the mean water vapor concentration in the particle-free airflows as well as in the measurement section.
-
Two different optical sensors to determine cloud particle size distributions inside the measurements section: a white-light optical particle spectrometer and a 3D dual-phase Doppler anemometer.
After the measurement section, the whole flow is dried by means of an adsorption dehumidifying system, split up again into two airflows being driven by the radial blowers and cleaned by the particle filters.
Computational fluid dynamics (CFD) simulations accompany and complement the experimental LACIS-T studies. They are used, on the one hand, to determine suitable experimental parameters and, on the other hand, to interpret the experimental results. In detail, Large Eddy Simulations (LES) are performed in OpenFOAM® modeling heat, flow, and mass transfer as well as aerosol and cloud particle dynamics. In this context, a Euler–Lagrange approach is formulated tracking the growth of individual cloud particles along their trajectories through the simulation domain (see Niedermeier et al. (2020) for details).
Note that the experiments on the topic of formation and growth of cloud droplets require individualized conditions concerning the flow field and the thermodynamic parameters inside the measurement section. These conditions need to be characterized prior to the respective experiments. This includes high-resolution measurements of velocity and temperature (on the decimeter to millimeter scale), measurements of the mean relative humidity as well as numerical simulations.
Before the start of individual experiments, the measurement section has to be thoroughly cleaned. It is considered clean when the particle concentration is below 1 cm−3. To do so, dry air is circulated through the system for about 1 h and the aerosol particle number concentration is monitored by means of a CPC. Furthermore, blank experiments (i.e., without particle addition) are performed regularly during the experiments in order to check for the cleanliness of the system.
8.1.4 Example of a Simulation Chamber Study on the Influence of Turbulent Saturation Fluctuations on Droplet Formation and Growth
In the following section, an experimental study on droplet formation and growth using LACIS-T is presented which aimed at investigating how turbulent saturation fluctuations influence the formed droplet size distribution (Niedermeier et al. 2020). The experiment was conducted as follows: a temperature difference of ΔT = 16 K was set between the two particle-free airflows. The temperature and dew-point temperature of the airflows were set to 20 °C in branch A and 4 °C in branch B, respectively, so that RH = 100% in each air flow. Due to the mixing of both saturated air flows in the measurement section, super-saturation occurred. Based on earlier performed characterization experiments and corresponding LES in OpenFOAM® (not shown), the mean relative humidity (RH) was approximately 101.5%. For the investigations, size-selected, monodisperse NaCl particles with dry diameters Dp,dry of 100 nm, 200 nm, 300 nm, and 400 nm are applied. For each injected Dp,dry, the particle concentration is set to 1000 cm−3. The mean flow velocity inside the measurement section was 1.5 m s−1. A Welas 2300 sensor was used for determining droplet size distributions during this type of experiment with the sensor being positioned at center position inside the measurement section at z = 40 cm or z = 80 cm below the aerosol inlet. For each Dp,dry, the sizes and numbers of droplets formed were measured for 20 min in order to obtain meaningful counting statistics.
The size distributions determined at the two positions are shown in Fig. 8.8. In both plots, the normalized droplet number versus the particle diameter is displayed. The following observations can be made: (a) for each Dp,dry the formed droplets grow with increasing distance to the aerosol inlet; (b) all size distributions nearly fall together at z = 80 cm; (c) the size distributions are negatively skewed; and (d) we also observe a significant number of particles close to Dp = 300 nm, i.e., close to the Welas 2300 detection limit.
Droplet formation and growth of differently size-selected, monodisperse NaCl particles Dp,dry = 100 nm–400 nm) for ΔT = 16 K measured at two different positions below the aerosol inlet (left figure: z = 40 cm below the aerosol inlet and right figure: z = 80 cm below the aerosol inlet). The dotted lines represent the critical diameters Dp,crit for particle activation which are 1.2 µm, 3.4 µm, 6.3 µm, and 9.7 µm for Dp,dry = 100 nm, 200 nm, 300 nm, and 400 nm, respectively. (Figure reused with permission from Niedermeier et al. 2020 Open access under a CC BY 4.0 license, https://creativecommons.org/licenses/by/4.0/)
To start with the interpretation of these observations, we included the critical diameters Dp,crit for particle activation which are 1.2 µm, 3.4 µm, 6.3 µm, and 9.7 µm (dotted lines in Fig. 8.8 for Dp,dry = 100 nm, 200 nm, 300 nm, and 400 nm, respectively). Looking at Fig. 8.8, it can be seen that for Dp,dry = 100 nm and 200 nm most of the droplets feature sizes above the critical size and are therefore activated. However, the droplets grown on particles with dry sizes Dp,dry = 300 nm and 400 nm have sizes clearly below the critical size. These droplets are not activated, despite the mean super-saturation being sufficient for their activation. The droplet growth is kinetically limited which is the reason for this observation. In order to reach the respective critical diameter, the particles have to be exposed to a certain level of super-saturation for a given time frame (Chuang et al. 1997; Nenes et al. 2001). For the prevailing super-saturation, the needed time frame is on the order of several tens of seconds for the Dp,dry = 300 nm and 400 nm particles. However, it takes about 0.5 s to reach z = 80 cm inside LACIS-T which is too short for these particles to achieve their respective Dp,crit. Moreover, the given time frame also limits the growth of the droplets formed on the particles with Dp,dry = 100 nm and Dp,dry = 200 nm. Under the prevailing conditions and the sole observation of the grown droplet distributions, it is not possible to distinguish between the activated and non-activated droplet distributions. In other words, the droplet growth is kinetically limited, independent of whether the droplets are in the hygroscopic or dynamic growth mode and the dry particle size is of minor importance for the observed droplet distributions.
For the interpretation of the significant number of particles close to Dp = 300 nm and the negative skewness of the size distributions, we consider the results from the respective LES which yield a RH standard deviation in the order of 4% (absolute). It can be concluded from these simulations that the small particles (Dp < Dp,crit), on the one hand, are hygroscopically grown particles which did not experience super-saturated conditions and, on the other hand, are evaporating droplets because they experienced sub-saturated conditions in the fluctuating saturation field.
In conclusion, the turbulent saturation fluctuations broaden the droplet size distribution toward smaller diameters caused by evaporating droplets or less-grown droplets, i.e., turbulence influences cloud droplet activation and growth/evaporation. The obtained results also imply that droplet activation in a turbulent environment may be inhibited due to kinetic effects/limitations. On the other hand, locally elevated super-saturations may occur due to turbulence which might lead to an increase of the activated droplet number.
8.1.5 Example of Using a Simulation Chamber as Platform for Instrument Test and Intercomparison
One important role for simulation chambers is in providing a well-defined and controllable environment to test instrumentation. A performance evaluation of the Ultrafast Thermometer (UFT) 2.0 under turbulent cloudy conditions was undertaken as part of EUROCHAMP-2020 trans-national access (TNA) activity in 2019 at LACIS-T (PI Jakub Nowak, University of Warsaw, Poland). Specific experiments included the following:
-
the calibration of the UFT sensors against a reference thermometer;
-
the inspection of the accuracy, response, and orientation dependence in a turbulent flow by comparison with commercial sensors;
-
the examination of the character and likelihood of wetting under cloudy conditions as well as the estimation of its dependence on the incidence angle; and
-
the investigation of the influence of salt deposition on wetting and instrument performance.
The main conclusions of the study can be summarized as follows:
-
1.
All studied UFT versions provide accurate and consistent temperature readings when being linearly calibrated against the reference sensor. The accuracy and response of the UFTs allow for studying details of mixing between air masses differing in temperature.
-
2.
The effect of the incidence angle, i.e., tilting the sensors by a chosen angle with respect to the mean flow in the LACIS-T, has a negligible effect on the mean temperature but significantly influences the obtained temperature fluctuations.
-
3.
In super-saturated air, water vapor condensation has a major contribution to the sensor wetting in contrast to collisions of cloud droplets. The wetting manifests in a decreasing time response due to the growing total heat capacity.
-
4.
Salt deposited on the sensors does not exert a measurable effect on the temperature measurement and the probability of wetting. However, it might contribute to the mechanical deterioration of the instrument which results in floating calibration and intensifies the chance of entire sensor damage. Excessive salt deposition, although unlikely for atmospheric conditions, can trigger hygroscopic condensation already at the relative humidity of about 76% which is well below saturation level.
This study has provided valuable information concerning the properties and performance of the whole family of the UFT thermometers. It has been the first experiment in which all the versions were systematically compared with a reference and between each other in controlled turbulent flow with well-defined thermodynamic conditions. Further work will involve the improvement of the design, in particular, introducing a mechanism preventing the instrument from condensational wetting, e.g., with hydrophobic coatings or alternate heating in a double-sensor device to periodically evaporate collected water.
