Abstract
Different migration mechanisms of chloride in concrete are introduced and various kinds of influences on diffusion coefficient, including temperature, relative humidity and age are also elaborated. Based on Fick second law and the boundary condition of close-end pipe pile, the analytical solution to chloride migration equation due to the process of both diffusion and convection is deduced. Applying this result to a typical jacked PHC pile, the chloride concentration is analyzed and the effect, which the seepage velocity, thickness of pipe pile and the diffusion coefficient have on distribution of chloride concentration, is also discussed. This example provides a computational basis for the corrosion of steel bar and some reference values for durability design of pipe pile.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keyword
1 General Introductions
Chloride ions are transmitted from the surrounding environment to the interior of the concrete through the pores and micro-cracks in the concrete, so that the chloride ion concentration on the surface of the steel bar gradually increases. After reaching the critical concentration, the steel bar will corrode. Collepardi et al. [2] used the Laplace transform method to obtain a one-dimensional closed solution under strictly restricted conditions, assuming that the concrete specimen was a semi-infinite space body, and the analytical solution was described by a transcendental function.
The two major transport mechanisms of chloride ion, diffusion and convection, are first expounded in this paper, pointing out various factors that affect the chloride ion diffusion coefficient, including temperature, relative humidity and age. Then, according to the boundary conditions of the pipe pile, the transport equations under the action of diffusion and convection are solved, analyzing the influence of various parameters. Moreover, the obtained results are verified with the measured data.
2 Transport Mechanism of Chloride Ion in Concrete
2.1 Diffusion
Diffusion refers to the phenomenon of directional migration of ions in solution under the action of a chemical potential gradient.
According to Fick’s first law, \(\vec{J} = - D \cdot \nabla C\); Based on the conservation equation \(\frac{\partial C}{{\partial t}} + \nabla \cdot \vec{J} = 0\) of ions in solution, Fick’s second law is obtained,
In the formula, \(\vec{J}\) is the chloride ion diffusion flux, \(C\) refers to the free chloride ion concentration (mol/L), and \(D\) is the diffusion coefficient (m2/s).
2.2 Convection
Convection refers to the phenomenon in which ions migrate as a whole with a solution.
The convective flux of chloride ions can be expressed as \(\vec{J} = - C \cdot \vec{v}\), where \(\vec{v}\) is the seepage velocity of the pore liquid in the concrete pores.
Pressure
The directional flow of pore fluid in porous media under external pressure difference obeys Darcy’s law.
In the formula, \(\vec{Q}\) is the volume flow rate of pore fluid (m3/s), and k is the permeability coefficient (m/s). \(\eta\) refers to the liquid viscosity coefficient (Pa s), and p is the pressure head (m).
Capillarity
The Capillarity can be equivalent to the pressure seepage in the calculation, which also conforms to Darcy’s law.
Electromigration
Electromigration refers to the process that ions in pore fluid perform directional migration under the action of an applied potential.
After the movement speed of chloride ion tends to be stable, the stable speed is \(\vec{v} = \frac{{ze\vec{E}}}{K}\), and the ion flux is \(\vec{J} = - C \cdot \frac{{zFD\vec{E}}}{RT}\). In the formula, K is the viscosity coefficient of the ion (N·s/m), F is the Faraday constant (96,485.3 mol/L), and e is the electron charge (C). z is the ion charge, R is the universal gas constant (8.31 J/(mol·K)), and T is the absolute temperature (K).
The chloride ion transport equation considering the processes of diffusion, convection and electromigration can be expressed as:
\(\vec{v}_{p}\) is the pore fluid velocity (m/s) caused by pressure seepage and \(\vec{v}_{c}\) refers to the pore fluid velocity (m/s) caused by capillary action.
3 Influencing Factors of Chloride Ion Diffusion Coefficient
The chloride ion transport process in actual concrete is very difficult to describe with an idealized model due to its complexity.
After comprehensively considering the effects of temperature, humidity and age, the effective chloride ion diffusion coefficient is:
\(f_{1} \left( T \right)\) is the temperature influence factor, and North American Life-365 (2018) suggested that \(f_{1} \left( T \right) = \exp \left[ {\frac{U}{R}\left( {\frac{1}{{T_{0} }} - \frac{1}{T}} \right)} \right]\). Stephen et al. (1998) suggested that \(f_{1} \left( T \right) = \frac{T}{{T_{0} }}\exp \left[ {q\left( {\frac{1}{{T_{0} }} - \frac{1}{T}} \right)} \right]\).
