Abstract
Genome-wide association studies have identified SNPs reproducibly associated with type 2 diabetes (T2D). We examined the effect of genetic predisposition to T2D on insulin sensitivity and secretion using detailed phenotyping in overweight individuals with no diagnosis of T2D. Furthermore, we investigated whether this genetic predisposition modifies the responses in beta-cell function and insulin sensitivity to a 24-week dietary intervention. We genotyped 25 T2D-associated SNPs in 377 white participants from the RISCK study. Participants underwent an IVGTT prior to and following a dietary intervention that aimed to lower saturated fat intake by replacement with monounsaturated fat or carbohydrate. We composed a genetic predisposition score (T2D-GPS) by summing the T2D risk-increasing alleles of the 25 SNPs and tested for association with insulin secretion and sensitivity at baseline, and with the change in response to the dietary intervention. At baseline, a higher T2D-GPS was associated with lower acute insulin secretion (AIRg 4% lower/risk allele, P = 0.006) and lower insulin secretion for a given level of insulin sensitivity, assessed by the disposition index (DI 5% lower/risk allele, P = 0.002), but not with insulin sensitivity (Si). T2D-GPS did not modify changes in insulin secretion, insulin sensitivity or the disposition index in response to the dietary interventions to lower saturated fat. Participants genetically predisposed to T2D have an impaired ability to compensate for peripheral insulin resistance with insulin secretion at baseline, but this does not modify the response to a reduction in dietary saturated fat through iso-energetic replacement with carbohydrate or monounsaturated fat.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) results from a combination of insulin resistance and impaired ability of the pancreatic beta-cell to secrete sufficient insulin (Kahn 2003). Diet composition, especially the amount and type of dietary fat, is a recognised environmental risk factor for T2D, which particularly affects peripheral insulin sensitivity (Parillo and Riccardi 2004). The quality of dietary carbohydrate is also important with a higher glycaemic index (GI) diet being associated with an increased risk of T2D (Barclay et al. 2008). Genome-wide association studies (GWAS) have identified loci that show robust association with increased risk of developing T2D (Saxena et al. 2007; Sladek et al. 2007; Voight et al. 2010; Zeggini et al. 2007) and with glycaemic traits (Dupuis et al. 2009). Most of these SNPs reside in or near genes that have a presumed role in pancreatic beta-cell dysfunction (Billings and Florez 2010). These loci have mostly been identified using overt measures of impaired glucose tolerance or frank diabetes; however, the impact on insulin sensitivity and beta-cell function has only been assessed using proxy measures. More detailed phenotyping has the advantage of identifying early-stage defects that exist before impaired fasting glucose becomes apparent.
In this study, we examined the effect of a T2D genetic predisposition score (GPS) on beta-cell function and peripheral insulin sensitivity, assessed by intravenous glucose tolerance test (IVGTT) in a cohort of overweight non-diabetic participants at increased cardiometabolic risk (Jebb et al. 2010). This was in order to determine the effect of SNPs that have been most robustly associated with T2D in GWAS on early-stage defects that can precede T2D. Furthermore, we examined whether this genetic predisposition to T2D influenced changes in insulin sensitivity and beta-cell function in response to changes in dietary fat and carbohydrate intake. This was tested in participants in a 24-week dietary intervention study to lower saturated fat intake through iso-energetic replacement with low or high GI carbohydrate or monounsaturated fat (MUFA). The objective of this analysis was to assess whether the T2D-GPS can act to modify dietary associated changes in peripheral insulin sensitivity and beta-cell function.
