Abstract
Despite efforts to increase agricultural production sustainably in sub-Saharan Africa, large gaps remain between actual and potential yield of food crops. Adding biochar to degraded cropland soils in the African tropics has significant potential to enhance crop productivity. Biochar-based farming can also mitigate climate change, through soil carbon storage. This study involved six smallholder farms at sites in eastern, central, and western Kenya that are characterized by different pedo-climatic conditions. We examined the response of non-fertilized and fertilized maize monoculture to three dosages of biochar that are realistic for domestic production by farmers at each of the sites over four growing seasons. Commonly available biomass wastes in each agro-ecosystem (coconut shells, coffee husks, maize cobs) were used as feedstock for biochar, which was applied at 1, 5, and 10 Mg ha−1 at the start of the experiment. Across seasons and fertilizer treatments, maize grain yield (dry matter) showed consistently positive responses, with an average increase of 1.0, 2.6, and 4.0 Mg ha−1, respectively, above the control for the three biochar application rates. Absolute responses of maize grain yield to specific biochar doses were similar across the four investigated seasons and replicate farms within sites, and uncorrelated to yield levels in the control treatment. Here, we show for the first time that yield response to biochar decreased with increasing application rate, indicating that it may be better to spread a given amount of biochar over a large area rather than concentrating it to a smaller area, at least when biochar is applied along plant rows at rates ≥1 Mg ha−1, as in our experiment. This study demonstrated that application of biochar, locally produced from available biomass residues, is a promising approach to enhance agricultural production and carbon storage on smallholder farms under a wide range of pedo-climatic conditions in Kenya.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The population of sub-Saharan Africa (SSA) is predicted to grow annually by 2.4% during the next decade (OECD/FAO 2019) and may more than double by 2050, which will be challenging for food security (Lal 2009; Van Ittersum et al. 2016). Existing gaps between actual and potential yield of agricultural crops have to be reduced to secure food supply and achieve “Zero Hunger by 2030,” one of the 17 Sustainable Development Goals set by the United Nations. To achieve this, “a uniquely African Green Revolution,” recognizing the diversity of peoples, soils, and farming practices, is needed (Tittonell et al. 2011).
Maize (Zea mays L.) is a major staple food in almost all countries in SSA. In Kenya, average maize grain yield has been hovering around 1.4–1.8 Mg ha−1 over the past decade (2010–2019) (www.fao.org/faostat/). Smallholder farmers are the main producers of staple food in Kenya, but have limited access to investment capital, knowledge, and agricultural inputs. Therefore, average maize yield is only about 1 Mg ha−1 on small-scale farms in Kenya, which is lower than the country-wide average and far below the yield of 6–8 Mg ha−1 that could be attained with recommended management practices (Kiboi et al. 2019; Mutuku et al. 2020). The gap in crop yield is largely attributable to soil nutrient depletion caused by long-term nutrient mining and land degradation, exacerbated by the old age and highly weathered state of Kenyan soils (Tittonell et al. 2008; Vanlauwe et al. 2014), temporal and spatial variability in rainfall (Rockström et al. 2010), and sub-optimal cropping practices (Mupangwa et al. 2012).
Adding biochar made from excess biomass wastes to cropland is a potential strategy for improving food security, soil stewardship, and climate mitigation, but has not been extensively studied in smallholder farming systems in SSA. Globally, crop yield responses to soil biochar application vary from negative to positive, depending on type of biochar, soil, and climate, with studies in sub-tropical and tropical regions consistently reporting positive responses (Biederman and Harpole 2013; Liu et al. 2013; Jeffery et al. 2017; Ye et al. 2020; Schmidt et al. 2021). However, potential trade-offs and drawbacks for other ecosystem processes must be considered, since yield reductions and unfavorable changes in soil chemical, physical, and biological properties following biochar addition have been reported (Wardle et al. 2008; Mukherjee and Lal 2014). It is also important to consider the source of the biomass, to ensure that biochar production does not place additional pressure on other environmental aspects, such as trees and shrubs in agricultural landscapes.
Biochar is a carbonized organic material that remains after pyrolysis, i.e., thermal decomposition of biomass under a low-oxygen headspace (Lehmann et al. 2006). Incubation studies have shown that a small fraction of biochar decomposes in a short time (Carlsson et al. 2012). According to a meta-analysis by Wang et al. (2016), this labile carbon pool comprises only about 3% on average, while the remaining 97% of biochar has a long residence time in soil that exceeds the residence time of classic organic amendments such as compost, manures, or raw crop residues (Kimetu and Lehmann 2010) or bulk soil organic matter. Based on data from long-term field experiments, it has been estimated that pyrogenic organic matter is 1.6 times more stable than bulk organic matter (Lutfalla et al. 2017). Thus, application of biochar to soil may also contribute to climate change mitigation through long-term carbon storage in soil (Njenga et al. 2017; Minx et al. 2018).