8.2 Application of Simulation Chambers to the Study of Processes at the Air–Sea Interface
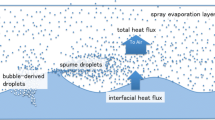
The coupling between oceans and the atmosphere influences a broad range of processes, from nutrient balance for marine biology, to climate. Logically, as the oceans cover most of the Earth’s surface, they also exert a major control on the atmospheric concentration of many trace gases. In fact, air–sea exchanges are key for the atmospheric chemistry, physics, and the biogeochemistry of the oceans (Liss and Johnson 2014). The exchange of trace gases between the oceans and the troposphere is a multifaceted process involving several physical, chemical, and biological processes in each media. In this context, wind speed is a crucial parameter as it influences bubble bursting, waves, rain, and surface films (Garbe et al. 2014). Bubble bursting contributes largely to the marine aerosol budget through the injection of small droplets into the atmosphere (de Leeuw et al. 2011), while surface films influence the air–sea gas exchange via several mechanisms due to their particular characteristics.
The top layer of the oceans operationally defined as the top 1 µm to 1 mm of the ocean is often called the sea surface microlayer (SML). It possesses different chemical and physical properties than the underlying water due to reduced mixing in this region. The SML is enriched in organic and inorganic matter, mainly hydrophobic in nature, but also of associated microorganisms (Cunliffe et al. 2011; Liss and Duce 1997). This surface layer is chemically reactive, as it contains a significant fraction of dissolved organic matter (DOM), containing a high proportion of functional groups such as carbonyls, aromatic moieties, and carboxylic acids (Sempere and Kawamura 2003; Stubbins et al. 2008) and can be conceived as a complex gelatinous film (Cunliffe et al. 2013).
This section will present a chamber-based strategy to investigate the chemical processes, at low wind speed or in other words with a focus on understanding the associated interfacial chemistry without bubble bursting. After designing a dedicated chamber, we will go through some examples showing the impact on VOC emissions and organic aerosol formation.
8.2.1 Design of a Simulation Chamber Dedicated to Air–Sea Processes Study
One of the more significant artifacts in chamber investigations is due to the influence of walls on the observed chemistry. Therefore, investigating the air/water interfacial chemistry in a chamber can be achieved by turning a drawback into an advantage, i.e., by placing the interface of interest on (ideally all) the walls of a given chamber. In this case, we will investigate photochemical processes on a liquid surface mimicking the SML on top of bulk water, based on in situ monitoring of gases and particles, i.e., the experimental samples will be made of bulk water, containing a photosensitizer of interest; the interface, enriched with a given surfactant; and the overlying gas phase.
For this purpose, a 2 m3 chamber (1 (l) × 1 (L) × 2 (h) m) made of FEP (fluorinated ethylene propylene) film was built for this purpose (Fig. 8.9). To mimic the ocean, a glass container can be placed at the bottom of the chamber giving a reactive surface to be investigated. In the specific example presented here, the glass container had a capacity of 89 L and an exposed surface of 0.64 m2.
The chemical processes occurring on this surface have to be dominant compared to those occurring on the remaining walls in order to obtain valuable information. Therefore, operating the chamber under clean conditions is essential, but made complicated due to the high intrinsic relative humidity in such experiments (i.e., experimental runs with a significant volume of liquid water).
The experimental chamber and water basin have to be scrubbed using ethanol, then rinsed with water and dried thoroughly before each experiment. After cleaning, the chamber can be flushed with 40 L min−1 of N2 for more than 48 h after which RH < 5% is maintained. After flushing, background checks have to be performed using a 7.5 L min−1 flow of N2 and turning on and off visible and UV light (see below for the light specifications) and with and without the presence of 0.6–5.0 ppm of O3 and water. Clean chamber conditions could be considered as met if particle concentration remained below 1 cm−3 and the sum of NO and NO2 concentrations (NOx) is kept at <0.6 ppb. After cleaning, 30 L of water (resistivity of 18.2 MΩ cm) can be introduced into the basin at a liquid flow rate of ~1 L min−1 using a peristaltic pump and Teflon plumbing lines. Background checks can again be performed using UV irradiation and ozone injection before and after filling the basin with pure water. The concentration of NOx always should remain <1 ppb and particle concentrations <10 cm−3 during water injection. After all background levels were established, the basin was emptied, dried, and then refilled with the solution to be investigated.
The actual experiment starts with filling with the basin with ca. 30 L of aqueous samples (i.e., water + photosensitizer) again at a liquid flow rate of ~1 L min−1 to which a surfactant can be added, through a septum installed immediately before the basin, at a concentration leading to mono- to multi-layer coverage at the air/water interface. These surfactants covered the 0.6 m2 water surface in excess such that surfactant lenses were in equilibrium with a monolayer at its respective equilibrium spreading pressure. The chamber is then flushed again (40 L min−1 N2) for hours or days and returned to experimental conditions (7.5 L min−1 N2) for another day before commencing UV irradiation of the surfactant interface. The systems investigated are listed in Table 8.1.
12 UV lamps (OSRAM lamps, Eversun L80W/79-R) are positioned in two banks as the light source, with six lamps mounted on two opposite sides. UV light irradiated the chamber at 8 W m−2 measured between 300 and 420 nm in wavelength. Figure 8.10 shows the photon flux measured with a calibrated spectrophotometer (Barsotti et al. 2015).
Particle size distribution and number concentration were monitored by means of a scanning mobility particle sizer (SMPS Model 3936, TSI) consisting of a long differential mobility analyzer (DMA 3081, TSI) and a condensation particle counter (CPC 3772, TSI, d50 > 10 nm). In addition, to follow the formation of ultrafine particle (diameter > 2.5 µm) at the bottom of the chamber (30 cm above the liquid), a specific particle counter (UCPC 3776, TSI) is used. To observe particle growth, the SMPS inlet is placed an additional 150 cm higher. Gas-phase concentrations of volatile organic compounds (VOCs) are monitored using a high-resolution proton transfer reaction mass spectrometer (PTR-TOF–MS 8000, Ionicon Analytik), while various standard analyzers were used for NOx, ozone, humidity, and temperature.
Ozonolysis reactions at the air/water interface and in the gas phase with unsaturated compounds, photochemically produced from the air/water interface, can be triggered by injecting ozone into the chamber up to a concentration of 600 ppb. Ozone is either generated using a corona discharge (Biozone Corporation, USA) or a UV light generator (Jelight Model 600). Fast introduction of ozone in the chamber, reaching the desired concentration, is achieved in a few minutes. This concentration is used to initiate SOA particle nucleation and verify the presence of unsaturated VOCs, although it is higher than typically observed at the Earth’s surface. The concentration and size distribution of new particle formation over time subsequent to ozone injection can therefore be monitored.
After each experiment, the chamber was cleaned for at least 24 h by flushing purified air at high flow rates in presence of ozone, at several ppm, under maximum irradiation. The glass container was also evacuated and rinsed with alkaline ([NaOH] = 10 mM) and ultra-pure water several times, in order to promote the dissolution of organic materials.
8.2.2 Example of a Simulation Chamber Study of a Photosensitized Production of Aerosol at the Air–Sea Interface
Using this simulation chamber, we were able to investigate aerosol formation from photosensitized reactions at the air–water interface (Alpert et al. 2017; Bernard et al. 2016; Rosignol et al. 2016). For this purpose, aqueous solutions of humic acid (HA), used as a proxy for dissolved organic matter and hence as photosensitizer, and nonanoic acid (NA) as a surfactant, were introduced into the chamber. Then the lights were switched on to trigger the targeted photochemical processes.
The NA concentrations ranged from 0.1 mM to 10 mM, while humic acid was added in the range from 1 to 10 mg L−1. After introducing 15 L of ultra-pure water (for 20 min), NA was injected. The formation of small “organic islands” of NA was minimized, but not avoided, by using a very slow injection rate. Over time, these islands agglomerated, increasing their size, and simultaneously decreasing their number concentration. At 0.1 mM of NA, they rapidly disappeared after the introduction of the acid. HA was usually injected around 25 min after NA. Allowing enough to the equilibrium time (ca. 90 min) of NA between the gas and liquid phases, lamps were switched on. The actual experiment lasted for at least 14 h. During the irradiation period, temperature and relative humidity were stable at about 300 K and 84%, respectively.
Particle formation
Figure 8.11 shows a typical experiment where photosensitized production of aerosol was observed, while the list of experiments and corresponding initial conditions are summarized in Table 8.2. Particles in the chamber were subject to dilution, wall loss, and coagulation processes, which represented sink processes. Reported particle concentrations were not corrected for these losses. Background concentrations of particles before the irradiation period were found to be in the range of <50–500 cm−3. As shown in Fig. 8.11, a significant production of secondary organic aerosol was observed rapidly after the injection of ozone. It is noteworthy to underline that the initial composition of the gas phase did not carry any chemical functionality that was expected to react through ozonolysis. In other words, compounds reacting with ozone were produced through photosensitized chemistry at the air–water interface.