Methods
Original RISCK trial study design
The RISCK trial (ISRCTN29111298) has been described in detail previously (Jebb et al. 2010). Briefly, men and women aged 30–70 years (n = 720) at increased cardiometabolic risk (according to a study-specific scoring system) but with no diagnosis of T2D were recruited from the general population (Jebb et al. 2010). All participants underwent a 4-week run-in period on the ‘reference’ diet (REF), designed to reflect the high saturated fat intake of a ‘Western diet’. Participants were then randomised to the REF diet, or one of four diets designed to achieve a target reduction in saturated fat intake from ~18% of energy (REF diet) to ~10% of energy, for 24 weeks; the actual mean reduction achieved was 7–8% (Jebb et al. 2010). The REF and intervention diets described in detail previously (Moore et al. 2009) were designed to be iso-energetic, but varied in the amount and type of fat and carbohydrate as follows: high saturated fat and high GI (REF); high MUFA/high GI; high MUFA/low GI; low fat/high GI; and low fat/low GI diets. Measurements taken after the run-in REF diet are referred to in this study as ‘baseline’ measurements. At baseline, and following the dietary intervention, a fasting blood sample was collected, anthropometry measured, and an IVGTT performed.
Ethical approval for the RISCK study was granted from the National Research Ethics Service and written informed consent including subsequent genetic analyses was obtained from participants.
Study cohort
Of the 720 participants, 549 completed the study, and DNA was available for 512 participants. To reduce heterogeneity in genetic background, 412 individuals of white European ancestry, based on self-reported ethnicity, were included in the analysis. All other ethnic sub-groups were excluded from these analyses due to their limited size. Following genotyping quality control procedures (see below), 405 participants were available for analysis at baseline, and 376 completed the dietary intervention. Data from the IVGTT was available for 377 participants at baseline and for 354 of those who completed the study.
Of these participants, 48% had fasting glucose >5.6 mM (impaired fasting glucose), and no participants had fasting glucose >7 mM (T2D). The characteristics of the participants included in these analyses are presented in Table 1.
IVGTT
A short IVGTT protocol was used and described in more detail elsewhere (Jebb et al. 2010). The area under the plasma insulin curve up to 19 min was computed to indicate the degree of endogenous insulin secretion in response to the glucose challenge (AIRg). Insulin sensitivity (Si) was estimated using the MINMOD Millennium programme (Version 6.02). The disposition index (DI) was calculated as the product of Si and AIRg and is a measure of the beta-cell’s ability to compensate for changes in Si (Bergman et al. 2002).
SNP selection and genotyping
Twenty-eight SNPs were identified from GWAS to be associated with T2D risk (Dupuis et al. 2009; Saxena et al. 2007; Sladek et al. 2007; Voight et al. 2010; Zeggini et al. 2007). SNPs were only selected from GWAS with at least 1,000 individuals in the discovery stage, which after follow-up reached the threshold of genome-wide significance of P < 5 × 10−8. Where multiple SNPs resided in or near the same gene, only SNPs in low linkage disequilibrium (LD r 2 < 0.3) were selected.
Genotyping was performed by KBiosciences (Hoddesdon, Herts, UK) using a fluorescence-based competitive allele-specific PCR (KASPar) technology, and all SNPs had a call rate >95%. Individuals were excluded if genotyping was unsuccessful in >10% of SNPs (n = 7). Genotype distributions of all SNPs were tested for deviation from the Hardy–Weinberg Equilibrium using the log-likelihood ratio Chi-square test (1 df) for association. Using a cut-off of P < 0.001 excluded three SNPs (rs1470579-IGFBP2; rs13266634-SLC30A8; rs8042680-VPS33B) from analyses due to deviation. The remaining 25 SNPs in or near 24 genes were included in the current analyses (Table 2).
Genetic predisposition score
We defined the risk allele of a SNP as the allele associated with increased risk of developing T2D or with raised fasting plasma glucose concentration in previous GWAS (Dupuis et al. 2009; Saxena et al. 2007; Sladek et al. 2007; Voight et al. 2010; Zeggini et al. 2007). An individual’s genotype was coded as 0, 1 or 2 depending on the number of the risk alleles an individual carried for that particular SNP. For each individual, a GPS was calculated by adding the number of risk alleles of the 25 SNPs (Table 2). As there is currently no evidence for interaction between SNPs, a simple addition of the associated risk alleles for each trait has been commonly adopted (Hamrefors et al. 2010; Li et al. 2010; Talmud et al. 2009). For participants with missing genotypes (<10%), the average count of risk alleles for the respective SNP was substituted for the missing genotype for the purpose of calculating the GPS. The GPS was normally distributed. The SNPs selected for analysis are presented in Table 2 showing the risk-allele frequency.
