Abstract
In this chapter, we will describe practical approaches to the evaluation of adrenal masses detected as incidental masses, as well as in other clinical scenarios in the oncological and non-oncological patient. The most commonly used clinical and imaging techniques and procedures used in the evaluation of these masses and their common appearances will also be described. Current European and US guidelines and their limitations will be briefly addressed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Adrenal
- Biopsy
- Cancer
- Computed tomography
- CT
- Functioning
- Incidentaloma
- Mass
- Magnetic resonance imaging
- MRI
- MIBG
- PET-FDG
- Tumor
- Machine learning
10.1 Introduction
Learning Objectives
-
Provide an overview for the evaluation of an adrenal mass in various clinical scenarios
-
Provide an understanding of the different imaging techniques and procedures available for the detection and characterization of adrenal masses
-
Understand the differentiating features between benign and malignant adrenal masses
-
Outline the current recommendations and limitations of European and US clinical practice guidelines for incidental adrenal masses, including treatment options
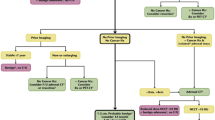
In this chapter, we will define an adrenal “incidentaloma,” describe imaging techniques and procedures used to evaluate adrenal masses, discuss hyperfunctioning lesions/tumors of the gland, and illustrate these with examples, as well as outline current clinical practice guidelines for incidental and functioning adrenal masses including treatment options.
When an adrenal nodule/mass is detected on imaging, its appearance as well as some detailed history as listed below may help at arriving at an initial list of diagnoses.
-
1.
Presence of morphological/internal features such as presence of macroscopic fat, fluid density, or other specific features?
-
2.
Known underlying or history of a prior malignancy?
-
3.
Stability of adrenal mass compared to prior imaging exams
-
4.
Adrenal hyperfunction
This chapter will be divided into:
-
(1) The incidental adrenal mass in non-oncology, (2) the adrenal mass detected in patients with an underlying malignancy, and (3) Imaging evaluation in patients suspected of harboring hyperfunctioning adrenal lesions.
10.2 Incidental Adrenal Mass: No Underlying Malignancy
An adrenal incidentaloma can be defined as “an unsuspected and asymptomatic mass (measuring ≥1 cm in short axis) detected on imaging exams obtained for purposes other than detection of adrenal disease.”
The overwhelming majority of incidentalomas are benign, i.e., non-functioning adrenal cortical adenomas [1, 2]. Other common benign adrenal masses include myelolipoma, cyst, and adrenal hemorrhage, neurogenic tumors among other rare lesions. If an adrenal incidentaloma has >50% of macroscopic fat [myelolipoma as shown in Fig. 10.1a, b] or features of a simple cyst (≤20 HU increase on enhanced CT compared to unenhanced CT), these are specific diagnosis, for which no additional workup or follow-up imaging is needed, except in cases of large myelolipomas which are being managed conservatively, some of which may grow and undergo hemorrhage, and therefore need follow-up imaging. Variable amounts of macroscopic fat can be seen in myelolipomas, with a small amount also seen in degenerated adenomas and rarely in adrenal cancers [3–4]. If an adrenal mass is of high density (higher than that of paraspinal musculature) on unenhanced images and shows <10 HU change between pre- and post-contrast enhanced images, the possibility of adrenal hemorrhage should be suspected in the appropriate clinical setting. Follow-up imaging is essential to exclude hemorrhage into an underlying tumor, except when the hemorrhage is due to trauma or stress such as surgery [5].
There are two features of adenomas that are helpful in their characterization on CT and MRI: (1) the presence of intracellular lipid—low density on unenhanced CT, and loss of signal intensity and chemical shift (CSI) out-of-phase (OOP) MRI and (2) CT contrast washout features—rapid washout on contrast-enhanced CT.