Comparison of particle formation measured with the ultrafine condensation particle counter from smog chamber experiments conducted in the presence of humic acid (HA) and nonanoic acid (NA) (Exp. 2 in Table 8.2) compared to nonanoic acid (NA) only (Exp. 3 in Table 8.2). The yellow sections are the periods when the lights were on
These observations contrast sharply with our blank experiments. In the absence of any surfactant, HA (20 mg L−1) photochemistry did not produce any particles and their concentration did not exceed background levels. It is important to note that the HA concentration used in the blank experiment was higher than for mixed HA and NA experiments. Also, with NA only, no significant dark particle formation (as compared to the results shown below) was observed after introducing ozone. Some residual photochemistry of NA films was observed and avoided by using low concentrations. This highlights the involvement of HA in the photochemical transformation of NA and thus demonstrates that particle formation originated from the photosensitized reaction.
The gaseous temporal profiles of volatile organic compounds were monitored by means of the PTR–ToF–MS instrument. A large number of products were identified with various chemical functionalities, such as saturated aldehydes (C7–C9), unsaturated aldehydes (C6–C9), alkanes (C7–C9), alkenes (C5–C9), and dienes (C6–C9).
Interestingly, in the absence of ozone, these gas products only lead to a small SOA production, with particle number concentrations ranging from 150 to 700 cm−3 and close to the background levels prior to irradiation, showing that direct photochemical processes were not important under our experimental conditions.
Lights were turned off and 30 min after ozone was added in the dark. OH radical formation might be scavenged by gaseous NA (kOH+NA = 9.76 × 10–12 cm3 molecule−1 s−1) (Cui et al. 2019). For all the experiments with the combined presence of NA and HA, new particle formation was observed, confirming the production of SOA precursors among all produced VOCs. The observed maximum background subtracted number concentrations ranged from 68 to 3060 cm−3. The lowest number concentration was observed with the lowest concentration of NA (0.1 mM) and HA (1 mg L−1), while the highest one was logically with the highest concentrations of both NA (2 mM) and HA (10 mg L−1). This highlights the fact that both bulk and surface concentrations are key drivers in the observed SOA formation. The total particle mass concentration (∆M0) formed under these experimental conditions did not exceed 1 μg m−3 during the dark ozonolysis reaction. This chemistry led to the formation of condensable organic vapors of volatility low enough to induce the formation of new particles. Such compounds have been referred to as extremely low-volatility organic compounds (LVOC) (Ehn et al. 2014). New particle formation is characterized by a significant increase in particle number, with low mass concentrations. The observed SOA production is in agreement with the formation of products, bearing one or several unsaturated sites, which are potential SOA precursors.
SOA formation potential from photosensitized reactions
A surfactant will alter the surface tension of a liquid as described by the Gibbs adsorption isotherm, where the surface excess concentration of nonanoic acid (NA) is as follows (Donaldson and Anderson 1999):
where \(\Gamma\) NA is the surface excess concentration of NA (in molecules cm−2), representing the amount of NA at the air–sea interface; CNA the bulk concentration of NA (in mol cm−3); γ is the surface tension (in N m−1); R is the gas constant; and T is the temperature in Kelvin. The surface tensions of the systems used in this study were previously measured (Ciuraru et al. 2015), leading to surface excess concentrations in the range from 1.66 × 1014 to 3.99 × 1014 molecules cm−2 in this work.
Assuming that the humic acids are evenly distributed in the solution, at pH ≈ 4, HA are fully soluble in the concentration range used (Klaviņš and Purmalis 2014), and the surface concentration of the surfactant drives the chemical formation, then a correlation between the measured number particles and the chemical formation rate can be expected (Boulon et al. 2013). In fact, assuming that both nonanoic and humic acids were in large access, i.e., constant during the experiment, the chemical production rate of gaseous products Pg (in molecule cm−3 s−1), neglecting the influence of mass transport or dilution in the chamber, can be simplified as
where k (cm3 molecule−1 s−1) corresponds to the overall rate coefficient for the photosensitized reaction of NA in the presence of HA, including reactions kinetics, product yields and phase transfer kinetics, A is the surface area of the liquid (in m2), and V is the internal volume of the chamber (in m3).
The amount of condensable vapor (here simply named [LVOC]) is then related to the ozonolysis of unsaturated products (functionalized alkenes). Assuming that the ozonolysis reaction occurred under pseudo-first-order conditions, the concentration of condensable products (in molecule cm−3) can be expressed as
where kO3 corresponds to the bimolecular reaction rate coefficient of ozone reacting with unsaturated compounds (in cm3 molecule−1 s−1), tirr is the irradiation time (in s), and tind is the induction time (in s) corresponding to the time interval between the introduction of ozone and particle measurements.
Hereby, we assumed that the ratio of the SOA precursor concentration to the total amount of products is similar whatever the initial liquid-phase concentrations are. Figure 8.12 shows indeed a correlation between the number of particles and the concentration of condensable vapors, similar to Boulon et al. (2013). Under our experimental conditions, the formation of particles was in fact photochemically controlled by the photochemical interfacial process, and not the ozone concentration, which was always in excess.
This example highlights the peculiar photochemistry chemistry occurring at the SML, which in fine affects the emission of oceanic VOC. In the field, decoupling such processes from physical ones (mixing though waves, wind, bubble bursting, etc.) would be quite challenging. In this study, the use of a multiphase atmospheric simulation chamber has proven to be a reliable approach to explore the in situ formation of gases and particles from photo-induced chemical processes at the air–water interface. Therefore, investigating processes occurring at the air–sea interface in a dedicated multiphase chamber opens new routes for characterizing specifically interfacial chemical pathways.
8.3 Application of Simulation Chambers to the Study of Cryosphere–Atmosphere Interface
8.3.1 Introduction
The upper surface of sea ice is one of the important interfaces with the atmosphere (Law et al. 2013) and the role of snow and ice in mediating important aspects of atmospheric chemistry continues to be an important topic (Abbatt et al. 2012). At its lower surface, sea ice forms the boundary with the ocean and hence mediates the transport of a variety of physical (e.g., energy, momentum) and chemical components (gas, particles) between the atmosphere and the polar ocean. Not only is sea ice an interface between the atmosphere and ocean, it is also an important environment in its own right and is the location for many important biological and chemical processes (Fritsen et al. 1994; Garnett et al. 2019; King et al. 2005; Vancoppendle et al. 2013).
Natural sea ice is difficult and expensive to access. Sea ice is also extremely heterogeneous in space and time (Miller et al. 2015) with interesting phenomena occurring during formation and melting. The lack of observational data means that many scientific questions remain to be addressed (Swart et al. 2019). Observing laboratory-grown sea ice, where experimental conditions can be carefully controlled in sea-ice tanks, is one way of addressing this knowledge gap. A variety of experimental approaches over a range of scales have been developed and have recently been reviewed by Thomas et al. (2021). However, the enclosed tank environment poses its own challenges (e.g., Thomas 2018; Thomas et al. 2021). Wall effects can alter the sea-ice freeboard and stresses within the sea ice. Severe super-cooling can occur in the “ocean” of sea-ice tanks, damaging instrumentation and hindering measurements. Additionally, as salt is partitioned between the sea ice and the ocean, the ocean salinity can increase to unrealistic levels (Cox and Weeks 1975).
This protocol is relevant for any facility growing artificial sea ice. Though facility specific issues may limit the implementation of some of the procedures grown here, experimenters will need to keep the issues raised in mind when designing experiments and contextualizing their results.
Tank effects must be mitigated to some degree in order to grow artificial sea ice that is scientifically relevant and that can be reasonably compared to numerical models. The generality of sea-ice tank results is increased if the artificial sea ice closely approximates natural sea ice, at least for relevant experimental parameters. If the artificial sea ice is being observed to evaluate numerical models, then experimenters must be careful that key model assumptions are satisfied.
8.3.2 Preparation of Synthetic Sea-Ice Growth
To grow artificial sea ice, researchers must first make their artificial ocean, with a realistic and quantified salinity and composition.
Secondly, they should have a facility that allows a downward freezing of the ocean surface. The ocean is contained in a tank and cooled to near its freezing point. Further cooling should only affect the ocean surface, which will result in the formation of sea ice at its surface. Additional cooling of this sea-ice layer will result in sea-ice growth and a thickening of the sea-ice layer as the sea-ice/ocean interface advances downward.
There are two important aspects of natural sea-ice growth that are difficult to accomplish in the laboratory. First, the upper part of natural sea ice is exposed to extremely cold atmosphere (≤−60 °C) while the bottom part is constantly at the seawater freezing (−1.86 (around −2 °C)). In a tank experiment, the challenge is to expose the surface ocean and sea ice to freezing temperature while maintaining the bulk-underlying ocean at or just above the freezing point. Maintaining the ocean above freezing prevents super-cooling effect.
Second, natural sea ice is generally free floating, with a freeboard of around 10% of the sea-ice thickness. In the laboratory, sea ice tends to attach to the tank walls, which increases the hydrostatic pressure into the tank due to volume expansion of the sea-ice ocean system. Non-floating sea ice induces generally an artificial upward movement of seawater moving into the ice, which floods the sea-ice surface (Rysgaard et al. 2014).