Statistical analysis
Distributions of traits were tested for normality; and because of right skewness Si, AIRg and DI were natural log-transformed for analyses and presented in figures as the geometric mean and 95% confidence intervals. For interpretation of the effect of T2D-GPS on traits, the coefficient of association from the linear regression analysis of log(n)-transformed traits equated to the percentage change per risk allele.
Due to insufficient power to examine associations of individual SNPs, we focussed our study on the GPS, which provides more power. Linear regression analysis was used to test for associations between GPS (a continuous variable according to the number of risk alleles) and traits at baseline, assuming an additive effect of each additional risk allele, while adjusting for age, gender, centre and BMI. We additionally tested for curvature in the model by inclusion of GPS-squared term in the model.
Next, we tested for the effect of GPS to modify the change in Si, AIRg and DI following 24 weeks of dietary intervention by an interaction between T2D-GPS and dietary intervention group. This was used in a linear regression model of the association between the GPS and change in Si, AIRg and DI following intervention, adjusted for baseline values, age, gender, centre, baseline BMI, diet and change in weight. The four intervention diets were compared to the REF diet group.
Associations between the individual SNPs and traits at baseline, and the interaction effects of SNPs and dietary intervention group on change in trait in response to the dietary intervention, were tested with linear regression in the same way GPS were tested, adjusting for the same covariates. This exploratory analysis was conducted despite the assumption that we would have low power to detect small effects of individual SNPs to illustrate the contribution to the effects of GPS.
Statistical analysis was conducted using Stata 11 (StataCorp, TX, USA). A Bonferroni correction was applied to the six tests between T2D-GPS and the three traits at baseline, and to change in response to the intervention (P = 0.008). We did not correct the associations of individual SNPs as we decided to only report the summary statistics for future research, rather than to interpret them on their own due to the limited statistical power to detect small individual effects when corrected for false positive chance.
Results
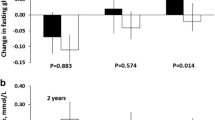
At baseline, we observed no association of T2D-GPS with peripheral insulin sensitivity (Si) (Fig. 1a). However, the T2D-GPS was associated with lower acute insulin secretion (4% per risk allele, P = 0.006, Fig. 1b) and with a lower disposition index (5% per risk allele, P = 0.002, Fig. 1c).
Effect of type 2 diabetes genetic predisposition score (T2D-GPS) on insulin sensitivity (Si), acute insulin secretion (AIRg) and disposition index (DI) at baseline. The participants were stratified by T2D-GPS, and the number of participants in each GPS stratum is shown in panel d. The effect of T2D-GPS on (a) Insulin sensitivity index (Si) (b) Acute insulin secretion (AIRg) and (c) Disposition index (DI) is presented as geometric mean and 95% CI. Data were log(n)-transformed for analysis, and the per allele effect was determined by linear regression analysis with age, gender, BMI and centre as confounding variables. The effect is presented as the beta-coefficient from the linear regression of the log(n)-transformed trait (which equated to percentage difference) and the P value. These measures were conducted after a 1-month period on a ‘reference’ high SFA diet prior to the dietary intervention to lower SFA intake
Of all the SNPs tested, only the TCF7L2 SNP rs7901695 (Table 2) showed a convincing association with both AIRg (13% lower per risk allele, P = 0.00005) and DI (11% lower per risk allele, P = 0.001), but not with Si. To examine whether the associations observed for the GPS with AIRg and DI were driven by the effect of the TCF7L2 SNP, we excluded this SNP from the GPS and tested for association again. The associations remained for AIRg (3% lower per risk allele, P < 0.05) and DI (4% lower per risk allele, P = 0.02), but the effect was diminished, and they were no longer significant when accounting for multiple testing.