10.2.1 Unenhanced CT
Most incidental adenomas are lipid-rich adenomas (measuring ≤10 HU on unenhanced CT) although between 20%–30%, are lipid-poor adenomas measuring >10 HU [6, 7]. Unenhanced CT density measurement of ≤10HU is highly specific (>95%) for the diagnosis of adenoma; however, some studies have shown that even higher density numbers could be used to diagnose benign adrenal lesions [8, 9]. In a recent study of 250 patients with incidental adrenal masses, who either underwent surgery or follow-up for at least 1 year, it was shown that even if the density threshold on unenhanced CT was raised to <20 HU for lesions <3 cm, and <15 HU for lesions <4 cm, a specificity of 100% for predicting benign lesions could be achieved [8]. However, this threshold, currently lacks sufficient evidence for routine clinical use.
10.2.2 CT Contrast-Washout
Although a threshold of ≤10 HU is used to diagnose lipid-rich adenomas, lipid-poor adenomas do not contain adequate lipid and cannot be diagnosed by non-contrast CT using this threshold as there is overlap in density with primary malignancies and metastases.
As an alternative imaging strategy, differences in CT contrast enhancement and washout, can be used to diagnose non-hypervascular adenomas and differentiate them from metastases. Lipid-rich and lipid-poor adenomas both have rapid washout with intravenous contrast (iodinated CT contrast or MR gadolinium chelates) whereas most metastases do not [7, 10, 11]. Using density measurements, from images obtained at various time points after injection of intravenous contrast, washout calculations can be performed.
Absolute percent washout (APW) % values are calculated by the formula:
A threshold washout value ≥60% is diagnostic of an adenoma.
Relative percent washout (RPW) % can be used when a non-contrast CT is not available, and the dynamic enhanced values are compared to 15-min delayed scans. RPW % is calculated by the formula:
A threshold washout value ≥40% is diagnostic of adenoma.
Specificity for adenoma diagnosis using these washout threshold values was >90%, when first reported in a few small studies that compared adenomas with small numbers of metastases and other malignant lesions, i.e., not incidentalomas [7, 10, 11]. A more recent study of 336 incidentalomas in 299 patients, however showed that for differentiating benign from malignant adrenal nodules (including pheochromocytomas) absolute CT contrast-washout % had a sensitivity of only 77.5% and specificity of 70% [12, 13], as some metastatic hypervascular nodules such as from clear cell renal cell cancer (CCRCC) and hepatocellular carcinoma (HCC), as well approximately 20–30% pheochromocytomas had washout values like adenomas [14, 15]. So, the routine use of CT washout to distinguish between benign and malignant adrenal nodules in incidentally discovered adrenal lesions has limitations but may still be useful in the setting of oncology patients.
10.2.3 Dual Energy CT
Dual energy CT has also been used to characterize lipid-rich adenomas using density measurements from virtual unenhanced images, but this has slightly lower specificity than conventional unenhanced density measurements, as there is a tendency for the technique to overestimate the native unenhanced attenuation values due to incomplete iodine subtraction [16].
10.2.4 MRI
Chemical-shift MRI[CSI-MRI] or out-of-phase (OOP) images can detect of intracellular lipid and diagnose lipid-rich adenomas with a high degree of specificity, demonstrating loss of signal intensity on OOP images as shown in Fig. 10.2a, b. In a meta-analysis study of 1280 adrenal nodules, CSI-MRI, had a pooled sensitivity of 94% and specificity of 95% [17]. However, this high specificity diminishes with lipid-poor adenomas, especially those whose unenhanced CT density exceeds 30 HU [18]. Intracellular lipid-containing metastases from clear cell renal cell carcinoma (CCRCC) and some hepatocellular carcinomas [HCC] [19], can mimic adenomas on CSI-MRI. But imaging characteristics on other sequences, such as increased signal intensity and lesion heterogeneity on T2 images can be used to distinguish these metastases from adenomas [20]. Importantly, these two primary neoplasms (CCRCC, HCC) often have a known primary and other coexisting metastatic disease.
