Abstract
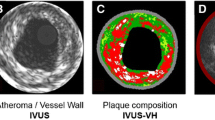
Cardiovascular imaging has recently expanded to the search for unstable plaques at high risk of rupturing and causing acute coronary events. A vulnerable plaque has been described histologically as a plaque with a large lipid core, a thin fibrous cap, and inflammation at the margins of the plaque. One of the most interesting recent findings is the discovery that plaques exhibiting high-risk characteristics may be associated with inducible myocardial ischemia even in the absence of luminal obstruction. Currently, anatomical and functional imaging of coronary atherosclerosis can be performed with computed tomography angiography and positron emission tomography, as briefly reviewed in this chapter.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Cardiovascular imaging has recently expanded to the search for unstable plaques at high risk of rupturing and causing acute coronary events. A vulnerable plaque has been described histologically as a plaque with a large lipid core, a thin fibrous cap, and inflammation at the margins of the plaque. One of the most interesting recent findings is the discovery that plaques exhibiting high-risk characteristics may be associated with inducible myocardial ischemia even in the absence of luminal obstruction. Currently, anatomical and functional imaging of coronary atherosclerosis can be performed with computed tomography angiography and positron emission tomography, as briefly reviewed in this chapter.
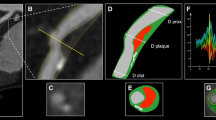
Molecular imaging with positron emission tomography (PET) allows investigators to image some of the components of a high-risk plaque. The PET tracers most used are 18F-fluoro-deoxy-glucose (FDG) and sodium fluoride (18F-NaF). Of these, 18F-fluoro-deoxy-glucose (FDG) is a glucose analogue actively taken up by cells with a high metabolic rate but has the inherent limitation of its preferential uptake by the myocardium that clouds the uptake by the much smaller coronary vessels. 18F-NaF has been in use for a long time to image bone metastases, but it was only recently discovered as a tracer to image atherosclerosis. 18F-NaF accumulates in plaques accruing calcium apatite and all nascent plaques accrue microcalcifications that may evolve into larger deposits. The initial proof that 18F-NaF could identify patients with vulnerable plaques was provided in a study that involved patients in the acute setting. In a study performed with 18F-NaF imaging has been employed in patients with either acute coronary syndromes, or stable angina and/or undergoing carotid endarterectomy. 18F-NaF uptake localized in culprit arteries of patients with acute coronary syndromes, and carotid arteries with ruptured plaques in patients with cerebrovascular events. A similar carotid artery finding was reported in a second publication of patients with stroke or transient ischemic attacks.
5.1 Plaque Imaging
Case 67
History
-
A 61-year-old male presented with chest pain. He had a previous history of an anterior ST elevation myocardial infarction treated with a drug eluting stent to the left anterior descending coronary artery.
-
He underwent invasive coronary angiography (Fig. 5.1) which demonstrated in-stent thrombosis and plaque rupture proximal to the LAD stent. Thrombus was aspirated and a drug eluting stent was inserted into the LAD.
-
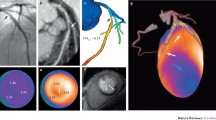
He underwent PET-MRI with 18F-sodium fluoride as part of a research study (Fig. 5.2).
Coronary Angiography
PET/MR Images
Findings
-
18F-sodium fluoride PET-MRI showed radiotracer uptake in the proximal LAD (arrow) at the site of the recent plaque rupture.
Differential Diagnosis
-
ST elevation myocardial infarction caused by plaque rupture and treated with a drug eluting stent.
Correlative Imaging
This patient underwent CCTA (Fig. 5.3).
Management
-
The patient recovered well from his invasive coronary angiography and will be seen in the cardiology clinic.
Teaching Points
-
18F-sodium fluoride uptake can be identified in recent plaque rupture in patients with acute myocardial infarction.
-
18F sodium fluoride is a marker of microcalcification that can be identified in the arteries and valves.
-
PET/MRI offers the opportunity to assess both structure and function, with reduced radiation dose compared to PET/CT.
Further Reading
-
Joshi N, Vesey A, Williams M, Shah A, Calvert P, Craighead F, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. The Lancet. 2014;383:705–713.
-
Irkle A, Vesey A, Lewis D, Skepper J, Bird J, Dweck M, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nature Communications. 2015;6:74–95.
-
Robson P, Dey D, Newby D, Berman D, Li D, Fayad Z, et al. MR/PET Imaging of the Cardiovascular System. JACC: Cardiovascular Imaging. 2017;10:1165–1179.
5.2 Cardiac Toxicity After Chemo/Radiation
5.2.1 Cardiac Involvement at Oncological PET
Case 68
History
-
81-year-old male with diagnosis of beta follicular Non-Hodgkin’s Lymphoma (NHL) undergoing chemotherapy which included anthracycline.
-
He was referred for FDG PET/CT to monitor response to therapy (Fig. 5.4).