Gasification energy technologies can reduce the demand for firewood, lowering the pressure on forests, which causes rapid declines of tree cover across sub-Saharan Africa (Leblois et al. 2017). Small gasifier cooking stoves have been shown to be more energy efficient than preparing meals on traditional three-stone open fire systems (Njenga et al. 2016). The pressure on forests can be lowered further by using on-farm produced biomass like crop residues and tree prunings as the primary feedstock for biochar (Sundberg et al. 2020). Although agricultural residues are utilized for a variety of purposes on smallholder farms, such as fodder, mulch, or construction, there are rather large quantities of excess agricultural wastes and residues in rural areas, as found recently in a study in eastern Uganda (Roobroeck et al. 2019). Since biochar is rich in energy, it can be used as charcoal, replacing other energy sources, so application of biochar to agricultural land is only justified when this practice significantly improves crop yields (Woolf et al. 2016; Njenga et al. 2017).
The present study was motivated by findings from a field experiment in central and western Kenya, where grain yield of maize and soybean demonstrated an average increase of 1.2 and 0.4 Mg ha−1, respectively, over 10 years after one-time application of 50+50 Mg ha−1 of biochar derived from acacia charcoal (Kätterer et al. 2019). These yield increases occurred irrespective of whether mineral fertilizer was applied or not, and under favorable and unfavorable rainfall conditions. Improvements of soil porosity, pH, plant-available phosphorus (P), and soil water-holding capacity were identified as potential reasons for the observed yield responses to biochar addition (Kätterer et al. 2019).
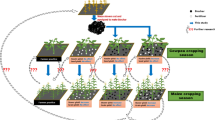
New experiments were set up on nine farms, three each in eastern, central, and western Kenya, to test the effect of biochar produced from locally available feedstocks on crop performance under maize monoculture (Fig. 1). Assuming that a typical smallholder family can produce 0.3 Mg biochar per year with a gasifier cookstove if all food is prepared this way, it would take one decade to cover 0.5 hectare of farmland (common smallholder farm size) with 6 Mg biochar (Sundberg et al. 2020). Thus, rates of biochar application in this experiment (1, 5, and 10 Mg ha−1) reflect the quantity that could be produced from resources available on smallholder farms to enrich the entire area of cropland within a reasonable period.
Biochar was produced from cobs and stovers from maize, coconut shells, and coffee husks, the most common agricultural residues in the study region. Responses of maize grain yield were tracked over four growing seasons after soil biochar amendment. Our specific hypotheses were that (i) maize grain yield increases with increasing biochar dose, (ii) the yield response depends on the feedstock/s used to produce the biochar, (iii) the response to biochar is independent of mineral fertilizer dose, i.e., yield responses to biochar and mineral fertilizer are additive, and (iv) absolute yield increases compared with negative control treatments in response to biochar application are similar regardless of site-specific soil fertility. We also scrutinized the effects of biochar addition on soil physical properties and fertility functions by measuring soil acidity and by considering existing data on bulk density and water retention from two previous trials, at our sites in central and western Kenya (Pühringer 2016).
2 Material and methods
2.1 Selection and characterization of field sites
The fields in which the experiment was conducted were selected based on-farm management data collected in a survey in 2014 of 60 households in three counties, Kwale, Siaya, and Embu, in eastern, western, and central Kenya, respectively, as part of the overall project. Key selection criteria were fields located on flat or gently sloping topography and fields under similar management during the previous 2–3 cropping seasons, i.e., under maize monoculture with low amounts of organic and/or mineral fertilizers. For experimental fields in Siaya, a further selection criterion was that they were free of commonly occurring parasitic weeds (Striga sp.), to avoid confounding effects. Nine farms were selected but, before the end of the first growing season (2015), one farmer in Kwale discontinued the trial due to a land dispute, bringing the total number of field sites to eight (Table 1). For different reasons, one farmer in Embu and one in Kwale dropped out later in the experimental period.
At the Embu and Siaya sites, there are two growing seasons per year, long rains (LR) from February to July and short rains (SR) from September to December. In Kwale, there is one growing season per year, from April to August. Mean annual precipitation is normally slightly lower in Embu (1200 mm) than in Siaya (about 1350 mm), and lowest in Kwale (about 1050 mm, but with large variation within the county) (www.climatedata.eu). In the SR season of 2016 (SR-2016), Embu and Siaya experienced a very late start of rains and low levels of precipitation, so it was decided to let the experimental fields lie fallow for this cycle. By the end of experiment, we had collected crop yield data from four seasons for five fields (two in Embu and three in Siaya), from three seasons for one field in Kwale, from two seasons for one field in Embu, and from one season for one field in Kwale (Table 2).