To ensure freezing from the seawater surface, eliminate super-cooling and avoid ice formation along the walls, the tank sides need to be heavily insulated from the cold atmosphere and/or slightly heated (Naumann et al. 2012; Wettlaufer et al. 1997; Cox and Weeks 1975). Trial and error is required to find the right level of insulation and heating. Our best methodology is to mount heating pads between the glass and the surrounding instrumentation (Fig. 8.13). If sea ice is observed to creep down the tank sides, or if sea ice forms on instrumentation or the corners/base of the tank, the insulation and heating were not sufficient. If the sea ice forms a bowl shape, with greatly reduced thickness at the sides of the tank, the heating is too strong. Bowl-shaped sea ice and creeping sea ice are both visible in Fig. 8.14. With heating pads placed in direct contact with the water, the heating was too strong and local, where a heating pad broke in this run sea ice can be seen to creep down the side of the tank.
Heating the sides of the tank may be sufficient to maintain free-floating sea ice. Such an approach is particularly effective when the tank sides are smooth (glass, for example) or if they are angled such that the sea ice forms in a wedge shape, wider at the top than the bottom, and so floats up. Free floating sea ice bobs when pushed and when a hole is cut in the surface the water line is shallower than the surface.
We recommend having temperature probes recording the temperature in the atmosphere to ensure that temperature stay below freezing. We advise to also monitor the temperature along the tank walls to have a better control of the heat input and avoid freezing on the wall. Finally, monitoring the seawater salinity and bulk temperature with a CTD is necessary to detect potential super-cooling effect.
Our main limitation is linked to the absence of a dilution reservoir. When sea ice forms, it rejects salt into underlying water, which causes an increase in salinity of our artificial ocean. To maintain a constant salinity in our artificial ocean during a sea-ice growth experiment, we need to install a dilution system.
8.3.3 Step-by-Step Procedure for Growing Synthetic Sea-Ice
The first step in growing artificial sea ice is to prepare the tank. First, we place heating film (220 W/m2) on the outside surface of tank walls (Fig. 8.13). Secondly, we insulate the tank sides with quilt insulation and 10 cm of Dow Floormate 500A foam. This setup is sufficient for us to prevent super-cooling in the ocean and to maintain free-floating sea ice up to at least 20 cm thickness.
Ocean instrumentation is mounted on a fixed pole, while sea-ice instrumentation is mounted on a pole that is free to rise in the vertical (Fig. 8.14). As sea ice grows, it is therefore free to rise and maintain a natural freeboard. Cables for all instrumentation are run out of the tank through the ocean and a smaller tank attached to the main tank. These cables therefore do not disturb the sea-ice surface. Pumps are also installed that allow mixing of the ocean. These pumps face each other so at to generate turbulence while minimizing currents (Loose et al. 2011).
Once the insulation and instrumentation are in place, the tank is filled with some artificial ocean. The salinity of this ocean should be realistic (28–35 g kg–1) and the salt composition should be well characterized. Knowing the precise salt composition allows the freezing point of the ocean to be accurately modeled. We often use pure NaCl. When a natural salt composition is required, we use filtered, real seawater, or some aquarium salt mix (Tropic Marin). Salts are mixed with deionised water using the pumps, generally taking around a day to dissolve.
The ocean then needs to be cooled to near its freezing point. We set the coldroom to −20 °C and run the pumps on full during this cooling period to ensure to have a well-mixed seawater before the start of an experiment. When we are ready to start the experiment, we turn the pumps off or set them to their minimum flow rate. Sea-ice formation then begins within an hour or so providing the ocean is within a few tenths of a degree of its freezing point.
During an experiment it is best to enter the coldroom as infrequently as possible. Each time the door is opened there is an influx of warm, moist air into the coldroom, disrupting experimental conditions. Similar to Naumann et al. (2012), when the pumps are on, a layer of grease ice will form and with pumps off nilas will form (Fig. 8.15). A few periodic checks may be necessary, depending on the nature of the experiment. Whether or not the sea ice is free floating can be checked by gently pressing the sea ice at one corner. If it bobs it is free floating. When sampling sea ice, the freeboard can be checked and compared with that expected from the thickness of the sea ice. A shiny wet upper surface is a sign that the sea ice may have fixed to the tank sides and that the surface has flooded. Super-cooling can be inferred by precisely measuring the ocean temperature and salinity, and comparing the in situ temperature to the salinity-dependant freezing point. Severe super-cooling tends to make the ocean salinity and temperature readings increasingly noisy.
In some experiments, sea ice is grown from a cold plate (Wettlaufer et al. 1997; Eide and Martin 1975; Niederauer and Martin 1979; Middleton et al. 2016) in direct contact with the sea-ice surface. The position of the upper interface is defined in this case and the freeboard of the sea ice can only be maintained by adjusting the ocean volume using a hydrostatic pressure release valve. In small tanks, the heating required to maintain free-floating sea ice may affect the sea-ice growth to such a degree as to be prohibitive.
Future work involves the deployment of atmospheric measurements above the sea ice in order to qualify the impact of sea-ice growth on decay on atmospheric chemistry. The facility is already equipped with several dedicated gas analyzers. A Los Gatos greenhouse gas analyzer (Los Gatos 30R-EP) measures CO2, CH4, and H2O vapor. A T200 UP Teledyne measures NOx, a T200 U Teledyne measures NOy, and there is an ozone analyzer (T400 Teledyne) and generator. A lighting rack sits already between 1.5 m above sea-ice tank surface to allow atmospheric photochemical experiments. Solar spectrum LED (FluenceSolar Max), UV-Aa (Cleo performance 100 W), and UV-B (Phillips broadband TL100W) fluorescent bulbs are evenly spaced over the tank in sets of three, with 24 lights in total (Fig. 8.16). Currently, we can create an artificial atmosphere above the main tank by attaching cuboid 50 μm FEP Teflon atmosphere. FEP Teflon is transparent in the visible and UV spectrum, and chemically inert, making it ideal for many photochemical experiments. When the tank is covered with an artificial atmosphere, the temperature and the humidity of the contained headspace increase. The increase of temperature decreases drastically the ice-growing process and the increase of humidity causes ice formation on surfaces in the headspace inducing condensation and refreezing on the Teflon atmosphere. To pursue measurements in the artificial atmosphere, we should need to develop a system extracting the heat and humidity trap in the headspace during ice growth.to extract heat and moisture from the headspace.
References
Abbatt, J.P.D., Benz, S., Cziczo, D.J., Kanji, Z., Lohmann, U., Möhler, O.: Solid ammonium sulfate aerosols as ice nuclei: a pathway for cirrus cloud formation. Science 313, 1770–1773 (2006). https://doi.org/10.1126/science.1129726
Abbatt, J.P.D., Thomas, J.L., Abrahamsson, K., Boxe, C., Granfors, A., Jones, A.E., King, M.D., Saiz-Lopez, A., Shepson, P.B., Sodeau, J., Toohey, D.W., Toubin, C., von Glasow, R., Wren, S.N., Yang, X.: Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmos. Chem. Phys. 12, 6237–6271 (2012). https://doi.org/10.5194/acp-12-6237-2012