More recently diffusion-weighted imaging has been used to try and differentiate between adenomas and malignant masses, but with limited success [21].
10.2.5 FDG PET/CT
FDG-PET/CT is used as a secondary tool to exclude adrenal malignancy using the SUV max tumor/liver ratio and based on the results of a study of non-cancerous patients found that a threshold of less than 1.5, was suggestive of a benign lesion [22, 23]. Adrenal metastases tend to demonstrate increased metabolic activity, with higher tracer uptake relative to the liver or background, while most benign adenomas do not. This imaging technique has extremely high sensitivity, but the specificity is lower (87–97%), as few adenomas can have mildly increased FDG uptake, mimicking malignant lesions [22, 23].
10.2.6 Lesion Morphology
Risk factors for malignancy include lesion size, and characteristics such as enhancement, heterogeneity, irregular margins, interval change in size, as well as prior a history of malignancy. Current management guidelines used data that suggested the risk of adrenal cortical carcinoma (ACC) based on size was 2%, 6%, and 24% for lesions <4 cm, 4–6 cm, and >6 cm, respectively [24,25,26]. But a recent study of risk assessment in 2219 patients found that the risk is much lower being 0.1%, 2.4%, and 19.5% risk for lesions <4 cm, between 4 and 6 cm, and >6 cm, respectively [27]. However, in addition to size, a patient’s age should also be taken into consideration when risk of ACC is assessed, as incidentalomas are uncommon in patients <40 years. of age and in these patients, additional evaluation to exclude a malignancy is warranted. Lesion characteristics such as margin, heterogeneity, contrast enhancement, have high specificity for the diagnosis of malignant lesions, but the low sensitivity precludes routine application in clinical practice [28].
10.2.7 Adrenal Biopsy
Non-invasive imaging as described above has been employed to successfully characterize most incidentally discovered adrenal masses. Adrenal biopsy is usually employed to definitively diagnose metastatic disease and stage patients with suspected malignancy. It is not recommended in patients with incidental indeterminate adrenal masses, as (1) the diagnosis of adrenal cortical cancer cannot be made definitively with percutaneous needle biopsies, and (2) in cases, where a pheochromocytoma has not been excluded by biochemical evaluation, a biopsy can precipitate an adrenal crisis. CT-guided adrenal biopsy has however been shown to be a safe procedure, with a diagnostic accuracy of 96% and a 3% complication rate although it has a non-diagnostic rate of between 3% and 8.7% [29].
10.2.8 Management
The American College of Radiology Whitepaper on incidental adrenal nodules recommends no further imaging follow-up for patients with no history of malignancy, and small (<4 cm) incidentally discovered homogeneous adrenal masses, measuring <10 HU on unenhanced images, and for other benign lesions such as small myelolipomas and adrenal cysts [30]. The American and European Endocrine Societies, however both recommend a biochemical workup to exclude mild autonomously functioning adrenal lesions, for all adrenal incidentalomas [2, 31]. In many centers in the USA, biochemical evaluation is not routinely performed in asymptomatic patients with small incidental adrenal adenomas. The European Society of Endocrinologists have recently changed their previous guidelines and now do not recommend routine follow-up imaging, for incidental adrenal adenomas, as several recent studies have shown that these lesions rarely grew or became malignant tumors such as an adrenocortical carcinoma [32,33,34,35,36]. The need for follow-up biochemical evaluation is also controversial, and the European Society of Endocrinology now recommends no routine follow-up biochemical evaluation, unless new clinical signs of endocrine activity or comorbidities develop. Patients with mild autonomous cortisol secretion, at initial evaluation, however, do need clinical follow-up evaluation, as they are at risk for developing significant comorbidities of cortisol excess such as hypertension, stress fractures, and diabetes [33,34,35]. In patients with mild autonomous cortisol excess, an adrenalectomy is usually not felt to be necessary. But in the rare circumstance, when an adrenalectomy is thought to be beneficial, and is planned, a follow-up biochemical evaluation is recommended to confirm autonomous cortisol excess prior to surgery.