2.2 Experimental design and treatments
The study was initiated in March–April 2015 and treatments were arranged at the field sites in a split-plot design with three replicate blocks. There were eight treatments, comprising no biochar (control) and three biochar application rates (1, 5, and 10 Mg ha−1) in main plots randomized in each block and two fertilizer treatments (without and with) in subplots randomized across main plots. Di-ammonium phosphate (DAP: 18% N, 20% P) was used as fertilizer, at a rate 60 kg N and 67 kg P per hectare and growing season. The 24 plots containing the different treatments each measured 3 m × 4 m in Embu and 4 m×5 m in Kwale and Siaya, with a buffer strip of 0.5 m surrounding each plot. At the onset of each growing season, the soil was prepared by manual hoeing, as is local practice. The improved hybrid maize variety DH04 was grown in Kwale and Siaya, and variety H513 in Embu. Maize was planted at a spacing of 0.75 m between rows and 0.25 m within rows (Table 2). At the beginning of the first cropping season, biochar (produced from site-specific substrates as detailed below) was placed along the length of each planting row in a furrow 0.15 m deep, thus increasing the concentration in the root zone of the crop. In the fertilized treatments, DAP was spot-applied every season at 0.10-m depth below the planting holes for maize. Two seeds were placed at 0.05-m depth inside each planting hole and covered with soil in all treatments. The plots were managed by the farmers, under the direction of the research team. Thinning/gap filling was carried out in the first two weeks after planting, bringing the density in maize stands to 5.7 plants m−2. Two rounds of weeding were carried out, at about three and 10 weeks after planting, in all plots at all sites. When necessary, the maize was sprayed with chemical agents to control insect pests (cutworm, fall army worm), according to local recommendations. All experiments were conducted under rain-fed conditions.
2.3 Biochar production and properties
To comply with the study aims, the biochar used in the experiments was produced from biomass wastes that are abundant locally and have little alternative use, such as animal feed. The available feedstock used for producing biochar differed between the sites, with maize cobs and stovers used in Siaya, coffee husks in Embu, and coconut shells in Kwale (Table 3). Biomass waste resources were collected on farms close to the experimental fields and processed into biochar at one location in Maseno. All feedstocks were first sun-dried on top of polythene bags until constant weight was attained, and then converted into biochar using converted oil drums with holes around the base, a grate at the bottom, and a chimney to control airflow. Temperature in kiln reactor typically reaches 400–500 °C. In this study, the pyrolysis process was allowed to run for about 2.5 h for all biomass feedstocks, which produced roughly 10 kg of biochar per kiln. Water was poured into the kiln until self-lighting and combustion ceased. The biochar was then sun-dried and crushed into pieces of around 5-mm diameter. A composite biochar sample was taken for each type of biomass feedstock and sent to a laboratory in Sweden for analysis. The biochar samples were fused with lithium metaborate and dissolved in acid, before quantification of total content of ash, oxides, and metals by ICP-SFMS (ALS Scandinavia AB).
2.4 Soil sampling and analyses
At the start and end of the experiment, 10 soil cores were sampled to 0.15-m depth with an auger within the net plot area used for yield measurements in each experimental plot on each farm. The 10 cores were thoroughly mixed and a composite sample was taken for drying and analyses. Baseline soil characteristics were determined on the composite samples taken from all subplots, with analysis taking place in 2015 (Table 1). Fractions of sand, silt, and clay in soil were determined through sedimentation and pipetting (Gee and Bauder 1986). Total carbon (Tot-C) and total nitrogen (Tot-N) contents in soil were measured by dry combustion (LECO Corp., USA), P content was analyzed colorimetrically in Mehlich-3 and Olsen extracts (Mehlich 1984; Olsen et al. 1954), and extractable base cations (Ca, K, Mg) were measured in 1 M ammonium acetate extract buffered at pH 7. Soil pH was measured in 2019 in air-dried archived samples taken in 2015 and 2017 in all plots in the six experimental fields that were still running in 2017. These pH measurements were conducted in one batch. Soil samples were mixed with distilled water at a mass ratio of 1:2.5 and pH was measured using a glass-membrane electrode.
2.5 Yield measurements
When 95% of the maize crop had dried in the field after reaching physiological maturity, a 4.5 m2 area in the center of each subplot was harvested by taking plants from a 1.5-m length of four maize rows. In the field, all fully grown maize cobs were separated from stover and total fresh weight of grain and of total crop biomass were measured using a mechanical spring balance, after which representative subsamples of six stover and six cobs were taken. In the laboratory, grains were separated from cores for all cobs, desiccated in an oven at 65 °C for 48 h, and measured for dry weight using an electronic table-top balance. Yield of maize grain per hectare was calculated by multiplying total fresh weight of cobs by the proportion of dry kernels per unit fresh weight, based on analysis of subsamples. After harvesting maize crops, all aboveground residues were removed from the field trials. All yield measurements are reported as dry matter yield.