Alpert, P.A., Ciuraru, R., Rossignol, S., Passananti, M., Tinel, L., Perrier, S., Dupart, Y., Steimer, S.S., Ammann, M., Donaldson, D.J., George, C.: Fatty acid surfactant photochemistry results in new particle formation. Sci. Rep. 7, 12693 (2017). https://doi.org/10.1038/s41598-017-12601-2
Augustin, S., Wex, H., Niedermeier, D., Pummer, B., Grothe, H., Hartmann, S., Tomsche, L., Clauss, T., Voigtländer, J., Ignatius, K., Stratmann, F.: Immersion freezing of birch pollen washing water. Atmos. Chem. Phys. 13, 10989–11003 (2013). https://doi.org/10.5194/acp-13-10989-2013
Augustin-Bauditz, S., Wex, H., Kanter, S., Ebert, M., Niedermeier, D., Stolz, F., Prager, A., Stratmann, F.: The immersion mode ice nucleation behavior of mineral dusts: a comparison of different pure and surface modified dusts. Geophys. Res. Lett. 41, 7375–7382 (2014). https://doi.org/10.1002/2014GL061317
Barsotti, F., Brigante, M., Sarakha, M., Maurino, V., Minero, C., Vione, D.: Photochemical processes induced by the irradiation of 4-hydroxybenzophenone in different solvents. Photochem. Photobiol. Sci. 14, 2087–2096 (2015). https://doi.org/10.1039/c5pp00214a
Bernard, F., Ciuraru, R., Boréave, A., George, C.: Photosensitized formation of secondary organic aerosols above the air/water interface. Environ. Sci. Technol. 50, 8678–8686 (2016). https://doi.org/10.1021/acs.est.6b03520
Bodenschatz, E., Malinowski, S.P., Shaw, R.A., Stratmann, F.: Can we understand clouds without turbulence? Science 327, 970–971 (2010). https://doi.org/10.1126/science.1185138
Bohren, C.F., Albrecht, B.A.: Atmospheric Thermodynamics. Oxford University Press, New York (1998)
Boulon, J., Sellegri, K., Katrib, Y., Wang, J., Miet, K., Langmann, B., Laj, P., Doussin, J.F.: Sub-3 nm particles detection in a large photoreactor background: possible implications for new particles formation studies in a smog chamber. Aerosol Sci. Technol. 47, 153–157 (2013). https://doi.org/10.1080/02786826.2012.733040
Bruggemann, M., Hayeck, N., Bonnineau, C., Pesce, S., Alpert, P.A., Perrier, S., Zuth, C., Hoffmann, T., Chen, J.M., George, C.: Interfacial photochemistry of biogenic surfactants: a major source of abiotic volatile organic compounds. Faraday Discuss. 200, 59–74 (2017). https://doi.org/10.1039/c7fd00022g
Brüggemann, M., Hayeck, N., George, C.: Interfacial photochemistry at the ocean surface is a global source of organic vapors and aerosols. Nat. Commun. 9, 2101 (2018). https://doi.org/10.1038/s41467-018-04528-7
Chai-Mei, J.: Ambient Air Treatment by Titanium Dioxide (TiO2) Based Photocatalyst in Hong Kong The Chinese University of Hong Kong, Hong KongTender Ref. AS 00–467 (2002)
Chandrakar, K.K., Cantrell, W., Chang, K., Ciochetto, D., Niedermeier, D., Ovchinnikov, M., Shaw, R.A., Yang, F.: Aerosol indirect effect from turbulence-induced broadening of cloud-droplet size distributions. Proc. Natl. Acad. Sci. 113, 14243–14248 (2016). https://doi.org/10.1073/pnas.1612686113
Chang, K., Bench, J., Brege, M., Cantrell, W., Chandrakar, K., Ciochetto, D., Mazzoleni, C., Mazzoleni, L.R., Niedermeier, D., Shaw, R.A.: A laboratory facility to study gas–aerosol–cloud interactions in a turbulent environment: the Π chamber. Bull. Am. Meteor. Soc. 97, 2343–2358 (2016). https://doi.org/10.1175/bams-d-15-00203.1
Chang, K., Bench, J., Brege, M., Cantrell, W., Chandrakar, K., Ciochetto, D., Mazzoleni, C., Mazzoleni, L.R., Niedermeier, D., Shaw, R.A.: A Laboratory facility to study gas–aerosol–cloud interactions in a turbulent environment: the Π chamber. Bull. Am. Meteor. Soc. 97, 2343–2358 (2017). https://doi.org/10.1175/bams-d-15-00203.1
Chuang, P.Y., Charlson, R.J., Seinfeld, J.H.: Kinetic limitations on droplet formation in clouds. Nature 390, 594–596 (1997). https://doi.org/10.1038/37576
Ciuraru, R., Fine, L., van Pinxteren, M., D’Anna, B., Herrmann, H., George, C.: Photosensitized production of functionalized and unsaturated organic compounds at the air-sea interface. Sci. Rep. 5, 12741 (2015). https://doi.org/10.1038/srep12741
Connolly, P.J., Saunders, C.P.R., Gallagher, M.W., Bower, K.N., Flynn, M.J., Choularton, T.W., Whiteway, J., Lawson, R.P.: Aircraft observations of the influence of electric fields on the aggregation of ice crystals. Q. J. r. Meteorol. Soc. 131, 1695–1712 (2005). https://doi.org/10.1256/qj.03.217
Connolly, P.J., Möhler, O., Field, P.R., Saathoff, H., Burgess, R., Choularton, T., Gallagher, M.: Studies of heterogeneous freezing by three different desert dust samples. Atmos. Chem. Phys. 9, 2805–2824 (2009). https://doi.org/10.5194/acp-9-2805-2009
Connolly, P.J., Emersic, C., Field, P.R.: A laboratory investigation into the aggregation efficiency of small ice crystals. Atmos. Chem. Phys. 12, 2055–2076 (2012). https://doi.org/10.5194/acp-12-2055-2012
Cox, G.F., Weeks, W.: Brine Drainage and Initial Salt Entrapment in Sodium Chloride Ice (1975)
Cui, L., Li, R., Fu, H., Li, Q., Zhang, L., George, C., Chen, J.: Formation features of nitrous acid in the offshore area of the East China Sea. Sci. Total Environ. 682, 138–150 (2019). https://doi.org/10.1016/j.scitotenv.2019.05.004
Cunliffe, M., Upstill-Goddard, R.C., Murrell, J.C.: Microbiology of aquatic surface microlayers. FEMS Microbiol. Rev. 35, 233–246 (2011). https://doi.org/10.1111/j.1574-6976.2010.00246.x
Cunliffe, M., Engel, A., Frka, S., Gašparović, B., Guitart, C., Murrell, J.C., Salter, M., Stolle, C., Upstill-Goddard, R., Wurl, O.: Sea surface microlayers: a unified physicochemical and biological perspective of the air–ocean interface. Prog. Oceanogr. 109, 104–116 (2013). https://doi.org/10.1016/j.pocean.2012.08.004
Cziczo, D.J., Ladino, L., Boose, Y., Kanji, Z.A., Kupiszewski, P., Lance, S., Mertes, S., Wex, H.: Measurements of ice nucleating particles and ice residuals. Meteorol. Monogr. 58, 8.1–8.13, https://doi.org/10.1175/amsmonographs-d-16-0008.1 (2017)
de Leeuw, G., Andreas, E.L., Anguelova, M.D., Fairall, C.W., Lewis, E.R., O’Dowd, C., Schulz, M., Schwartz, S. E.: Production flux of sea spray aerosol. Rev. Geophys. 49, https://doi.org/10.1029/2010RG000349 (2011)
DeMott, P.J., Rogers, D.C.: Freezing nucleation rates of dilute solution droplets measured between −30° and −40 °C in laboratory simulations of natural clouds. J. Atmosph. Sci. 47, 1056–1064 (1990). https://doi.org/10.1175/1520-0469(1990)047%3c1056:fnrods%3e2.0.co;2
DeMott, P.J., Möhler, O., Cziczo, D.J., Hiranuma, N., Petters, M.D., Petters, S.S., Belosi, F., Bingemer, H.G., Brooks, S.D., Budke, C., Burkert-Kohn, M., Collier, K.N., Danielczok, A., Eppers, O., Felgitsch, L., Garimella, S., Grothe, H., Herenz, P., Hill, T.C.J., Höhler, K., Kanji, Z.A., Kiselev, A., Koop, T., Kristensen, T.B., Krüger, K., Kulkarni, G., Levin, E.J.T., Murray, B.J., Nicosia, A., O’Sullivan, D., Peckhaus, A., Polen, M.J., Price, H.C., Reicher, N., Rothenberg, D.A., Rudich, Y., Santachiara, G., Schiebel, T., Schrod, J., Seifried, T.M., Stratmann, F., Sullivan, R.C., Suski, K.J., Szakáll, M., Taylor, H.P., Ullrich, R., Vergara-Temprado, J., Wagner, R., Whale, T.F., Weber, D., Welti, A., Wilson, T.W., Wolf, M.J., Zenker, J.: The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): laboratory intercomparison of ice nucleation measurements. Atmos. Meas. Tech. 11, 6231–6257 (2018). https://doi.org/10.5194/amt-11-6231-2018
Donaldson, D.J., and Anderson, D.: Adsorption of atmospheric gases at the air−water interface. 2. C1−C4 alcohols, acids, and acetone. J. Phys. Chem. A 103, 871–876, https://doi.org/10.1021/jp983963h (1999)
Ehn, M., Thornton, J.A., Kleist, E., Sipilä, M., Junninen, H., Pullinen, I., Springer, M., Rubach, F., Tillmann, R., Lee, B., Lopez-Hilfiker, F., Andres, S., Acir, I.-H., Rissanen, M., Jokinen, T., Schobesberger, S., Kangasluoma, J., Kontkanen, J., Nieminen, T., Kurtén, T., Nielsen, L.B., Jørgensen, S., Kjaergaard, H.G., Canagaratna, M., Maso, M.D., Berndt, T., Petäjä, T., Wahner, A., Kerminen, V.-M., Kulmala, M., Worsnop, D.R., Wildt, J., Mentel, T.F.: A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014). https://doi.org/10.1038/nature13032