The American College of Radiology Whitepaper on incidental adrenal masses [30], and the European Society of Endocrinology recommend that if a known adrenal lesion is enlarging or develops a change in morphology or internal features such as necrosis, degeneration, or hemorrhage, then suspicion for malignancy should be raised, and additional biochemical and imaging workup is needed.
In patients with no history of cancer and an indeterminate adrenal mass >4 cm in size, resection should be considered and although this is the current standard, in both Europe and the USA, some recent studies suggest that this should be re-evaluated as the risk of adrenal cancer in masses of this size may have been overestimated. If there is a history of prior cancer, then a PET/CT scan and as indicated, an adrenal biopsy could be performed to exclude metastases [30].
10.3 Evaluation of Adrenal Mass in Patient with Known Extra-Adrenal Malignancy
Evaluation of adrenal gland masses in the oncology patient is problematic because it is not only a frequent site of adenomas but also metastases [estimated risk of metastases is between 26% and 36%] [34, 36]. CT, MRI, PET-FDG, and adrenal biopsy can be used to evaluate adrenal masses in these patients to diagnose adenomas and differentiate them from metastases, as described in the above sections.
Key Points
-
All incidental adrenal nodules/masses should undergo biochemical evaluation (In the absence of clinical symptoms, patients with small incidental adenomas do not undergo biochemical evaluation in many centers in the USA)
-
Lipid-rich adenomas can be differentiated from many metastases using unenhanced CT and CSI-MRI
-
CT washout calculations helpful to distinguish lipid-poor adenomas from non-hypervascular metastases, but limited role in distinguishing lipid-poor adenomas from some metastases and some pheochromocytomas.
-
Majority of non-functioning adenomas (especially small) usually need no follow-up imaging or biochemical evaluation.
-
In patients with mild autonomous cortisol excess, a follow-up biochemical evaluation is needed only if new clinical signs of endocrine activity or comorbidities develop.
-
In oncology patients with indeterminate adrenal imaging findings, on CT and MRI, a PET-FDG and/or adrenal biopsy may be required to accurately stage the patient to help determine optimal treatment.
-
Current guidelines both in the USA and in Europe suggest that indeterminate masses >4 cm in patients, and with no history of a malignancy, should be surgically removed.
10.4 Evaluation of Patient with Suspected Adrenal Hyperfunction
10.4.1 Adrenal Cortical Hyperfunction
Cushing’s syndrome results from an overproduction of cortisol by the adrenal cortex and can be broadly divided into (1) ACTH-dependent and (2) ACTH-independent causes, resulting in elevated serum cortisol levels. Approximately 80% of Cushing’s is due to ACTH-dependent cause of overstimulation of the adrenal glands by a pituitary adenoma. Primary adrenal cortical tumors: adenoma and adrenal cortical carcinoma [ACC] are ACTH-independent cause of Cushing’s and account for approximately 20% of cases, with <1% being due to ectopic production of ACTH by a neoplasm, located either in the chest, abdomen, or pelvis.
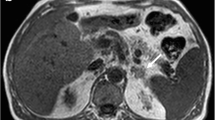
Adrenal cortical carcinomas are large, heterogeneous, and may have areas of calcification. On contrast-enhanced imaging (CT and MRI), they have heterogenous regions of enhancement as shown in Fig. 10.3a, b, and also show increased uptake on FDG-PET imaging [37, 38]. Functioning adenomas causing Cushing’s are smaller in size than ACCs and have an imaging appearance like that of non-functioning adenomas.