2.6 Data analysis
Statistical analyses were carried out using SAS software (version 9.4; SAS Institute, USA). A linear mixed model was used to analyze the variation in maize grain yields between biochar application rates, fertilizer treatments, and site as main and interactive fixed factors, in which farm within site, replicate block within farm, and repeated season were used as random factors. Since substrates used for biochar production differed between sites, we tested corresponding models separately for each site before applying the model to the whole dataset. As responses to biochar were similar at the three sites (as reported below), we included site in the statistical model, which here represents both site-specific pedo-climatic conditions and biochar properties. In F tests, the degrees of freedom for the denominator were calculated using the Kenward-Roger approximation method. Since farms in eastern Kenya do not grow maize in SR seasons and since two farms dropped out during the experiment, least square means of yield differences between study areas were calculated across farms and seasons. Another linear model was used to analyze the variation in maize yields between biochar application rates and fertilizer treatment for each combination of farm and growing season, based on a split-plot design with biochar as main plot, fertilizer as subplot treatment, and replicate blocks within farmers’ fields as random variable. Differences in mean yield between the fixed factors in this model were analyzed using Tukey’s HSD test.
3 Results
3.1 Soil properties
The farms in Embu and Siaya were located on clay dominated soils, with an average clay content of 61 and 70%, respectively (Table 1). Farms in Kwale have sandy clay loam soils, with 13% clay. Initial soil C content was higher on farms in Embu (1.79–1.86%) and Siaya (1.81–2.02%) than on farms in Kwale (0.65–0.85%). Soil C/N ratio was lower in Embu and Kwale (11–13) than in Siaya (14–21). Extractable P was lower on farms in Siaya than on those in Embu. At the start of the experiment, soil pH was higher (>6) in the fields in Kwale than in those in Embu and Siaya. It significantly declined between seasons LR-2015 and LR-2017 at all sites, by between 0.02 and 0.63 pH units (Table 1), but the change over time was not significantly affected by fertilization or biochar application.
3.2 Biochar characteristics
Among the biochars produced from different feedstocks, coffee husks (Embu site) had the highest content of ash, mainly consisting of oxides, and was generally the richest biochar in terms of plant nutrients. Biochar from maize cobs was richer in elements than that from coconut shells, except for the heavy metals Cr, Pb, and Sn (Table 3). However, concentrations of potentially harmful heavy metals were low in the biochars produced from all feedstocks (Table 3). Concentrations of Cr, Cd, Hg, Ni, Pb, and Zn were far below even the premium grade limits for biochar according to the European Biochar Certificate (www.european-biochar.org), which is a voluntary standard in Europe. However, Cu content in coffee husks (278 μg g−1) exceeded this standard (<100 μg g−1).
3.3 Yield responses to biochar and fertilizer
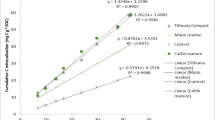
Maize grain yield in the control treatment (without biochar and fertilizer) was 0.70 Mg ha−1 on average across sites and seasons, and varied from 0.69 to 0.82 Mg ha−1 between the three sites (Fig. 2). Mean yields across all treatments and all seasons were significantly higher in Embu (3.65 Mg ha−1) than in Kwale (3.02 Mg ha−1) and Siaya (3.11 Mg ha−1). Maize grain yield increased significantly with biochar application rate across sites and seasons in both fertilized and unfertilized treatments. Yield responses varied significantly between the three rates of biochar amendment for both fertilized and unfertilized treatments. Statistical analysis revealed significant effects of biochar rate, fertilization, and site on grain yield, and also a weak but significant positive interaction between fertilizer and biochar application, indicating that N fertilizer was used more efficiently at the highest biochar application rate (Fig. 3, Table 4). The significant interaction between biochar and site was due to stronger yield response to biochar application in Embu than at the other two sites (Fig. 4). Although yields differed between seasons across sites and treatments, yield responses to biochar rates and fertilizer were similar between seasons (Fig. 2). The increase of grain yield per Mg of biochar applied, i.e., the efficiency, decreased with increasing application rate (Fig. 5). In absolute terms, the yield increase due to biochar addition was similar between sites and seasons, and was on average 1.0, 2.6, and 4.0 Mg ha−1 higher than in the corresponding fertilized and unfertilized control treatments for the 1, 5, and 10 Mg ha−1 biochar application rate, respectively. Absolute yield responses to biochar application did not show any significant correlation with yield in the unfertilized control treatment on individual farms (p-values varied between 0.14 and 0.89; not shown), which suggests that site-specific fertility, within the range investigated here, did not affect the biochar response.
Maize grain yield (dry matter) at different biochar application rates (0, 1, 5, and 10 Mg ha−1), with or without N fertilization, during four (three at Kwale) growing seasons (LR and SR represents long and short rainy season, respectively). Error bars denote standard error of means across farms per site. Bars with different letters (a, b, c, d) denote significant differences between biochar application rates per season and fertilizer treatment according to post-hoc (Tukey-Kramer) tests. Please note that the number of farms per site differed between seasons (see Table 2).