In response to the 24 week dietary interventions to lower saturated fat intake, there were no effects of T2D-GPS to modify the change in Si, AIRg or DI, accounting for baseline values, in response to any of the dietary interventions (Table 3).
Of all the SNPs tested, no convincing effect was observed on the change in Si, AIRg or DI in response to any of the dietary interventions (Supplementary Table 1).
Discussion
This study used detailed phenotyping from the IVGTT to characterise the effects of genetic predisposition to T2D on insulin secretion and sensitivity in non-diabetic overweight participants at increased cardiometabolic risk. Our results show that at baseline, a genetic predisposition to T2D was associated with an impairment of beta-cell function, but not with insulin sensitivity in participants on a run-in diet high in saturated fat, designed to reflect a ‘Western diet’. However, genetic predisposition to T2D did not modify changes in insulin sensitivity or beta-cell function in response to a dietary intervention to lower saturated fat intake by isoenergetic replacement with MUFA or carbohydrate.
Previous GWAS have identified genetic loci associated with T2D, the majority of which have a presumed role in beta-cell dysfunction (Billings and Florez 2010). Our findings are supportive of a mechanism contributing to impaired insulin secretion but not insulin sensitivity. The DI is a more informative measure of beta-cell function than acute insulin secretion; by taking into account the insulin sensitivity it is a measure of the ability of the beta-cell to compensate for the degree of insulin sensitivity (Bergman et al. 2002). This measure can also be more sensitive as differences in DI can be detected before changes in acute insulin secretion are apparent (Bergman et al. 2002). At baseline, genetic predisposition to T2D was associated with a lower acute insulin response, but also with a lower DI, which confirmed an impaired acute insulin response for the level of insulin resistance.
The dietary modifications in the RISCK study were designed to be achievable through simple dietary modification and implementable on a population scale, but produced no overall significant effect on insulin sensitivity (Jebb et al. 2010). Here we demonstrate that genetic predisposition to T2D did not moderate any effects of the dietary intervention on changes in insulin secretion or sensitivity. An effect of genetic predisposition on changes in these measures may have been evident with a more extreme intervention, particularly one that also achieved weight loss. For example, in response to a lifestyle intervention to decrease caloric intake and increase energy expenditure (TULIP, n = 1576), carriers of the rs7903146 (TCF7L2) risk allele, who had impaired glucose tolerance prior to the intervention, showed an increase in post-glucose-load insulin secretion (adjusted for change in BMI), which was not shown in non-carriers (Heni et al. 2010). The same locus had also previously been associated with increased risk of progression to T2D, which was lessened by metformin therapy in the diabetes prevention programme (DPP), a larger multi-ethnic cohort at high risk of developing T2D (n = 3548) (Florez et al. 2006). Several other SNPs were also identified to have nominal interactions with metformin on incidence of T2D (n = 2994) (Jablonski et al. 2010) or in treatment response (n = 3920) (Zhou et al. 2011). However, also in participants of the DPP (n = 2843), insulin sensitivity indices were studied using a genetic risk score (analogous to our GPS and with 23 of 34 SNPs covered in our study). Similar to the current study, they found a trend for a lower estimated insulin secretion (insulinogenic index) and oral DI (from a 2 h post-oral glucose load value) at baseline, but there was also no overt effect of GPS on change in insulin sensitivity or secretion indices following 1 year of intensive lifestyle modification or metformin treatment (Hivert et al. 2011). It appears that SNPs that are more strongly associated with a given trait in cross-sectional data may not be the most important SNPs in terms of change in trait, especially when improvements are seen in the trait that underlies the association.
