Abstract
Objectives
The aim of this study was to evaluate whether simple 2D measurements in axial slices of head and neck CT examinations correlate with generally established measurements of body composition in abdominal CT at the height of the third lumbar vertebra and thus allow for an estimation of muscle and fat masses.
Methods
One hundred twenty-two patients who underwent concurrent CT of the head and neck and the abdomen between July 2016 and July 2020 were retrospectively included. For a subset of 30 patients, additional bioelectrical impedance analysis (BIA) was available. Areas of paraspinal muscles at the height of the third (C3) and fifth cervical vertebrae (C5) as well as the total cross-sectional area at the height of C3 and at the submandibular level were correlated with the results of abdominal measurements and BIA. Furthermore, intra- and interreader variabilities of all measurements were assessed.
Results
Regarding adipose tissue, good correlations were found between the total cross-sectional area of the patient’s body at the submandibular level and at the height of C3 between both abdominal measurements and BIA results (r = 0.8–0.92; all p < 0.001). Regarding muscle, the total paraspinal muscle area at the height of C3 and C5 showed strong correlations with abdominal measurements and moderate to strong correlations with BIA results (r = 0.44–0.80; all p < 0.001), with the muscle area on C5 yielding slightly higher correlations.
Conclusions
Body composition information can be obtained with comparable reliability from head and neck CT using simple biplanar measurements as from abdominal CT.
Key Points
• The total paraspinal muscle area at the height of C3 and C5 correlates strongly with abdominal muscle mass.
• The total cross-sectional area at the submandibular level and at the height of C3 shows good correlations with abdominal fat mass.
• The described measurements facilitate a rapid, opportunistic assessment of relevant body composition parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Body composition and its pathological changes, such as sarcopenia, loss of muscle mass, and sarcopenic obesity, are established prognostic markers in various diseases. Their influence has been evaluated in different clinical scenarios ranging from oncological diseases to postoperative courses following several surgical procedures and cardiovascular and metabolic diseases as well as emergency settings [1,2,3,4].
Since chronically ill patients usually undergo radiological imaging on a regular basis, it seems appealing to obtain quantitative body composition information from clinically indicated cross-sectional imaging opportunistically. This offers the advantage of eliminating the necessity for patients to receive additional, dedicated examinations, which assess muscle and adipose tissue, such as dual-energy X-ray absorptiometry (DEXA) or bioelectrical impedance analysis (BIA). Imaging-based assessment of body composition is usually performed in abdominal cross-sectional examinations at the height of the third lumbar vertebra (L3) [5, 6]. Here, numerous studies have shown that results of simple 2D measurements and more elaborate segmentations as well as results of fully automated approaches are valid biomarkers of body composition [6,7,8,9,10].
However, many patients with neurological diseases receive only cross-sectional imaging of the head or the head and neck, hampering the opportunistic acquisition of body composition based on well-established abdominal parameters. Accordingly, studies on the influence of body composition on neurological diseases are relatively sparse. For example, there are only a very few studies that have investigated the relationship between body composition information obtained opportunistically from imaging data and outcome in patients with stroke, although this is a common and serious disease [11]. In contrast, there is much more data on the influence of body composition in abdominal diseases. This could be due, in part, to the fact that there have been few simple and validated measurements outside of abdominal imaging.
While a few studies have also demonstrated the possibility of obtaining reliable body composition information at the level of the chest, the data on the feasibility of obtaining data in patients with neck-only examinations is scarce [12,13,14]. A sporadic number of studies outlined that muscle areas measured at the level of the third cervical vertebra (C3) correlate with muscle areas measured at the level of L3 [15,16,17]. However, the approaches described so far to obtain body composition information from cervical CT scans either are relatively laborious, require additional clinical parameters, or cannot be obtained from visual muscle delineations alone, but require special preparations such as additional measurements of densities [15, 16]. Therefore, demand remains for a straightforward, easy-to-perform assessment of body composition in head and neck CT.