Analysis of individual farms and seasons revealed that biochar significantly affected maize grain yield for all 26 combinations of farms and seasons studied. Maize grain yield differed significantly between treatments with increasing biochar dose (1, 5, and 10 Mg ha−1) for 19 farm and season combinations. The effect of fertilizer was significant for 25 farm and season combinations. Significant positive interactions between biochar and fertilizer treatments were found for nine of the 26 farm and season combinations. Across seasons, the effects of biochar and fertilizer treatments were significant for all farms and interactions between biochar and fertilizer treatments were significant for two farms (data not shown). Fertilizer addition increased maize grain yield with 1.0 Mg ha−1 on average for all farms, seasons, and biochar rates. Averaged yield response to fertilizer input ranged from 0.8 to 1.3 Mg ha−1 on specific farms (data not shown). Averaged across farms and seasons, fertilizer yield response varied between 0.7 and 1.4 Mg ha−1 for different biochar application rates (Fig. 3).
4 Discussion
4.1 Maize yield response to biochar
In this experiment, we found that one-time biochar application consistently enhanced maize grain yield on smallholder farms across different sites in Kenya and over several seasons. Inter-seasonal yield variation was much lower than in our previous long-term study in Embu and Siaya (Kätterer et al. 2019). Because of very late and low rainfall during the SR-2016 season in Embu and Siaya, the farms were not cultivated to avoid non-representative treatment effects. The maize crop responded strongly to the lowest biochar application rate (1 Mg ha−1), which increased grain yield by about 1.0 Mg ha−1 on average over sites and fertilizer treatments. Yield response to biochar application increased consistently with biochar dose up to 10 Mg biochar ha−1 (Fig. 3), but the rate of yield gain decreased with biochar dose (Fig. 5), so yield increments per Mg biochar applied was greater at lower biochar doses. This is an important finding for practical implementation of biochar treatment in smallholder settings, as it indicates that it may be advantageous to spread the available amount of biochar over a larger area, at least in cases where the application rate is ≥1 Mg ha−1. It should furthermore be noted that the biochar in our experimental setting was placed in furrows along the length of each planting row at the start of the first season, thus increasing the concentration in the root zone of the crop. Although some biochar from the planting rows was dispersed by tillage, its placement along the furrow was kept intact so the rate underneath crops was higher than if it had been applied by broadcasting. This particular application method should be considered when comparing results from our experiment with those in other studies where other approaches are used.
Another important finding for practical implementation was that absolute yield response to biochar application was uncorrelated with yield in the control treatments, indicating that sites with different fertility respond similarly to application of biochar. The comparatively stronger yield response to biochar application rates observed in Embu is probably a result of the higher nutrient content in biochar produced from coffee husks at this site (Table 4). Since both biochar type and pedo-climatic conditions varied between the sites, their respective impact on yields cannot be separated in our study. Differences in yield responses however did not exceed 1 Mg ha−1 between the three sites which is relatively moderate (Fig. 4). Pyrolytic conditions during biochar production can have an important influence on its quality and thus the response of crops when applied to soil (Tan et al. 2017). Biochar used at the different sites was produced under similar conditions and this may have reduced the variation in this experiment.
The yield response to biochar application of 5 to 10 Mg ha−1, i.e., 2.6 and 4.0 Mg ha−1, was much stronger than for the long-term experiment at Embu and Siaya, where maize yield increased by 1.2 Mg ha−1 on average over 20 growing seasons after initial application of 100 Mg wood-derived biochar (Kätterer et al. 2019). Differences in biochar properties could explain these differences in yield response, but analytical results did not show obvious differences. The only apparent reason could be that the biochar used by Kätterer et al. (2019) was based on wood, produced in a traditional kiln, and contaminated with soil. Contrasting results on yield response to biochar have been reported in the literature, e.g., in one study in Zambia application of 0, 2, and 6 Mg biochar ha−1 had no significant effect on crop yield at three sites with sandy to loamy soil texture (Martinsen et al. 2014), whereas strong positive effects on maize yield were reported in another study in Zambia at maize cob biochar doses of 0.8 Mg ha−1 and wood biochar doses of 4 Mg ha−1 (Cornelissen et al. 2013). To improve soil fertility and maize production, further research is needed to identify where, when, and why soil biochar application is successful.
4.2 Biochar effects on physicochemical soil properties
Application of biochar, or fertilizer, did not significantly change soil pH between treatments, nor were these practices accountable for the significant decline of about 0.3 pH units over the experimental period. This is unlike expected, since the oxides of alkali earth metals in biochar (Table 3) would naturally increase soil pH, as reported in previous studies (e.g., Glaser et al. 2002; Kätterer et al. 2019). Our result is also surprising since the pH-buffering capacity of soil can be expected to increase with the rate of biochar addition (Xu et al. 2012). One possible reason may be that greater export of base cations through removal of maize grain and residue in the biochar treatments compared with the control has counteracted the increase of soil pH. The apparent pH decline could also be related to common seasonal dynamics (Olojugba and Fatubarin 2015) or duration of sample storage (Prodromou and Pavlatou-Ve 1998). The pH at the start of the experiment in LR-2015 was measured after 3 years, whereas that at the end of the experiment in LR-2017 was analyzed within less than half a year after sampling. Nonetheless, it could be that the positive yield response from biochar application was due to other causes than a liming effect.