A meta-analysis conducted by MAGIC (Meta-Analysis of Glucose- and Insulin-related traits Consortium) identified SNPs associated with increased glycaemia and insulin resistance in non-diabetic participants, using surrogate measures of beta-cell function (HOMA-B) and insulin sensitivity (HOMA-IR) in >35,000 participants (Dupuis et al. 2009). MAGIC identified more than twelve robust associations with fasting plasma glucose and beta-cell function, but only two with HOMA-IR or fasting insulin as a measure of insulin sensitivity. However, the studies included in this meta-analysis used proxy measures of insulin sensitivity and secretion, whilst in the current study, we used detailed phenotyping from the IVGTT to fully characterise insulin secretion and sensitivity by genetic predisposition to T2D, albeit in a small number of participants. Furthermore, examining these parameters before and in response to a dietary intervention showed that the effect of this genetic predisposition did not change the ability to respond to environmental changes. Only a small number of studies have examined the cumulative effect of loci most strongly associated with risk of T2D in response to an intervention or over time. The effects of a combined GPS in the DPP are discussed above (Hivert et al. 2011). An increased T2D risk score, composed of four alleles, has been associated with an accelerated age-related decline in beta-cell function in a longitudinal study (Haupt et al. 2009). In this case, a combined GPS from SNPs associated with 2 h post-load glucose was shown to contribute to a steeper age-related decline in glucose tolerance (Jensen et al. 2011).
Despite the small sample size and potential insufficient statistical power to examine effects of individual SNPs, in an exploratory analysis, we found significant associations of the TCF7L2 SNP with lower acute insulin secretion and DI, but no association between this SNP and the change in insulin secretion or DI in response to the intervention. The sensitivity analysis, in which we excluded the TCF7L2 SNP from the GPS, indicated that the accumulation of risk alleles, other than TCF7L2, still contributed to the impairment of beta-cell function, although the effect was not as strong. In participants from the DPP (n = 3548), the T2D risk-conferring allele of TCF7L2, which was associated with proxy measures of insulin secretion, but not sensitivity at baseline, also found no association with change in these measures in response to metformin or lifestyle interventions (Florez et al. 2006).
In conclusion, using detailed phenotyping from IVGTT, we demonstrated that genetic predisposition to T2D is associated with impaired beta-cell function in non-diabetic, overweight participants on a high saturated fat diet, which reflects an average ‘Western diet’. This genetic predisposition did not moderate effects of a reduction in dietary saturated fat by replacement with MUFA or carbohydrates on changes in insulin sensitivity or beta-cell function.
References
Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC (2008) Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 87(3):627–637
Bergman RN, Ader M, Huecking K, Van Citters G (2002) Accurate assessment of beta-cell function. Diabetes 51(suppl 1):S212–S220
Billings L, Florez JC (2010) The genetics of type 2 diabetes: what have we learned from GWAS? Ann NY Acad Sci 1212(1):59–77
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL et al (2009) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42:105–116
Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PIW, Pollin TI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D, Group ftDPPR (2006) TCF7L2 polymorphisms and progression to diabetes in the diabetes prevention program. N Engl J Med 355(3):241–250
Hamrefors V, Orho-Melander M, Krauss RM, Hedblad B, Almgren P, Berglund G, Melander O (2010) A gene score of nine LDL and HDL regulating genes is associated with fluvastatin-induced cholesterol changes in women. J Lipid Res 51(3):625–634
Haupt A, Staiger H, Schäfer S, Kirchhoff K, Guthoff M, Machicao F, Gallwitz B, Stefan N, Häring H, Fritsche A (2009) The risk allele load accelerates the age-dependent decline in beta cell function. Diabetologia 52(3):457–462
Heni M, Ketterer C, Thamer C, Herzberg-Schafer SA, Guthoff M, Stefan N, Machicao F, Staiger H, Fritsche A, Haring H-U (2010) Glycemia determines the effect of type 2 diabetes risk genes on insulin secretion. Diabetes 59(12):3247–3252
Hivert M-F, Jablonski KA, Perreault L, Saxena R, McAteer JB, Franks PW, Hamman RF, Kahn SE, Haffner S, the DC and others (2011) Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes 60(4):1340–1348