Thus, the aim of this study was to evaluate straightforward biplanar measurements in axial CT images of the neck with respect to their potential usefulness for body composition assessment and to compare them with well-established measurements at the level of L3 and non-imaging body composition information derived from BIA.
Materials and methods
Patient collective
Our retrospective, monocentric study was approved by the institutional review board. The requirement for informed consent was waived. A merged query to the radiological information system and the picture archiving and communication system was performed to identify adult patients, who were referred from the Department for Dermatology for CT-staging examination of the neck, chest, and abdomen between June 2016 and July 2020. For a subset of 30 patients, additional BIA body composition analysis was available in the context of a different, independent prospective study, which was approved by the institutional review board (No. 16-239, University of Cologne). For the subset of these 30 patients, written informed consent regarding BIA assessment was obtained from every patient.
Imaging protocol
All patients underwent CT-staging examinations as part of the standard clinical care on a 64-row spectral detector CT (IQon, Philips Healthcare). Scanning was performed in a head-first, supine patient position, with the head supported by a head cushion. Every patient was examined using a standardized examination protocol, which comprised a portal venous phase acquisition of the chest and abdomen, followed by a separated venous phase scan of the neck. The following scan settings were employed for the chest and abdomen examination: tube current modulation (DoseRight 3D-DOM, Philips Healthcare), tube voltage 120 kVp, rotation time 0.33 s, pitch 0.671, collimation 64 × 0.625 mm, and a matrix of 512 × 512. For the neck examination, the following scan settings were used: tube current modulation (DoseRight 3D-DOM, Philips Healthcare), tube voltage 120 kVp, rotation time 0.5 s, pitch 0.985, collimation 64 × 0.625, and a matrix of 512 × 512. Scanning parameters resulted in a CTDIvol of 11.7 mGy for scans of the neck and in a CTDIvol of 11.5 mGy for scans of the chest and abdomen. A total of 180 ml of iodinated contrast media (Accupaque, 350 mg/ml; GE Healthcare) is routinely administered as a bolus using a 20-G intravenous catheter. Here, 100 ml is allocated to the chest and abdomen scan and 80 ml to the subsequent neck scan. Images were reconstructed with a slice thickness of 2 mm and a 1-mm section increment using a hybrid iterative reconstruction algorithm (iDose 4, filter B, level 3, Philips Healthcare).
CT measurements
Reading was carried out by two radiologists with 4 and 5 years of experience. To determine intrareader reliability, one reader re-performed all measurements after 4 weeks to avoid recall bias. All 2D measurements were performed using a standard DICOM viewer (Impax EE R20, XVII SU1, Agfa Healthcare). Regions of interest (ROI) were created to determine muscle and total cross-sectional areas using a freehand annotation embedded in the viewing application.
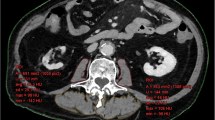
The abdominal measurements were carried out at the height of L3 in accordance with earlier, well-established methods: A sagittal plane was used to identify L3, and all measurements were then performed on an axial plane located centrally at this height. The area of the following regions was recorded: psoas major muscles, autochthonous spine muscles, and total cross-sectional area of the patient’s body. The combined total of all individual muscle areas was defined as the total abdominal paraspinal muscle area (see Fig. 1).
At the level of the neck, the total cross-sectional area of the patient’s body was measured at the height of C3 as well as submandibular. Furthermore, muscle areas of the sternocleidomastoid muscle and the autochthonous muscles were assessed at the height of the third and the fifth cervical vertebrae (C5). If the trapezius muscle was depicted at the level of C5, it was not considered. Analogously, the sum of all muscle areas was defined as the total cervical paraspinal muscle area at the height of C3 and C5, respectively. During ROI placement, small vessels or nerves within the muscles were included in measurements, as their contribution to the overall area appeared negligible. Figure 2 illustrates all cervical measurements.