Emersic, C., Connolly, P.J., Boult, S., Campana, M., Li, Z.: Investigating the discrepancy between wet-suspension- and dry-dispersion-derived ice nucleation efficiency of mineral particles. Atmos. Chem. Phys. 15, 11311–11326 (2015). https://doi.org/10.5194/acp-15-11311-2015
Frey, W., Hu, D., Dorsey, J., Alfarra, M.R., Pajunoja, A., Virtanen, A., Connolly, P., McFiggans, G.: The efficiency of secondary organic aerosol particles acting as ice-nucleating particles under mixed-phase cloud conditions. Atmos. Chem. Phys. 18, 9393–9409 (2018). https://doi.org/10.5194/acp-18-9393-2018
Fritsen, C.H., Lytle, V.I., Ackley, S.F., Sullivan, C.W.: Autumn bloom of antarctic pack-ice algae. Science 266, 782–784 (1994). https://doi.org/10.1126/science.266.5186.782
Gallagher, M.W., Connolly, P.J., Crawford, I., Heymsfield, A., Bower, K.N., Choularton, T.W., Allen, G., Flynn, M.J., Vaughan, G., Hacker, J.: Observations and modelling of microphysical variability, aggregation and sedimentation in tropical anvil cirrus outflow regions. Atmos. Chem. Phys. 12, 6609–6628 (2012). https://doi.org/10.5194/acp-12-6609-2012
Garbe, C.S., Rutgersson, A., Boutin, J., de Leeuw, G., Delille, B., Fairall, C. W., Gruber, N., Hare, J., Ho, D.T., Johnson, M.T., Nightingale, P.D., Pettersson, H., Piskozub, J., Sahlée, E., Tsai, W.-T., Ward, B., Woolf, D.K., Zappa, C.J.: Transfer across the air-sea interface. In: Liss, P.S., Johnson, M.T. (eds.), Ocean-Atmosphere Interactions of Gases and Particles. Springer, Berlin, Heidelberg, 55–112 (2014)
Garnett, J., Halsall, C., Thomas, M., France, J., Kaiser, J., Graf, C., Leeson, A., Wynn, P.: Mechanistic insight into the uptake and fate of persistent organic pollutants in sea ice. Environ. Sci. Technol. 53, 6757–6764 (2019). https://doi.org/10.1021/acs.est.9b00967
Grawe, S., Augustin-Bauditz, S., Hartmann, S., Hellner, L., Pettersson, J.B.C., Prager, A., Stratmann, F., Wex, H.: The immersion freezing behavior of ash particles from wood and brown coal burning. Atmos. Chem. Phys. 16, 13911–13928 (2016). https://doi.org/10.5194/acp-16-13911-2016
Grawe, S., Augustin-Bauditz, S., Clemen, H.C., Ebert, M., Eriksen Hammer, S., Lubitz, J., Reicher, N., Rudich, Y., Schneider, J., Staacke, R., Stratmann, F., Welti, A., Wex, H.: Coal fly ash: linking immersion freezing behavior and physicochemical particle properties. Atmos. Chem. Phys. 18, 13903–13923 (2018). https://doi.org/10.5194/acp-18-13903-2018
Hartmann, S., Niedermeier, D., Voigtländer, J., Clauss, T., Shaw, R.A., Wex, H., Kiselev, A., Stratmann, F.: Homogeneous and heterogeneous ice nucleation at LACIS: operating principle and theoretical studies. Atmos. Chem. Phys. 11, 1753–1767 (2011). https://doi.org/10.5194/acp-11-1753-2011
Hartmann, S., Augustin, S., Clauss, T., Wex, H., Šantl-Temkiv, T., Voigtländer, J., Niedermeier, D., Stratmann, F.: Immersion freezing of ice nucleation active protein complexes. Atmos. Chem. Phys. 13, 5751–5766 (2013). https://doi.org/10.5194/acp-13-5751-2013
Hartmann, S., Wex, H., Clauss, T., Augustin-Bauditz, S., Niedermeier, D., Rösch, M., Stratmann, F.: Immersion freezing of kaolinite: scaling with particle surface area. J. Atmos. Sci. 73, 263–278 (2016). https://doi.org/10.1175/jas-d-15-0057.1
Henning, S., Wex, H., Hennig, T., Kiselev, A., Snider, J.R., Rose, D., Dusek, U., Frank, G.P., Pöschl, U., Kristensson, A., Bilde, M., Tillmann, R., Kiendler-Scharr, A., Mentel, T.F., Walter, S., Schneider, J., Wennrich, C., and Stratmann, F.: Soluble mass, hygroscopic growth, and droplet activation of coated soot particles during LACIS Experiment in November (LExNo). J. Geophys. Res. Atmosph., 115, https://doi.org/10.1029/2009JD012626 (2010)
Heymsfield, A.J., Westbrook, C.D.: Advances in the estimation of ice particle fall speeds using laboratory and field measurements. J. Atmosph. Sci. 67, 2469–2482 (2010)
Hiranuma, N., Augustin-Bauditz, S., Bingemer, H., Budke, C., Curtius, J., Danielczok, A., Diehl, K., Dreischmeier, K., Ebert, M., Frank, F., Hoffmann, N., Kandler, K., Kiselev, A., Koop, T., Leisner, T., Möhler, O., Nillius, B., Peckhaus, A., Rose, D., Weinbruch, S., Wex, H., Boose, Y., DeMott, P.J., Hader, J.D., Hill, T.C.J., Kanji, Z.A., Kulkarni, G., Levin, E.J.T., McCluskey, C.S., Murakami, M., Murray, B.J., Niedermeier, D., Petters, M.D., O’Sullivan, D., Saito, A., Schill, G.P., Tajiri, T., Tolbert, M.A., Welti, A., Whale, T.F., Wright, T.P., Yamashita, K.: A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques. Atmos. Chem. Phys. 15, 2489–2518 (2015). https://doi.org/10.5194/acp-15-2489-2015
Hobbs, P.V.: research on clouds and precipitation past, present and future. Part II, Bull. Amer. Meteorol. Soc. 72, 184–191 (1991)
Eide, L.I., Martin, S.: The formation of brine drainage features in young sea ice. J. Glaciol. 14, 137–154 (1975). https://doi.org/10.3189/s0022143000013460
Kamm, S., Möhler, O., Naumann, K.H., Saathoff, H., Schurath, U.: The heterogeneous reaction of ozone with soot aerosol. Atmos. Environ. 33, 4651–4661 (1999). https://doi.org/10.1016/S1352-2310(99)00235-6
King, M.D., France, J.L., Fisher, F.N., Beine, H.J.: Measurement and modelling of UV radiation penetration and photolysis rates of nitrate and hydrogen peroxide in Antarctic sea ice: An estimate of the production rate of hydroxyl radicals in first-year sea ice. J. Photochem. Photobiol. A 176, 39–49 (2005). https://doi.org/10.1016/j.jphotochem.2005.08.032
Klaviņš, M., Purmalis, O.: Surface activity of humic acids depending on their origin and humification degree. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences., 67, 493–499, https://doi.org/10.2478/prolas-2013-0083 (2014)
Kreidenweis, S.M., Petters, M., Lohmann, U.: 100 years of progress in cloud physics, aerosols, and aerosol chemistry research. Meteorol. Monogr. 59, 11.11–11.72, https://doi.org/10.1175/amsmonographs-d-18-0024.1 (2019)
Law, C.S., Brévière, E., de Leeuw, G., Garçon, V., Guieu, C., Kieber, D.J., Kontradowitz, S., Paulmier, A., Quinn, P.K., Saltzman, E.S., Stefels, J., von Glasow, R.: Evolving research directions in surface ocean-lower atmosphere (SOLAS) science. Environ. Chem. 10, 1–16 (2013). https://doi.org/10.1071/EN12159
Liss, P.S., Duce, R.A.: The Sea Surface and Global Change. Cambridge University Press (1997)
Liss, P.S., Johnson, M.T.: Ocean-Atmosphere Interactions of Gases and Particles. Springer (2014)
List, R., Hallett, J., Warner, J., Reinking, R.: The future of Laboratory Research and Facilities for Cloud Physics and Cloud Chemistry 0003–0007, 1389–1403 (1986)
Liu, C., Lee Panetta, R., Yang, P.: The effects of surface roughness on the scattering properties of hexagonal columns with sizes from the Rayleigh to the geometric optics regimes. J. Quant. Spectrosc. Radiat. Transfer 129, 169–185 (2013). https://doi.org/10.1016/j.jqsrt.2013.06.011
Loose, B., Schlosser, P., Perovich, D., Ringelberg, D., Ho, D.T., Takahashi, T., Richter-Menge, J., Reynolds, C.M., McGillis, W.R., Tison, J.L.: Gas diffusion through columnar laboratory sea ice: implications for mixed-layer ventilation of CO2 in the seasonal ice zone. Tellus B: Chem. Phys. Meteorol. 63, 23–39 (2011). https://doi.org/10.1111/j.1600-0889.2010.00506.x
Malinowski, S.P., Andrejczuk, M., Grabowski, W.W., Korczyk, P., Kowalewski, T.A., Smolarkiewicz, P.K.: Laboratory and modeling studies of cloud–clear air interfacial mixing: anisotropy of small-scale turbulence due to evaporative cooling. New J. Phys. 10, 075020 (2008). https://doi.org/10.1088/1367-2630/10/7/075020
Mason, B.J., Ludlam, F.H.: The microphysics of clouds. Rep. Prog. Phys. 14, 147–195 (1951). https://doi.org/10.1088/0034-4885/14/1/306
Middleton, C.A., Thomas, C., De Wit, A., Tison, J.L.: Visualizing brine channel development and convective processes during artificial sea-ice growth using Schlieren optical methods. J. Glaciol. 62, 1–17 (2016). https://doi.org/10.1017/jog.2015.1
Miller, L.A., Fripiat, F., Else, B.G.T., Bowman, J.S., Brown, K.A., Collins, R.E., Ewert, M., Fransson, A., Gosselin, M., Lannuzel, D., Meiners, K. M., Michel, C., Nishioka, J., Nomura, D., Papadimitriou, S., Russell, L. M., Sørensen, L. L., Thomas, D.N., Tison, J.-L., van Leeuwe, M.A., Vancoppenolle, M., Wolff, E.W., Zhou, J.: Methods for biogeochemical studies of sea ice: the state of the art, caveats, and recommendations. Elementa Sci. Anthropocene 3, https://doi.org/10.12952/journal.elementa.000038 (2015)