Soil bulk density and soil water retention data for treatments with different biochar rates at two of the sites (Embu and Siaya) were taken during the first experimental season as part of another study (Pühringer 2016). Across biochar treatments the mean dry bulk density at 7.5-–12.5-cm depth measured 0.93 Mg m−3 at Embu and 1.0 Mg m−3 at Siaya, but findings did not show the expected decrease in bulk density with increasing biochar application rate. Soils enriched with biochar held slightly more plant-available water at both sites, but the effect of biochar was not significant (Pühringer 2016). The limited number of water potential values that were taken in that study may have led to that some critical parts of the water retention curve were missed. Other studies conversely found that biochar application can have varying impacts on soil water retention along the water retention curve (Fischer et al. 2019), with water potential values between 0.01 and 1 MPa being most affected (Downie et al. 2009).
4.3 Crop nutrient supply
About 15 kg N and 2 kg P ha−1 were exported from the field when harvesting 1 Mg of maize grain, assuming an N and P concentration of 15 and 2 mg g−1 of grain, respectively. Application of 10 Mg biochar ha−1 increased maize yield by on average 4 Mg ha−1, which corresponds to approximately 60 kg N and 8 kg P ha−1. For sustainability of the system, it is crucial to identify the sources of this additional nutrient export. The amount of P applied in 10 Mg biochar was 1.2, 14, and 20 kg ha−1 for coconut shells, maize cobs, and coffee husks, respectively (Table 3). As only part of this P is likely to be plant-available (Chintala et al. 2014), biochar will be a minor contributor to the additional P demand from higher yield, i.e., about 8 kg P for a yield increase of 4 Mg ha−1. We did not measure the N content in the biochars, but it is generally low since a part of N is volatized during pyrolysis (Li et al. 2018; Schulz and Glaser 2012) and plant availability of the N retained in biochar is probably low. Thus, the N demand for the biochar-induced yield increase must also have been met by sources other than the biochar. According to the literature, there are several potential mechanisms that could increase N retention and availability to plants following biochar application. These include improved efficiency of N utilization by the crop (Wang et al. 2015) due to enhanced root growth (Bruun et al. 2014) and suppression of nitrification (Plaimart et al. 2021) resulting in less leaching and reduced gaseous N emissions (He et al. 2017). Given that N fertilization enhanced grain yield by 1 Mg ha−1 in the treatment without biochar, as well as on average for all biochar application rates, the agronomic use efficiency of fertilizer N was largely unchanged at 25%, or 15 kg grain per 60 kg N fertilizer added, as also observed in a recent biochar study using 15N-labelled fertilizer (Abbruzzini et al. 2019). Although fertilizer N use efficiency was slightly higher (36%) in the treatment with 10 Mg biochar than in treatments with lower rates, the level remained relatively low, suggesting that tighter N cycling was probably not the only explanation for the observed yield response to biochar.
Enhanced N2 fixation is another mechanism that could explain the increase in N crop uptake. Application of biochar has been shown to increase symbiotic N fixation in common bean (Güereña et al. 2015) and may also affect non-symbiotic N fixation, as evidenced by biochar-induced changes in the community structure of diazotrophs reported by Liu et al. (2019). To our knowledge, N fixation by diazotrophs in response to biochar has not yet been quantified, but N fixation by root-associated diazotrophs can be substantial, e.g., in nutrient-poor soil it can contribute 29–82% of the N taken up by maize (Van Deynze et al. 2018).
Biochar-induced N mining from soil due to accelerated decomposition of native soil organic matter (priming) could also partly explain the observed increase in crop N acquisition (Wardle et al. 2008). Both positive and negative biochar priming effects have been reported in the literature, but low-fertility soils predominantly exhibit positive priming (increased rates of decay) (Wang et al. 2016). Persistent positive priming would deplete soil fertility in the long run. However, since priming effects are usually rather short-term (Luo et al. 2016) and positive yield responses to biochar can be sustained for a decade or more (Kätterer et al. 2019), it is unlikely that priming was the only explanation for the increased N availability in our study. Detailed studies on nutrient cycling in response to biochar are urgently needed to evaluate the potential effects of biochar on plant nutrient acquisition.