Overview of the cervical 2D measurements to assess body composition. All measurements were performed using a freehand ROI tool within the standard PACS. The total cross-sectional area was measured at the submandibular level and at height of the third cervical vertebra (C3, outlined in green). Paraspinal muscle areas were measured at the height of C3 and at the height of the fifth cervical vertebra (C5, outlined in orange)
Bioelectrical impedance analyses
BIA was conducted using a multi-frequency BIA apparatus (Seca mBCA 515, Seca) in overnight-fasted patients during the morning. Tetrapolar measurements of bioelectrical impedance were executed in a standardized, upright patient position with a total of 19 frequencies ranging from 1 kHz to 1 MHz. Waist circumference in centimeters was determined manually for each patient undergoing BIA.
Statistical analyses
All statistical analyses were conducted using R 3.4.0 with RStudio 1.0.136, and figures were created using the ggplot2 package. The Pearson correlation coefficient was employed to assess the correlation between cervical and abdominal CT measurements and further to assess the correlation between these CT measurements and BIA results. The concordance correlation coefficient was used to evaluate intra- and interreader reliability. Interpretation of the concordance correlation coefficient was as follows: excellent agreement (> 0.8), good agreement (> 0.6), moderate agreement (> 0.4), and poor agreement (≤ 0.4). Otherwise, continuous variables are reported as mean ± standard deviation (SD). Statistical significance was set as p ≤ 0.05.
Results
Patient characteristics
Of 122 included patients (mean age 63 ± 16 years), 69 were male with a mean age of 64 ± 17 years and 53 were female with a mean age of 63 ± 15 years. As their underlying disease, 114 patients had malignant melanoma, 5 patients had a Merkel cell carcinoma, 2 patients had a squamous cell carcinoma, and one patient each had a dermal sarcoma and a cutaneous B cell lymphoma. In the subset of 30 patients for which the results of BIA were available, all patients were diagnosed with malignant melanoma. The average time interval between BIA and CT examination was 21 ± 16 days.
Correlation of cervical and abdominal CT measurements
The total cross-sectional area of the patient’s body at the submandibular level and at the height of C3 both showed a strong correlation with the total cross-sectional area at the height of L3 of r = 0.83 and r = 0.81, respectively (both p < 0.001; see Fig. 3). For the estimation of the total cross-sectional area at the height of L3, the following equations were obtained:
Regarding the muscle areas, the total cervical paraspinal muscle area at the height of both C3 and C5 showed strong correlations with the total abdominal paraspinal muscle area of r = 0.74 and r = 0.8, respectively (both p < 0.001; see Fig. 4). For the estimation of muscle areas at the height of L3, the following equations were obtained:
Mean values of the measured areas are presented in Table 2.
Correlation of CT measurements and BIA
In the subset of 30 patients, for whom additional BIA information was available, both the total cross-sectional area of the patient’s body at the submandibular level and that at the height of C3 showed strong correlations with the total fat mass obtained from BIA (r = 0.82/r = 0.80; both p < 0.001) and the visceral fat mass (r = 0.92 and r = 0.90, both p < 0.001). Similarly, strong correlations between the total cross-sectional area of the patient’s body at the submandibular level and at the height of C3 and waist circumference were found (r = 0.92 and r = 0.86, both p < 0.001). Regarding the correlation between abdominal total cross-sectional area at the height of L3 and BIA measurements, strong correlations were found for total fat mass (r = 0.90, p < 0.001), for visceral fat mass (r = 0.90, p < 0.001), and for waist circumference (r = 0.94, p < 0.001).
Moderate to strong correlations of r = 0.44 and r = 0.72 were found between the total cervical paraspinal muscle area at the height C3 and C5 and muscle mass determined by BIA (p = 0.02 and p < 0.001). A correlation of r = 0.67 was found between the total abdominal paraspinal muscle area and the absolute muscle mass (p < 0.001). Detailed results are presented in Table 1 and ESM 1.