Mitchell, D.L., Mishra, S., Lawson, R.P.: Representing the ice fall speed in climate models: results from tropical composition, cloud and climate coupling (TC4) and the indirect and semi-direct aerosol campaign (ISDAC). J. Geophys. Res. Atmosph. 116, https://doi.org/10.1029/2010JD015433 (2011)
Möhler, O., Stetzer, O., Schaefers, S., Linke, C., Schnaiter, M., Tiede, R., Saathoff, H., Krämer, M., Mangold, A., Budz, P., Zink, P., Schreiner, J., Mauersberger, K., Haag, W., Kärcher, B., Schurath, U.: Experimental investigation of homogeneous freezing of sulphuric acid particles in the aerosol chamber AIDA. Atmos. Chem. Phys. 3, 211–223 (2003). https://doi.org/10.5194/acp-3-211-2003
Möhler, O., Büttner, S., Linke, C., Schnaiter, M., Saathoff, H., Stetzer, O., Wagner, R., Krämer, M., Mangold, A., Ebert, V., Schurath, U.: Effect of sulfuric acid coating on heterogeneous ice nucleation by soot aerosol particles. J. Geophys. Res. Atmosph. 110, https://doi.org/10.1029/2004JD005169 (2005)
Möhler, O., Field, P.R., Connolly, P., Benz, S., Saathoff, H., Schnaiter, M., Wagner, R., Cotton, R., Krämer, M., Mangold, A., Heymsfield, A.J.: Efficiency of the deposition mode ice nucleation on mineral dust particles. Atmos. Chem. Phys. 6, 3007–3021 (2006). https://doi.org/10.5194/acp-6-3007-2006
Möhler, O., Georgakopoulos, D.G., Morris, C.E., Benz, S., Ebert, V., Hunsmann, S., Saathoff, H., Schnaiter, M., Wagner, R.: Heterogeneous ice nucleation activity of bacteria: new laboratory experiments at simulated cloud conditions. Biogeosciences 5, 1425–1435 (2008). https://doi.org/10.5194/bg-5-1425-2008
Naumann, A.K., Notz, D., Håvik, L., Sirevaag, A.: Laboratory study of initial sea-ice growth: properties of grease ice and nilas. Cryosphere 6, 729–741 (2012). https://doi.org/10.5194/tc-6-729-2012
Nenes, A., Ghan, S., Abdul-Razzak, H., Chuang, P.Y., Seinfeld, J.H.: Kinetic limitations on cloud droplet formation and impact on cloud albedo. Tellus B Chem. Phys. Meteorol. 53, 133–149 (2001). https://doi.org/10.3402/tellusb.v53i2.16569
Niedermeier, D., Wex, H., Voigtländer, J., Stratmann, F., Brüggemann, E., Kiselev, A., Henk, H., Heintzenberg, J.: LACIS-measurements and parameterization of sea-salt particle hygroscopic growth and activation. Atmos. Chem. Phys. 8, 579–590 (2008). https://doi.org/10.5194/acp-8-579-2008
Niedermeier, D., Hartmann, S., Shaw, R.A., Covert, D., Mentel, T.F., Schneider, J., Poulain, L., Reitz, P., Spindler, C., Clauss, T., Kiselev, A., Hallbauer, E., Wex, H., Mildenberger, K., Stratmann, F.: Heterogeneous freezing of droplets with immersed mineral dust particles—measurements and parameterization. Atmos. Chem. Phys. 10, 3601–3614 (2010). https://doi.org/10.5194/acp-10-3601-2010
Niedermeier, D., Voigtländer, J., Schmalfuß, S., Busch, D., Schumacher, J., Shaw, R.A., Stratmann, F.: Characterization and first results from LACIS-T: a moist-air wind tunnel to study aerosol–cloud–turbulence interactions. Atmos. Meas. Tech. 13, 2015–2033 (2020). https://doi.org/10.5194/amt-13-2015-2020
Niedrauer, T.M., Martin, S.: An experimental study of brine drainage and convection in Young Sea ice. J. Geophys. Res. Oceans 84, 1176–1186 (1979). https://doi.org/10.1029/JC084iC03p01176
Niemand, M., Möhler, O., Vogel, B., Vogel, H., Hoose, C., Connolly, P., Klein, H., Bingemer, H., DeMott, P., Skrotzki, J., Leisner, T.: A particle-surface-area-based parameterization of immersion freezing on desert dust particles. J. Atmos. Sci. 69, 3077–3092 (2012). https://doi.org/10.1175/jas-d-11-0249.1
Petters, M.D., Wex, H., Carrico, C.M., Hallbauer, E., Massling, A., McMeeking, G.R., Poulain, L., Wu, Z., Kreidenweis, S.M., Stratmann, F.: Towards closing the gap between hygroscopic growth and activation for secondary organic aerosol—Part 2: theoretical approaches. Atmos. Chem. Phys. 9, 3999–4009 (2009). https://doi.org/10.5194/acp-9-3999-2009
Quaas, J., Bony, S., Collins, W.D., Donner, L., Illingworth, A., Jones, A., Lohmann, U., Satoh, M., Schwartz, S.E., Tao, W.-K., Wood, R.: Current understanding and quantification of clouds in the changing climate system and strategies for reducing critical uncertainties. In: Clouds in the Perturbed Climate System, pp. 557–573. MIT Press, Cambridge, MA, USA (2009)
Rossignol, S., Tinel, L., Bianco, A., Passananti, M., Brigante, M., Donaldson, D.J., George, C.: Atmospheric photochemistry at a fatty acid–coated air-water interface. Science 353, 699–702 (2016). https://doi.org/10.1126/science.aaf3617
Rysgaard, S., Wang, F., Galley, R.J., Grimm, R., Notz, D., Lemes, M., Geilfus, N.X., Chaulk, A., Hare, A.A., Crabeck, O., Else, B.G.T., Campbell, K., Sørensen, L.L., Sievers, J., Papakyriakou, T.: Temporal dynamics of ikaite in experimental sea ice. Cryosphere 8, 1469–1478 (2014). https://doi.org/10.5194/tc-8-1469-2014
Saathoff, H., Naumann, K.H., Möhler, O., Jonsson, Å.M., Hallquist, M., Kiendler-Scharr, A., Mentel, T.F., Tillmann, R., Schurath, U.: Temperature dependence of yields of secondary organic aerosols from the ozonolysis of α-pinene and limonene. Atmos. Chem. Phys. 9, 1551–1577 (2009). https://doi.org/10.5194/acp-9-1551-2009
Schnaiter, M., Linke, C., Möhler, O., Naumann, K.-H., Saathoff, H., Wagner, R., Schurath, U., Wehner, B.: Absorption amplification of black carbon internally mixed with secondary organic aerosol. J. Geophys. Res. Atmosph., 110, https://doi.org/10.1029/2005JD006046 (2005)
Seinfeld, J.H., Bretherton, C., Carslaw, K.S., Coe, H., DeMott, P.J., Dunlea, E.J., Feingold, G., Ghan, S., Guenther, A.B., Kahn, R., Kraucunas, I., Kreidenweis, S.M., Molina, M.J., Nenes, A., Penner, J.E., Prather, K.A., Ramanathan, V., Ramaswamy, V., Rasch, P.J., Ravishankara, A.R., Rosenfeld, D., Stephens, G., Wood, R.: Improving our fundamental understanding of the role of aerosol−cloud interactions in the climate system. Proc. Natl. Acad. Sci. 113, 5781–5790 (2016). https://doi.org/10.1073/pnas.1514043113
Sempéré, R., Kawamura, K.: Trans-hemispheric contribution of C2C10 , -dicarboxylic acids, and related polar compounds to water-soluble organic carbon in the western Pacific aerosols in relation to photochemical oxidation reactions. Global Biogeoch. Cycles Global Biogeochem Cycle, 17, https://doi.org/10.1029/2002gb001980 (2003)
Siebert, H., Franke, H., Lehmann, K., Maser, R., Saw, E.W., Schell, D., Shaw, R.A., Wendisch, M.: Probing finescale dynamics and microphysics of clouds with helicopter-borne measurements. Bull. Am. Meteor. Soc. 87, 1727–1738 (2006). https://doi.org/10.1175/bams-87-12-1727
Siebert, H., Shaw, R.A.: Supersaturation fluctuations during the early stage of cumulus formation. J. Atmos. Sci. 74, 975–988 (2017). https://doi.org/10.1175/jas-d-16-0115.1
Simpson, E.L., Connolly, P.J., McFiggans, G.: Competition for water vapour results in suppression of ice formation in mixed-phase clouds. Atmos. Chem. Phys. 18, 7237–7250 (2018). https://doi.org/10.5194/acp-18-7237-2018
Smith, H.R., Connolly, P.J., Baran, A.J., Hesse, E., Smedley, A.R.D., Webb, A.R.: Cloud chamber laboratory investigations into scattering properties of hollow ice particles. J. Quant. Spectrosc. Radiat. Transfer 157, 106–118 (2015). https://doi.org/10.1016/j.jqsrt.2015.02.015
Smith, H.R., Connolly, P.J., Webb, A.R., Baran, A.J.: Exact and near backscattering measurements of the linear depolarisation ratio of various ice crystal habits generated in a laboratory cloud chamber. J. Quant. Spectrosc. Radiat. Transfer 178, 361–378 (2016). https://doi.org/10.1016/j.jqsrt.2016.01.030
Steinke, I., Funk, R., Busse, J., Iturri, A., Kirchen, S., Leue, M., Möhler, O., Schwartz, T., Schnaiter, M., Sierau, B., Toprak, E., Ullrich, R., Ulrich, A., Hoose, C., Leisner, T.: Ice nucleation activity of agricultural soil dust aerosols from Mongolia, Argentina, and Germany. J. Geophys. Res. Atmosph. 121, 13,559–513,576, https://doi.org/10.1002/2016JD025160 (2016).