5 Conclusions
Recurrent increases in maize grain yield in response to realistic biochar application rates on smallholder Kenyan farms were recorded. Across seasons and fertilizer treatments, maize grain yield (dry matter) showed consistently positive responses over four growing seasons studied, with an average increase of 1.0, 2.6, and 4.0 Mg ha−1 above the local control for the three biochar application rates of 1, 5, and 10 Mg ha−1 at the start of the experiment. Absolute yield responses to biochar application were uncorrelated with yield in the control treatments, indicating that sites with different fertility may respond similarly to application of biochar. Yield responses to biochar rates were slightly stronger at one of the sites where the richest biochar in terms of plant nutrients was applied. However, the effects of biochar type and pedo-climatic conditions could not be separated in our study. In general, yield responses to biochar were similar across sites, growing season conditions, and feedstock used for producing biochar. Thus treating farmland with biochar made from common agricultural wastes represents a promising approach for increasing crop production sustainably and mitigating climate impacts in many rural communities in Kenya, and elsewhere. Here, we show for the first time that for optimized use of this valuable resource at farm scale, available biochar should be spread at a low rate (≥1 Mg ha−1) to a large area rather than at a high rate to a smaller area. It should be considered that biochar in our experimental setting was placed in furrows along the length of each planting row at the start of the first season, thus increasing the concentration in the root zone of the crop. Before large-scale implementation of this practice, its benefits and drawbacks must be thoroughly evaluated in system analyses considering food security and economic, social, and environmental dimensions of sustainability. These analyses should also consider potential effects on the long-term sustainability of soil nutrient cycling.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Abbruzzini TF, Davies CA, Toledo FH, Cerri CEP (2019) Dynamic biochar effects on nitrogen use efficiency, crop yield and soil nitrous oxide emissions during a tropical wheat-growing season. J Environ Manage 252:109638. https://doi.org/10.1016/j.jenvman.2019.109638
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214. https://doi.org/10.1111/gcbb.12037
Bruun EW, Petersen CT, Hansen E, Holm JK, Hauggaard-Nielsen H (2014) Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manage 30:109–118. https://doi.org/10.1111/sum.12102
Carlsson M, Andrén O, Stenström J, Kirchmann H, Kätterer T (2012) Charcoal application to arable soil: effects on CO2 emissions. Commun Soil Sci Plant Anal 43:2262–2273. https://doi.org/10.1080/00103624.2012.701687
Chintala R, Schumacher TE, McDonald LM, Clay DE, Malo DD, Papiernik SK, Clay SA, Julson JL (2014) Phosphorus sorption and availability from biochars and soil/biochar mixtures. Clean Soil Air Water 42:626–634. https://doi.org/10.1002/clen.201300089
Cornelissen G, Martinsen V, Shitumbanuma V, Alling V, Breedveld G, Rutherford D, Sparrevik M, Hale S, Obia A, Mulder J (2013) Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agron 3:256–274. https://doi.org/10.3390/agronomy3020256
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In: Biochar for environmental management: science and technology. Earthscan, London, pp 13–29
Fischer BMC, Manzoni S, Morillas L, Garcia M, Johnson MS, Lyon SW (2019) Improving agricultural water use efficiency with biochar – a synthesis of biochar effects on water storage and fluxes across scales. Sci Total Environ 657:853–862. https://doi.org/10.1016/j.scitotenv.2018.11.312
Gee GW, Bauder JW (1986) Particle-size analysis. In: Methods of soil analysis, Part I. Agronomy series no. 9, 2nd ed. Madison, Wisconsin
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol Fertil Soils 35:219–230. https://doi.org/10.1007/s00374-002-0466-4
Güereña DT, Lehmann J, Thies JE, Enders A, Karanja N, Neufeldt H (2015) Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol Fertil Soils 51:479–491. https://doi.org/10.1007/s00374-014-0990-z
He Y, Zhou X, Jiang L, Li M, Du Z, Zhou G, Shao J, Wang X, Xu Z, Hosseini Bai S, Wallace H, Xu C (2017) Effects of biochar application on soil greenhouse gas fluxes: a meta-analysis. GCB Bioenergy 9(4):743e755–743e755. https://doi.org/10.1111/gcbb.12376
Jeffery S, Abalos D, Prodana M, Bastos A, Van Groenigen JW, Hungate BA, Verheijen F (2017) Biochar boosts tropical but not temperate crop yields. Environ Res Lett 12:053001. https://doi.org/10.1088/1748-9326/aa67bd
Kätterer T, Roobroeck D, Andrén O, Kimutai G, Karltun E, Kirchmann H, Nyberg G, Vanlauwe B, Röing de Nowina K (2019) Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crops Res 235:18–26. https://doi.org/10.1016/j.fcr.2019.02.015