Both intra- and interreader reliability measurements showed excellent agreements, ranging from concordance correlation coefficients of 0.81 to 0.99 and from 0.82 to 0.99, respectively. Detailed results of intra- and interreader reliability measurements are provided in Table 2.
Discussion
Sarcopenia, sarcopenic obesity, and loss of muscle mass correlate positively with adverse outcomes and mortality in a variety of medical conditions [3, 18,19,20,21]. While surrogate markers in abdominal CT imaging have been extensively analyzed, such markers are unavailable for examinations of the head and neck. This substantiates the necessity for reliable and easy-to-perform approaches in patients who solely receive head and neck CT examinations. In this study, we report markers that can be easily grasped from head and neck CT that correlate closely with surrogate markers established in abdominal imaging and hence may provide specific benefit in assessment of sarcopenia and sarcopenic obesity in patients with neurological disorders.
We found strong correlations between both the total cross-sectional areas at the level of C3 and directly submandibular and the total cross-sectional area at the level of L3. For the latter, strong correlations with both absolute fat mass and visceral fat mass have already been demonstrated [6, 22, 23]. Likewise, both total cross-sectional areas at the neck correlated strongly with the fat masses determined by BIA. Here, a slightly better correlation of the submandibular total cross-sectional area compared to the area at the level of C3 was observed for all parameters. Strong correlations were also found between muscle areas at C3 and C5 and the reference measurement at L3. Compared with the fat masses, slightly weaker correlations were found between the cervical muscle areas and the muscle mass determined by BIA; however, correlations were still predominantly strong. One likely explanation might be that in BIA all muscles (including extremities) are included in the measurement, while imaging-based assessment is limited to the torso. Fat, on the other hand, likely follows a more even distribution and hence shows a better correlation between the modalities. The variation of correlations might as well be impacted by the limited sample size of patients undergoing BIA. Lastly, while BIA is an accepted and established reference standard, it is known to be erroneous if not carried out according to a standardized patient preparation with regard to hydration state (which has been considered in our cohort) [24, 25]. Overall, in our view, the measurements of the submandibular total cross-sectional area as well as the measurement of the paraspinal muscle areas at the level of C5 appear to be suitable parameters to measure body composition in neck CT examinations. A possible explanation for the better correlation of muscle area at the C5 level with abdominal muscle area and actual muscle masses found in this study might be the different positioning of the patients during the CT examination. Head flexion or reclination as well as deviation in the horizontal may have a stronger effect further distally than close to the trunk at the level of C5 and thus cause a higher degree of variance.
These results differ to some extent from those of previous studies, which proposed measurements of muscle areas at the level of C3. For example, Jung et al found a moderate correlation between cross-sectional muscle areas at the height of C3 and L3 [15]. However, additional consideration of extra parameters (age, sex, and weight) allowed for a more accurate prediction of abdominal muscle areas [15]. Strong correlations were described by Swartz et al between 2D measurements of muscle areas at the level of C3 and L3 in their study [16]. However, the proposed measurements by Swartz et al were performed with prior consideration of density limits and using a dedicated software solution [16]. Overall, it can be argued that such special preparations or the need to consider additional clinical parameters limits the usability and implementation in daily routine. However, in most cases, this information can certainly be determined with reasonable effort and the creation of an algorithm or simple software that includes these factors should be straightforward. Furthermore, previous studies did not investigate whether measurements in neck CT might be used to predict patients’ fat masses, but only focused on determining muscle masses and/or areas.
In this respect, the present study differs from previous work as the described 2D ROI measurements are easy to perform in any PACS application and allow estimation of both muscle and fat masses. Furthermore, to our knowledge, this is the first study to compare 2D measurements on head and neck CT with a non-imaging reference standard.