Hyperaldosteronism or Conn’s syndrome is suspected in a hypertensive patient with low serum potassium and is confirmed by measuring the serum aldosterone to renin ratio [39]. When the diagnosis is suspected based on biochemical assays, a CT scan is performed to exclude adrenal cortical carcinoma, as the etiology. In younger patients (<40 years), a CT may detect a unilateral small adrenal mass, and if the contralateral adrenal gland appears normal, a diagnosis of aldosterone-producing adenoma can be made with moderate accuracy. If CT findings are normal or equivocal for the detection of an adenoma, as is often the case especially in the older populations, patients with suspected hyperaldosteronism, undergo adrenal venous sampling to localize and lateralize the side of elevated aldosterone production is performed, prior to deciding further management [39].
10.4.2 Adrenal Medullary Hyperfunction
Pheochromocytomas originate from the adrenal medulla and are usually solitary and occur sporadically. Extra-adrenal paragangliomas can occur anywhere along the sympathetic chain. These tumors are seen in subjects with various syndromes such as MEN Type II, von Hippel-Lindau [vHL], and neurofibromatosis type I. More recent studies show that about 25% of pheochromocytomas may be familial. Subjects with mutations in the succinate dehydrogenase subunits are also at risk of developing pheochromocytomas and paragangliomas [38, 40].
The most appropriate first-line test is the measurement of plasma free or and urinary fractionated metanephrines. As >95% of pheochromocytomas originate in the adrenal glands, CT is the main modality that has been recommended. MRI examination can be performed when radiation dose is a consideration or if metastatic disease is suspected [11,12,13,14, 38, 40]. Most pheochromocytomas are moderate-sized tumors and have imaging appearances that overlap with that of other solid tumors such as ACC and metastases as shown in Fig. 10.4a [15, 38]. In patients with MEN and vHL, the tumors are small and multicentric [38].
While meta-iodobenzyl-guanidine (MIBG) scintigraphy has high specificity (>95%) for the diagnosis of pheochromocytoma, as shown in Fig. 10.4b, but its sensitivity only moderate, ranging between 77–90%. Recent studies have suggested that MIBG scintigraphy should be used selectively and only in patients with familial or hereditary disorders, in the detection of metastatic disease, and in patients with biochemical evidence for pheochromocytoma and negative CT or MRI. These studies also concluded that MIBG scintigraphy does not offer any added advantage in patients with biochemical evidence for a pheochromocytoma, and have an adrenal mass detected by CT or MRI but have no hereditary or familial diseases [40, 41].
The standard treatment of a biochemically active adrenal cortical and medullary tumors is laparoscopic or open surgical resection [34, 38].
Key Points
-
In patients with suspected biochemically active adrenal tumors, the role of imaging is primarily to detect the tumor and exclude malignancy (exception pheochromocytoma).
-
In patients with Conn’s syndrome adrenal venous sampling (AVS) is required to lateralize the side of hyperfunction, after a malignant adrenal lesion has been excluded by CT or MRI.
-
Adrenal cortical carcinomas are large heterogeneously enhancing masses.
-
MIBG-scintigraphy is not 100% accurate in detecting pheochromocytomas, but is useful in detecting pheochromocytomas and paragangliomas, in patients with hereditary or familial diseases and detecting metastases.
10.5 Future Directions
There has been a high degree of variability in both radiologist detection of adrenal masses and an even greater variation in radiologist and endocrinologist recommendations for management of incidentally discovered adrenal masses. The American College of Radiology (ACR) Whitepaper for adrenal masses and European Guidelines for management of the incidentally discovered adrenal mass have been published to reduce practice variation and create best practices. The ACR is also developing “at the elbow” tools to standardize reporting for incidental findings and a reporting tool for adrenal masses to assist the radiologist at their workstation is underway. Artificial Intelligence and Machine Learning have been applied in many areas of radiology and hold promise to aid in both the detection of abnormalities and consistent characterization of these imaging findings. A recent publication has shown that machine learning algorithms can accurately segment (find) the adrenal glands on abdominal CT and differentiate adrenal masses from normal glands [42]. This may assist busy radiologists faced with high workloads and provide more consistent care for our patients.