\(f_{2} \left( h \right)\) is the relative humidity influencing factor, Bitaraf and Mohammadi [1] suggested that \(f_{2} \left( h \right) = \left[ {1 + \frac{{\left( {1 - h} \right)^{4} }}{{\left( {1 - h_{0} } \right)^{4} }}} \right]^{ - 1}\).
\(f_{3} \left( t \right)\) is the age-related factor, and Thomas and Bamforth [6] suggested that \(f_{3} \left( t \right) = \left( {\frac{{t_{0} }}{t}} \right)^{m}\).
Among them, D0 refers to the diffusion coefficient of the reference chloride ion, which is measured by the test when the temperature is T0, the relative humidity is h0, and the age is t0. m is the attenuation coefficient. Usually, T0 is room temperature, which is 293 K; h0 is the critical relative humidity, which is 75%; t0 is 28 days. For ordinary concrete, m is taken as 0.25; for fly ash and slag concrete, m is taken as 0.6 [4].
In addition, water-cement ratio, stress state of concrete members [9], cracks [3], aggregate shape [5] and gradation, environmental changes and chloride binding properties [8] all have a certain impact on the transport process of chloride ions, which includes complex mechanisms.
4 Transport Law of Chloride Ion in Concrete Pipe Pile
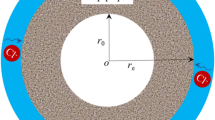
For long pipe piles, it can be assumed that the chloride ion diffusion in each cross-section is the same, thus transforming the three-dimensional problem into a two-dimensional problem. Considering the axial symmetry of the pipe pile, the chloride ion diffuses only in the radial direction (Fig. 1).
It is supposed that the wall thickness of the pipe pile is l, the outer wall is exposed to the chloride salt environment, and the environmental chloride ion concentration is u0. The inner wall is the closed surface, and the chloride ion concentration is 0. The initial chloride ion concentration inside the pipe pile is 0. No electric field acceleration exists in the natural environment. The pore fluid velocity caused by pressure seepage and capillary action is along the radial direction. From Formula (1), it can be obtained as follows.
Boundary conditions: \(C|_{x = 0} = u_{s}\), \(C|_{x = l} = 0\);
Initial conditions: \(C|_{t = 0} = 0\), (0 < x < l).
Setting \(v_{p} + v_{c} = v\) as the percolation velocity due to pressure and capillary action, and \(C\left( {x,t} \right) = u\left( {x,t} \right) + w\left( x \right)\). \(u\left( {x,t} \right)\) satisfies the secondary boundary condition, while \(w\left( x \right)\) satisfies the non-secondary boundary condition. Considering that the seepage velocity is positively opposed to the coordinate, a minus sign should be added before \(v\) when it is brought into Eq. (2), and there will be:
Then \(u\left( {x,t} \right)\) needs to satisfy:
Boundary conditions: \(u|_{x = 0} = 0\), \(u|_{x = l} = 0\);
Initial conditions: \(u|_{t = 0} = - w\left( x \right)\), (0 < x < l).
Setting \(u\left( {x,t} \right) = X\left( x \right)T\left( t \right)\), the separation variable method is used to solve the problem, which is transformed into the eigenvalue problem. Then, there will be
Among them.
Substitute (3) into (6) to get
Substitute (6) into (4) and add (3) to get
5 Analysis of Examples and Parameter Influence
The wall thickness of the PHC concrete pipe pile in marine engineering is l = 60 mm, the initial chloride ion concentration \(u_{0}\) is 0, and the boundary chloride ion concentration \(u_{s}\) is 1.0%. The diffusion coefficient D is 20 mm2/a, and the penetration rate is 2 mm/a. The calculation results that chloride ion concentration changes with time in the pipe pile are as follows (Fig. 2).
It can be seen from the calculation results that the overall trend of the chloride ion concentration in the pipe pile is increasing over time. Still, it is not higher than the ambient chloride ion concentration.