The measurements we describe may facilitate an improved evaluation of the influence of body composition parameters in patients who undergo CT of the neck only, such as patients with suspected stroke. For example, data investigating a link between detailed parameters of body composition and outcome in patients with stroke is sparse [11].
This study has further limitations besides its retrospective design, which need to be mentioned. First, the proportion of patients for whom additional BIA data were available is limited. A larger fraction would be desirable but, unfortunately, was not available. In addition, there are several approaches to determine body composition in abdominal cross-sectional imaging. In the present study, validated and easy-to-perform 2D measurements on single CT slices were chosen as reference. Other methods, such as a voxel-by-voxel analysis of all muscle and adipose tissue, may provide different results. Recently, more and more AI-based approaches have been described to determine body composition. Therefore, it can be argued that it would be more useful to develop fully automated algorithms that can obtain body composition information in head and neck CT without manual measurements. Manual segmentations are inherently more susceptible to a higher intra- and interreader variability compared to fully automated methods. Nevertheless, we found excellent agreements, which underline the simplicity of the measurements. Several studies have demonstrated an influence of contrast media application on the determination of body composition, mostly showing a modest but significant increase in muscle areas after contrast media administration. Since the present study included only examinations of the neck and chest and abdomen, there is also a potential for bias due to the double administration of contrast medium [10, 26, 27]. Furthermore, it cannot be excluded with certainty that the underlying oncological disease of the included patients caused an influence on the correlations between abdominal and cervical measurements. Furthermore, the measurements presented in this study have not yet been evaluated for their potential to identify sarcopenic patients which might be in the scope of future studies. Lastly, the described method may have limitations in patients with abnormal fat distribution, for example, under cortisone treatment.
In conclusion, this study describes measurements that can be easily performed on single CT slices of head and neck CT and correlate very well with established measurements of body composition in abdominal CT examinations and results of BIA. Therefore, the reported measurements allow for a simple and reliable estimation of body composition in patients who receive a neck CT only and may substantiate their aptitude in future studies.
Abbreviations
- BIA:
-
Bioelectrical impedance analysis
- C3:
-
Third cervical vertebra
- C5:
-
Fifth cervical vertebra
- DEXA:
-
Dual-energy X-ray absorptiometry
- L3:
-
Third lumbar vertebra
References
Lee K, Shin Y, Huh J et al (2019) Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol 20(2):205–217
Kottlors J, Zopfs D, Fervers P et al (2020) Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease - a multicenter feasibility study. Eur J Radiol 132:109274
Pinto Dos Santos D, Kloeckner R, Koch S et al (2020) Sarcopenia as prognostic factor for survival after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 32(5):626–634
Xia L, Zhao R, Wan Q et al (2020) Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med 9(21):7964–7978
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67
Zopfs D, Theurich S, Große Hokamp N et al (2020) Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol 30(3):1701–1708
Koitka S, Kroll L, Malamutmann E, Oezcelik A, Nensa F (2021) Fully automated body composition analysis in routine CT imaging using 3D semantic segmentation convolutional neural networks. Eur Radiol 31(4):1795–1804
Zopfs D, Bousabarah K, Lennartz S et al (2020) Evaluating body composition by combining quantitative spectral detector computed tomography and deep learning-based image segmentation. Eur J Radiol 130:109153
Burns JE, Yao J, Chalhoub D, Chen JJ, Summers RM (2020) A machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol 27(3):311–320
Fuchs G, Chretien YR, Mario J et al (2018) Quantifying the effect of slice thickness, intravenous contrast and tube current on muscle segmentation: implications for body composition analysis. Eur Radiol 28(6):2455–2463
Kim J-H, Choi K-H, Kang K-W et al (2019) Impact of visceral adipose tissue on clinical outcomes after acute ischemic stroke. Stroke 50(2):448–454