10.6 Concluding Remarks
Most incidentally discovered adrenal masses are benign. But in the setting of a known malignancy, differentiation between a metastases and adenoma is essential to guide management. CT, MRI, and PET-FDG imaging are the main imaging tools available currently in the evaluation and characterization of adrenal masses.
Functioning adrenal lesions can be detected by CT, MRI, and MIBG scintigraphy and in patients with suspected hyperaldosteronism, additional invasive testing with AVS.
Take-Home Messages
-
Incidental adrenal masses are common.
-
Most incidental adrenal masses are benign, non-functioning adenomas.
-
Current guidelines suggest biochemical evaluation for all incidentally discovered adrenal masses with a caveat for small lesions in asymptomatic patients.
-
Unenhanced CT is the most used first line imaging modality used worldwide to characterize incidental adrenal lesions.
-
Chemical shift MRI, CT washout, and PET-FDG are other imaging techniques used.
-
Adrenal venous sampling is used to lateralize the side of hyperfunction in patients with suspected hyperaldosteronism.
-
Pheochromocytomas can overlap the imaging appearance of other adrenal masses such as adrenal cortical carcinomas and metastases.
-
MIBG scintigraphy in the setting of the hereditary and familial diseases is useful in the detection of primary tumors and metastases.
References
Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1049 consecutive adrenal masses in patients with no known malignancy. Am J Roentgenol. 2008;190:1163–8.
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarkis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice guideline in collaboration with the European Network for the study of adrenal tumors. Eur J Endocrinol. 2016;175:G1–G34.
Sahdev A. Imaging incidental adrenal lesions. Br J Radiol. 2022;95:20220281.
Elsayes KM, Mukundan G, Narra V, et al. Adrenal masses: MR imaging features with pathological correlation. Radiographics. 2004;24:S73–86.
Jordan E, Poder L, Courtier J, et al. Imaging of non-traumatic adrenal hemorrhage. Am J Roentgenol. 2012;199:W91–8.
Korobkin M, Giordano TJ, Brodeur FJ. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200:743–7.
Caoili EM, Korobkin M, Francis IR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222:629–33.
Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439–46.
Hong AR, Kim JH, Park KS, et al. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of 2016 ENSAT guidelines in real clinical practice. Eur J Endocrinol. 2017;177:475–83.
Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. Am J Roentgenol. 1998;170:747–52.
Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Imaging characterization of adrenal incidentalomas: principles, techniques, and algorithms. Radiology. 2008;249:756–75.
Corwin MT, Badawy M, Elaine M Caoili EM et al. Incidental adrenal nodules in patients without known malignancy: prevalence of malignancy and utility of washout CT for characterization-A multi-institutional study. Am J Roentgenol 2022; 219:1–10.
Schloetelburg W, Ebert I, Petritsch B, et al. Adrenal wash-out CT: moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol. 2021;186:183–93.
Choi YA, Kim CK, Park BK, et al. Evaluation of adrenal metastases from RCC and HCC. Radiology. 2013;266:514–20.
Woo S, Suh CH, Kim SY, et al. Pheochromocytoma as a frequent false positive in adrenal washout CT; a systematic review and meta-analysis. Eur Radiol. 2018;28(3):1027–36.
Nagayama Y, Inoue T, Oda S, et al. Adrenal adenomas versus metastases: diagnostic performance of dual-energy spectral CT virtual non-contrast imaging and iodine maps. Radiology. 2020;296(2):324–32.
Platzek I, Sieron D, Plodeck V, et al. Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. Eur Radiol. 2019;29(2):806–17.
Seo JM, Park BK, Park SY, et al. Characterization of lipid-poor adrenal adenoma: chemical shift MRI and washout CT. Am J Roentgenol. 2014;202(5):1043–50.