PET/CT Imaging
Findings
-
The initial staging whole body FDG PET/CT scan demonstrated stage 3 NHL. In the last follow-up scan, there is complete remission of metabolic activity both in the neck and in the upper abdomen.
-
The images also show progressive increase of myocardial FDG uptake.
-
Serial echocardiography demonstrated a drop in LVEF after anthracycline therapy.
Differential Diagnosis
-
Cardiac toxicity from chemotherapy.
-
Non-specific cardiac FDG uptake.
-
Coronary artery disease.
-
SPECT MPI was ordered to exclude CAD (Fig. 5.5).
Findings
-
The SPECT MPI showed a dilated LV and an equivocal, small and mild perfusion defect in the inferolateral with apparent reversibility.
-
The rest LVEF was 48%.
Management and Teaching Points
-
The presence of myocardial FDG uptake following chemotherapy is a common finding.
-
While this finding could represent myocardial injury secondary to chemotherapy in some patients, the finding must be interpreted in the context of clinical symptoms and cardiac function.
-
In patients with frank LV dysfunction and heart failure, the heart switches substrate metabolism from preferential fatty acid utilization to glucose. In the case discussed above, a serial echocardiogram showed reduced LVEF which prompted the additional evaluation with SPECT MPI. However, SPECT MPI showed borderline normal LVEF.
Further Reading
-
Bauckneht M, Pastorino F, Castellani P, Cossu V, Orengo A, Piccioli P, et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. Journal of Nuclear Cardiology. 2019.
-
Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S, et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. Journal of Translational Medicine. 2018;16:295.
-
Bauckneht M, Ferrarazzo G, Fiz F, Morbelli S, Sarocchi M, Pastorino F, et al. Doxorubicin Effect on Myocardial Metabolism as a Prerequisite for Subsequent Development of Cardiac Toxicity: A Translational18F-FDG PET/CT Observation. Journal of Nuclear Medicine. 2017;58:1638–1645.
5.2.2 Pericarditis After Chemotherapy
Case 69
History
-
A 66-year-old female with Hodgkin’s Lymphoma (HL) undergoing interim PET/CT (Fig. 5.6) to monitor treatment response after two cycles ABVD (doxorubicin + bleomycin + vinblastine + dacarbazine).
This patient was then submitted to TTE (Fig. 5.7).
Findings
-
The PET/CT images showed no evidence of FDG uptake in mediastinal lymph nodes.
-
There was a small pericardial effusion with moderate FDG uptake in the pericardium.
-
Transthoracic echocardiography performed 4 days after PET scan showed an interval increase in pericardial effusion.
Management
-
The patient received anti-inflammatory therapy.
Teaching Points
-
Pericarditis can be a late, dose dependent complication of mediastinal irradiation. It can also be an adverse effect from chemotherapy (e.g., doxorubicin). While it can also result from heart failure, this patient had normal LV systolic function.
-
The presence of FDG uptake is consistent with active pericardial inflammation.
-
Asymptomatic pericardial effusion during chemotherapy or radiation therapy can pose challenging management decisions. The relatively small pericardial effusion and the prompt response to anti-inflammatory therapy required only a minor delay in her chemotherapy.
Further Reading
-
Floyd J, Nguyen D, Lobins R, Bashir Q, Doll D, Perry M. Cardiotoxicity of Cancer Therapy. Journal of Clinical Oncology. 2005;23:7685–7696.
-
Laursen AH, Elming MB, Ripa RS, Hasbak P, Kjær A, Køber L, Marott JL, et al. Rubidium-82 positron emission tomography for detection of acute doxorubicin-induced cardiac effects in lymphoma patients. J Nucl Cardiol. 2018 Oct 8. https://doi.org/10.1007/s12350-018-1458-6. [Epub ahead of print].
-
Bauckneht M, Pastorino F, Castellani P, Cossu V, Orengo A, Piccioli P, et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. J Nucl Cardiol. 2019 Feb 8. https://doi.org/10.1007/s12350-019-01618-x. [Epub ahead of print].
-
Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc Imaging. 2018 Aug;11(8):1173–1186. https://doi.org/10.1016/j.jcmg.2018.06.003. Review. Erratum in: JACC Cardiovasc Imaging. 2019 Jan;12(1):224.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the [NameOfOrganization], its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the [NameOfOrganization], provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the [NameOfOrganization] that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the [NameOfOrganization]'s name for any purpose other than for attribution, and the use of the [NameOfOrganization]'s logo, shall be subject to a separate written license agreement between the [NameOfOrganization] and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Di Carli, M.F. et al. (2022). Emerging Applications. In: Di Carli, M.F., Dondi, M., Giubbini, R., Paez, D. (eds) IAEA Atlas of Cardiac PET/CT. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-64499-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-662-64499-7_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-64498-0
Online ISBN: 978-3-662-64499-7
eBook Packages: MedicineMedicine (R0)