5.1 Comparison with Origianl Survey Data
The comparison results between the predicted values in this paper and the origianl survey data [7] are shown in Fig. 3. The comparison results show that the predicted values in this paper are better conformed with the measured data.
5.2 The Influence of Seepage
With or without influence of seepage, different results show that convection has a great impact on the diffusion of chloride ions in a design life of 10 and 30 years (Fig. 4).
5.3 The Influence of Seepage Velocity
The greater the seepage velocity is, the stronger the chloride ion erosion will be, and the influence on the distribution of chloride ion concentration in deep concrete is more prominent (Fig. 5).
5.4 The Influence of Pipe Pile Thickness
For pipe piles with different thicknesses, the distribution of chloride ion concentration in shallow concrete is basically the same. Affected by the chloride ion concentration in the inner wall of the pipe pile, the distribution of chloride ion concentration in the deep concrete is quite different (Fig. 6).
5.5 The Influence of Diffusion Coefficient
The diffusion of chloride ions significantly influences the distribution of chloride ion concentration in shallow concrete. The greater the diffusion coefficient is, the faster the chloride ion erosion will be. However, the influence of chloride ion concentration in deep concrete is just the opposite. The larger the diffusion coefficient is, the slower the chloride ion erosion will be (Fig. 7).
6 Conclusion
-
(1)
For concrete pipe piles, considering the diffusion effect, the chloride ion transport law under the influence of convection can better reflect the objective reality, which can guide the design more economically and reasonably.
-
(2)
The greater the seepage velocity, the faster the chloride ion will be transported into the concrete. The seepage velocity significantly influences the chloride ion concentration distribution in the deep concrete. The diffusion influence of chloride ions obviously influences the distribution of chloride ion concentration in shallow concrete. The greater the diffusion coefficient is, the faster the chloride ion will be transported. The influence on chloride ion concentration in deep concrete is just the opposite.
-
(3)
For pipe piles with the same diffusion coefficient but different thicknesses, the distribution of chloride ion concentration in shallow concrete is basically the same, and the chloride ion concentration in deep concrete is significantly affected by the chloride ion concentration in the inner wall of pipe piles.
References
Bitaraf M, Mohammadi S (2008) Analysis of chloride diffusion in concrete structures for prediction of initiation time of corrosion using a new meshless approach. Constr Build Mater 22(4):546–556
Collepardi M, Marcialis A, Turrizzani R (1970) The kinetics of penetration of chloride ions into the concrete. ll Cem 4:157–164
Jin ZQ, Zhao TJ, Zhuang QC. et al. (2012) Chloride transport in splitting cracked concrete at marine tidal zone. J Civil Archit Environ Eng 34(2):52–57
Life-365 Service Life Prediction Model [CP/OP]. http://www.life-365.org/download.html
Peng GJ, Zheng JJ, Zhou YQ (2009) A numerical method for predicting the chloride diffusion coefficient of concrete with aggregate shape. Adv Sci Technol Water Resour 29(6):13–16
Thomas MDA, Bamforth PB (1999) Modeling chloride diffusion in concrete—effect of fly ash slag. Cem Concr Res 29(4):487–495
Wang DD, Wang CQ, Shi BL et al (2008) Field sampling, analysis and research on durability of large diameter cylindrical piles. China Harbour Eng 1:39–43
Yu HF, Sun W (2006) Model research on chloride ion diffusion on concretes. J Southeast Univ (Nat Sci Ed 36(Sup(II)) 68–76
Yuan CB, Zhang DF, Liu RG et al (2003) Diffusivity of chloride in concrete in different stress states. J Hohai Univ (Nat Sci) 31(1):51–54
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 Crown
About this chapter
Cite this chapter
Yang, B. (2023). Analysis on Migration Mechanism of Chloride Ions in Concrete Close-End Pipe Piles. In: Yang, Y. (eds) Advances in Frontier Research on Engineering Structures. Lecture Notes in Civil Engineering, vol 286. Springer, Singapore. https://doi.org/10.1007/978-981-19-8657-4_33
Download citation
DOI: https://doi.org/10.1007/978-981-19-8657-4_33
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8656-7
Online ISBN: 978-981-19-8657-4
eBook Packages: EngineeringEngineering (R0)