Tariq U, Poder L, Carlson D, Courtier J, Joe BN, Coakley FV. Multimodality imaging of fat-containing adrenal metastasis from hepatocellular carcinoma. Clin Nucl Med. 2012;37:e157–9.
Schieda N, Krishna S, McInnes MDF, et al. Utility of MRI to differentiate clear cell renal cell carcinoma adrenal metastases from adrenal adenomas. Am J Roentgenol. 2017;209(3):W152–9.
Sandrasegaran K, Patel AA, Ramaswamy R, et al. Characterization of adrenal masses with diffusion weighted imaging. Am J Roentgenol. 2011;197(1):132–8.
Guerin C, Pattou F, Brunaud L, et al. Performance of F-FDG PET/CT in the characterization of adrenal masses in non-cancer patients: a prospective study. J Clin Endocrinol Metab. 2017;102:2465–72.
Boland GW, Blake MA, Holalkere NS, Hahn PF. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative versus quantitative accuracy in 150 consecutive patients. Am J Roentgenol. 2009;192:956–62.
Barzon L, Sonino N, Fallo F, et al. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–85.
Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–40.
Cawood TJ, Hunt PJ, O’Shea D, et al. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is like the risk of the adrenal lesion becoming malignant; time for a rethink. Eur J Endocrinol. 2009;161:513–27.
Kahramangil B, Kose E, Remer EK, et al. A modern assessment of cancer risk in adrenal incidentalomas. Analysis of 2219 patients. Ann Surg. 2022;275(1):e238–44.
Song JH, Grand DJ, Beland MD, et al. Morphologic features of 211 adrenal masses at initial contrast-enhanced CT: can we differentiate benign from malignant lesions using imaging features alone? Am J Roentgenol. 2013;201:1248–53.
Bancos I, Tamhane S, Shah M, et al. Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R65–80.
Mayo-Smith WW, Song JH, Boland GL, et al. Management of Incidental adrenal masses: a white paper of the ACR incidental findings committee. Am Coll Radiol. 2017;14:1038–44.
Zeiger MA, Thompson GB, Duh QY, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons: medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1)
Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25(2):178–92.
Cambos S, Tabarin A. Management of adrenal incidentalomas: working through uncertainty. Best Pract Res Clin Endocrinol Metab. 2020;34:101427.
Bancos I, Prete A. Approach to the patient with adrenal incidentaloma. J Clin Endocrinol Metab. 2021;106(11):3331–53.
Elhassan YS, Alahdab F, Prete A, et al. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess. Ann Intern Med. 2019;171:107–11.
Mao JJ, Dages KN, Suresh M, et al. Presentation, disease progression and outcomes of adrenal gland metastases. Clin Endocrinol. 2020;93(5):546–54.
Bharwani N, Rockall AG, Sahdev A, et al. Adrenocortical carcinoma: the range of appearances on CT and MRI. AJR Am J Roentgenol. 2011;196(6):W706–14.
Fassnacht M, Assie G, Baudin G, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2020;31(11):1476–90.
Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–196.
Lenders JWM, Duh QY, EIsenhofer G et al. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99(6):1915–1942.
Greenblatt DY, Shenker Y, Chen H. The utility of meta-iodobenzylguanidine (MIBG) scintigraphy in patients with pheochromocytoma. Ann Surg Oncol. 2008;15:900–5.
Robinson-Weiss C, Patel J, Bizzo BC, et al. Machine learning for adrenal gland segmentation and classification of normal and adrenal masses at CT. Radiology. 2023;306:e220101. Published online September 2022.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Francis, I.R., Mayo-Smith, W.W. (2023). Adrenal Diseases. In: Hodler, J., Kubik-Huch, R.A., Roos, J.E., von Schulthess, G.K. (eds) Diseases of the Abdomen and Pelvis 2023-2026. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-031-27355-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-27355-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27354-4
Online ISBN: 978-3-031-27355-1
eBook Packages: MedicineMedicine (R0)