Jablonski KA, McAteer JB, de Bakker PIW, Franks PW, Pollin TI, Hanson RL, Saxena R, Fowler S, Shuldiner AR, Knowler WC et al (2010) Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes 59(10):2672–2681
Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, Bluck LJ, Williams CM, Sanders TAB (2010) Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr 92(4):748–758
Jensen AC, Barker A, Kumari M, Brunner EJ, Kivimaki M, Hingorani AD, Wareham NJ, Tabak AG, Witte DR, Langenberg C (2011) Associations of common genetic variants with age-related changes in fasting and postload glucose. Diabetes 60(5):1617–1623
Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46(1):3–19
Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw K-T, Ong KK, Wareham NJ, Loos RJ (2010) Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am J Clin Nutr 91(1):184–190
Moore C, Gitau R, Goff L, Lewis FJ, Griffin MD, Chatfield MD, Jebb SA, Frost GS, Sanders TAB, Griffin BA et al (2009) Successful manipulation of the quality and quantity of fat and carbohydrate consumed by free-living individuals using a food exchange model. J Nutr 139(8):1534–1540
Parillo M, Riccardi G (2004) Diet composition and the risk of type 2 diabetes: epidemiological and clinical evidence. Br J Nutr 92(01):7–19
Saxena R, Voight BF, Lyssenko V, Burtt NlP, de Bakker PIW, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316(5829):1331–1336
Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445(7130):881–885
Talmud PJ, Drenos F, Shah S, Shah T, Palmen J, Verzilli C, Gaunt TR, Pallas J, Lovering R, Li K et al (2009) Gene-centric Association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am J Hum Genet 85(5):628–642
Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G et al (2010) Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42(7):579–589
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JRB, Rayner NW, Freathy RM et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316(5829):1336–1341
Zhou K, Bellenguez C, Spencer C, Bennett A, Coleman R, Tavendale R, Hawley S, Donnelly LA, Schofield C, Groves CJ et al (2011) Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 43(2):117–120
Acknowledgments
The RISCK dietary intervention trial was funded by the Food Standards Agency project number NO2031. Genetic analyses were funded by the participating centres including UK Medical Research Council (grant codes U105960389, U1052.00.014). The RISCK Study Group comprised of: Carmel Moore, Mark Chatfield, Christine Williams, Hannah Farrant, Claire Lawrence, Edel Magee, Kit Tsoi, Darren Cole, Steve Austin, Hanneke Mfuni, Kate Guberg, Anna Gent, Celia Greenberg, Caroline Stokes, Mario Siervo, Rosemary Hall, Louise Goff, Claire Howard, Namrata Dhopatkar, Bushra Siddiqui, Anne Dornhurst, Fiona Lewis, Samantha Bowen, L Chen, Robert Gray, Nuala Booth, Gary Moore, Roy Sherwood, Anthony Leeds, A Shah, G Saran, J Niehuser-Saran, JA Cockburn, Rachel Gitau, Katie Newens, Sean Lovegrove, Ana Rodriguez-Mateos, John Wright, Margaret Griffin and Nicola Harman. Special thanks to Aseel Al Saleh (Kings College London) for DNA extraction of samples. Thanks to Ashley Olson for statistical advice.
Conflict of interest
The authors and their research groups have a number of links with the food industry. In a personal capacity, GSF is a consultant to Coca-Cola, Premier Foods, and Unilever, and TABS is a member of the external scientific review committee of the Malaysian Palm Oil Board. TABS, BAG, JAL, SAJ and GSF have received ad hoc honoraria for lectures or writing articles. In a non-personal capacity, BAG was formerly a member of an expert group known as the Fat Panel, which was supported by Dairy Crest, Kerry Gold and Unilever; SAJ and JAL sit on government advisory boards that also include food industry members. All research groups received products from a range of food companies gratis for research purposes, including Archer Daniel Mills, Croda, Matthews Foods, Nestle, PepsiCo, Jordan, GSK, and Unilever. CGW, RJLF, APM and LJB reported no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the RISCK study group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Walker, C.G., Loos, R.J.F., Mander, A.P. et al. Genetic predisposition to type 2 diabetes is associated with impaired insulin secretion but does not modify insulin resistance or secretion in response to an intervention to lower dietary saturated fat. Genes Nutr 7, 529–536 (2012). https://doi.org/10.1007/s12263-012-0284-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12263-012-0284-8