Stevens, B.: Atmospheric moist convection. Annu. Rev. Earth Planet. Sci. 33, 605–643 (2005). https://doi.org/10.1146/annurev.earth.33.092203.122658
Stratmann, F., Kiselev, A., Wurzler, S., Wendisch, M., Heintzenberg, J., Charlson, R.J., Diehl, K., Wex, H., Schmidt, S.: Laboratory studies and numerical simulations of cloud droplet formation under realistic supersaturation conditions. J. Atmos. Oceanic Tech. 21, 876–887 (2004). https://doi.org/10.1175/1520-0426(2004)021%3c0876:lsanso%3e2.0.co;2
Stratmann, F., Möhler, O., Shaw, R.A., and Wex, H.: Laboratory cloud simulation: capabilities and future directions, in: clouds in the perturbed climate system: their relationship to energy balance, atmospheric dynamics, and precipitation. In: Heintzenberg, J., Charlson, R.J. (eds.) Clouds in the Perturbed Climate System, pp. 149–172. MIT Press, Cambridge, MA, USA (2009)
Stratmann, F., Bilde, M., Dusek, U., Frank, G.P., Hennig, T., Henning, S., Kiendler-Scharr, A., Kiselev, A., Kristensson, A., Lieberwirth, I., Mentel, T.F., Pöschl, U., Rose, D., Schneider, J., Snider, J.R., Tillmann, R., Walter, S., Wex, H.: Examination of laboratory-generated coated soot particles: an overview of the LACIS Experiment in November (LExNo) campaign. J. Geophys. Res. Atmosph., 115, https://doi.org/10.1029/2009JD012628 (2010)
Stubbins, A., Hubbard, V., Uher, G., Law, C.S., Upstill-Goddard, R.C., Aiken, G.R., Mopper, K.: Relating carbon monoxide photoproduction to dissolved organic matter functionality. Environ. Sci. Technol. 42, 3271–3276 (2008). https://doi.org/10.1021/es703014q
Swart, S., Gille, S.T., Delille, B., Josey, S., Mazloff, M., Newman, L., Thompson, A.F., Thomson, J., Ward, B., du Plessis, M.D., Kent, E.C., Girton, J., Gregor, L., Heil, P., Hyder, P., Pezzi, L.P., de Souza, R.B., Tamsitt, V., Weller, R.A., Zappa, C.J.: Constraining southern ocean air-sea-ice fluxes through enhanced observations. Front. Marine Sci. 6, https://doi.org/10.3389/fmars.2019.00421 (2019)
Tajiri, T., Yamashita, K., Murakami, M., Saito, A., Kusunoki, K., Orikasa, N., Lilie, L.: A novel adiabatic-expansion-type cloud simulation chamber. J. Meteorol. Soc. Japan. Ser. II(91), 687–704 (2013). https://doi.org/10.2151/jmsj.2013-509
Thomas, M.: Brine and pressure dynamics in growing sea ice: measurements and modelling in the Roland von Glasow air-sea-ice chamber. School of Environmental Sciences, University of East Anglia, Doctoral (2018)
Thomas, M., France, J., Crabeck, O., Hall, B., Hof, V., Notz, D., Rampai, T., Riemenschneider, L., Tooth, O.J., Tranter, M., Kaiser, J.: The roland von glasow air-sea-ice chamber (RvG-ASIC): an experimental facility for studying ocean–sea-ice–atmosphere interactions. Atmos. Meas. Tech. 14, 1833–1849 (2021). https://doi.org/10.5194/amt-14-1833-2021
Ulanowski, Z., Kaye, P.H., Hirst, E., Greenaway, R.S., Cotton, R.J., Hesse, E., Collier, C.T.: Incidence of rough and irregular atmospheric ice particles from Small Ice Detector 3 measurements. Atmos. Chem. Phys. 14, 1649–1662 (2014). https://doi.org/10.5194/acp-14-1649-2014
Ullrich, R., Hoose, C., Möhler, O., Niemand, M., Wagner, R., Höhler, K., Hiranuma, N., Saathoff, H., Leisner, T.: A new ice nucleation active site parameterization for desert dust and soot. J. Atmos. Sci. 74, 699–717 (2017). https://doi.org/10.1175/jas-d-16-0074.1
Vancoppenolle, M., Meiners, K.M., Michel, C., Bopp, L., Brabant, F., Carnat, G., Delille, B., Lannuzel, D., Madec, G., Moreau, S., Tison, J.-L., van der Merwe, P.: Role of sea ice in global biogeochemical cycles: emerging views and challenges. Quatern. Sci. Rev. 79, 207–230 (2013). https://doi.org/10.1016/j.quascirev.2013.04.011
Wagner, R., Benz, S., Möhler, O., Saathoff, H., Schnaiter, M., Schurath, U.: Mid-infrared extinction spectra and optical constants of supercooled water droplets. J. Phys. Chem. A 109, 7099–7112 (2005). https://doi.org/10.1021/jp051942z
Wettlaufer, J.S., Worster, M.G., Huppert, H.E.: the phase evolution of Young Sea Ice. Geophys. Res. Lett. 24, 1251–1254 (1997). https://doi.org/10.1029/97GL00877
Wex, H., Kiselev, A., Stratmann, F., Zoboki, J., Brechtel, F.: Measured and modeled equilibrium sizes of NaCl and (NH4)2SO4 particles at relative humidities up to 99.1%. J. Geophys. Res. Atmosph., 110, https://doi.org/10.1029/2004JD005507 (2005)
Wex, H., Hennig, T., Salma, I., Ocskay, R., Kiselev, A., Henning, S., Massling, A., Wiedensohler, A., Stratmann, F.: Hygroscopic growth and measured and modeled critical super-saturations of an atmospheric HULIS sample. Geophys. Res. Lett., 34, https://doi.org/10.1029/2006GL028260 (2007)
Wex, H., Petters, M.D., Carrico, C.M., Hallbauer, E., Massling, A., McMeeking, G.R., Poulain, L., Wu, Z., Kreidenweis, S.M., Stratmann, F.: Towards closing the gap between hygroscopic growth and activation for secondary organic aerosol: Part 1—Evidence from measurements. Atmos. Chem. Phys. 9, 3987–3997 (2009). https://doi.org/10.5194/acp-9-3987-2009
Ziese, M., Wex, H., Nilsson, E., Salma, I., Ocskay, R., Hennig, T., Massling, A., Stratmann, F.: Hygroscopic growth and activation of HULIS particles: experimental data and a new iterative parameterization scheme for complex aerosol particles. Atmos. Chem. Phys. 8, 1855–1866 (2008). https://doi.org/10.5194/acp-8-1855-2008
Zink, P., Knopf, D.A., Schreiner, J., Mauersberger, K., Möhler, O., Saathoff, H., Seifert, M., Tiede, R., Schurath, U.: Cryo-chamber simulation of stratospheric H2SO4/H2O particles: Composition analysis and model comparison. Geophys. Res. Lett. 29, 46–41–46–44, https://doi.org/10.1029/2001GL013296 (2002)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Alpert, P.A. et al. (2023). Application of Simulation Chambers to Investigate Interfacial Processes. In: Doussin, JF., Fuchs, H., Kiendler-Scharr, A., Seakins, P., Wenger, J. (eds) A Practical Guide to Atmospheric Simulation Chambers. Springer, Cham. https://doi.org/10.1007/978-3-031-22277-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-22277-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22276-4
Online ISBN: 978-3-031-22277-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)