Derstine BA, Holcombe SA, Goulson RL et al (2017) Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J Nutr Health Aging 21(10):180–185
Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC (2018) Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8(1):11369
Nemec U, Heidinger B, Sokas C, Chu L, Eisenberg RL (2017) Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter Aortic Valve Replacement. Acad Radiol 24(9):1154–1161
Jung AR, Roh J-L, Kim JS, Choi S-H, Nam SY, Kim SY (2019) Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol 95:95–99
Swartz JE, Pothen AJ, Wegner I et al (2016) Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol 62:28–33
Zwart AT, van der Hoorn A, van Ooijen PMA, Steenbakkers RJHM, de Bock GH, Halmos GB (2019) CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle 10(5):1060–1069
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. Lancet 393(10191):2636–2646
Ubachs J, Ziemons J, Minis-Rutten IJG et al (2019) Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 10(6):1165–1174
Hsu C-S, Kao J-H (2018) Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol 12(12):1229–1244
Hwang F, McGreevy CM, Pentakota SR et al (2019) Sarcopenia is predictive of functional outcomes in older trauma patients. Cureus 11(11):e6154
Xia Y, Ergun DL, Wacker WK, Wang X, Davis CE, Kaul S (2014) Relationship between dual-energy X-ray absorptiometry volumetric assessment and X-ray computed tomography-derived single-slice measurement of visceral fat. J Clin Densitom 17(1):78–83
Geraghty EM, Boone JM (2003) Determination of height, weight, body mass index, and body surface area with a single abdominal CT image. Radiology 228(3):857–863
Ward LC (2019) Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr 73(2):194–199
Coppini LZ, Waitzberg DL, Campos ACL (2005) Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care 8(3):329–332
Morsbach F, Zhang Y-H, Martin L, Lindqvist C, Brismar T (2019) Body composition evaluation with computed tomography: contrast media and slice thickness cause methodological errors. Nutrition 59:50–55
Troschel AS, Troschel FM, Fuchs G et al (2021) Significance of acquisition parameters for adipose tissue segmentation on CT images. AJR Am J Roentgenol 217(1):177–185
Funding
Open Access funding enabled and organized by Projekt DEAL. Supported by the Cologne Clinician Scientist Program (CCSP) / Faculty of Medicine / University of Cologne. Funded by the German Research Foundation (DFG, FI 773/15-1). Supported by the Koeln Fortune Program / Faculty of Medicine, University of Cologne (330/2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Nils Große Hokamp.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Nils Große Hokamp receives research support from Philips Healthcare unrelated to this project. NGH is on the speakers bureau of Philips Healthcare. Nils Große Hokamp is a consultant for Bristol Myers Squibb.
David Zopfs and Simon Lennartz received research support from Philips Healthcare unrelated to this project.
Roman Klöckner has received consultancy fees from Boston Scientific, Bristol-Myers Squibb, Guerbet, Roche, and SIRTEX and lectures fees from BTG, EISAI, Guerbet, Ipsen, Roche, Siemens, SIRTEX, and MSD Sharp & Dohme.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
For a subset of 30 patients, written informed consent was obtained from every patient in the context of a different, independent prospective study, which was approved by the institutional review board (No. 16-239, University of Cologne). Otherwise, written informed consent was waived by the institutional review board due to the retrospective character.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
The upper row shows correlations between muscle mass determined by bioelectrical impedance analysis (BIA) and the paraspinal muscle area at the height of the third cervical vertebra (C3, upper row on the left) and at the height of the fifth cervical vertebra (C3, upper row on the right). The bottom row depicts correlations between the total fat mass determined by BIA and the total cross-sectional muscle area at the height of the third cervical vertebra (C3, bottom row on the left) and at the submandibular level (bottom row on the right). BIA values are reported in kg, CT measurements in mm2 (PNG 2252 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zopfs, D., Pinto dos Santos, D., Kottlors, J. et al. Two-dimensional CT measurements enable assessment of body composition on head and neck CT. Eur Radiol 32, 6427–6434 (2022). https://doi.org/10.1007/s00330-022-08773-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08773-9