Kiboi MN, Ngetich KF, Fliessbach A, Muriuki A, Mugendi DN (2019) Soil fertility inputs and tillage in fl uence on maize crop performance and soil water content in the Central Highlands of Kenya. Agric Water Manage 217:316–331. https://doi.org/10.1016/j.agwat.2019.03.014
Kimetu JM, Lehmann J (2010) Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Aust J Soil Res 48:577–585. https://doi.org/10.1071/SR10036
Lal R (2009) Soils and food sufficiency. a review. Agron Sustain Dev 29:113–133. https://doi.org/10.1051/agro:2008044
Leblois A, Damette O, Wolfersberger J (2017) What has driven deforestation in developing countries since the 2000s? Evidence from new remote-sensing data. World Dev 92:82–102. https://doi.org/10.1016/j.worlddev.2016.11.012
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial Ecosystems: a review. Mitig Adapt Strat Gl 11:403–427. https://doi.org/10.1007/s11027-005-9006-5
Li S, Barreto V, Li R, Chen G, Hsieh YP (2018) Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J Anal Appl Pyrolysis 133:136–146. https://doi.org/10.1016/j.jaap.2018.04.010
Liu XY, Zhang AF, Ji CY, Joseph S, Bian RJ, Li LQ, Pan GX, Paz-Ferreiro J (2013) Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil 373:583–594. https://doi.org/10.1007/s11104-013-1806-x
Liu XY, Liu C, Gao WH, Xue C, Guo ZH, Jiang L, Li F, Liu Y (2019) Impact of biochar amendment on the abundance and structure of diazotrophic community in an alkaline soil. Sci Total Environ 688:944–951. https://doi.org/10.1016/j.scitotenv.2019.06.293
Luo Z, Wang E, Sun OJ (2016) A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biol Biochem 101:96–103. https://doi.org/10.1016/j.soilbio.2016.07.011
Lutfalla S, Abiven S, Barré P, Wiedemeier DB, Christensen BT, Houot S, Kätterer T, Macdonald AJ, van Oort F, Chenu C (2017) Pyrogenic carbon lacks long-term persistence in temperate arable soils. Front Earth Sci 5:96. https://doi.org/10.3389/feart.2017.00096
Martinsen V, Mulder J, Shitumbanuma V, Sparrevik M, Borresen T, Cornelissen G (2014) Farmer-led maize biochar trials: effect on crop yield and soil nutrients under conservation farming. J Plant Nutr Soil Sci 177:681–695. https://doi.org/10.1002/jpln.201300590
Mehlich A (1984) Mehlich-3 Soil test extractant - a modification of Mehlich-2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Minx JC, Lamb WF, Callaghan MW, Fuss S, Hilaire J, Creutzig F, Amann T, Beringer T, de Oliveira GW, Hartmann J, Khanna T, Lenzi D, Luderer G, Nemet GF, Rogelj J, Smith P, Vicente JL, Wilcox J, del Mar Zamora Dominguez M (2018) Negative emissions – part 1: research landscape and synthesis. Environ Res Lett 13(6):063001. https://doi.org/10.1088/1748-9326/aabf9b
Mukherjee A, Lal R (2014) The biochar dilemma. Soil Res 52:217–230. https://doi.org/10.1071/SR13359
Mupangwa W, Twomlow S, Walker S (2012) Reduced tillage, mulching and rotational effects on maize (Zea mays L.), cowpea (Vigna unguiculata (Walp) L.) and sorghum (Sorghum bicolor L. (Moench)) yields under semi-arid conditions. Field Crops Res 132:139–148. https://doi.org/10.1016/j.fcr.2012.02.020
Mutuku EA, Roobroeck D, Vanlauwe B, Boeckx P, Cornelis WM (2020) Maize production under combined conservation agriculture and integrated soil fertility management in the sub-humid and semi-arid regions of Kenya. Field Crops Res 254:107833. https://doi.org/10.1016/j.fcr.2020.107833
Njenga M, Iiyama M, Jamnadass R, Helander H, Larsson L, de Leeuw J, Neufeldt H, Röing de Nowina K, Sundberg C (2016) Gasifier as a cleaner cooking system in rural Kenya. J Clean Prod 121:208–217. https://doi.org/10.1016/j.jclepro.2016.01.039
Njenga M, Mahmoud Y, Mendum R, Iiyama M, Jamnadass R, Roing de Nowina K, Sundberg C (2017) Quality of charcoal produced using micro gasification and how the new cook stove works in rural Kenya. Environ Res Lett 12(9):095001. https://doi.org/10.1088/1748-9326/aa7499
OECD/FAO (2019) Agricultural Outlook 2019-2028. OECD Publishing, Paris. Food and Agriculture Organization of the United Nations, Rome. https://doi.org/10.1787/agr_outlook-2019-en
Olojugba MR, Fatubarin AR (2015) Effects of seasonal dynamics on the chemical properties of the soil of a Northern Guinea savanna ecosystem in Nigeria. J Soil Sci Environ Manage 6:100–107. https://doi.org/10.5897/JSSEM13.0414
Olsen R, Cole CV, Watanbe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ. 939 USDA, Government printing office, Washington, DC
Plaimart J, Acharya K, Mrozik W, Davenport RJ, Vinitnantharat S, Werner D (2021) Coconut husk biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ Pollut 268:115684. https://doi.org/10.1016/j.envpol.2020.115684
Prodromou KP, Pavlatou-Ve AS (1998) Changes in soil pH due to the storage of soils. Soil Use Manage 14:182–183
Pühringer H (2016) Effects of different biochar application rates on soil fertility and soil water retention in on-farm experiments on smallholder farms in Kenya. Master’s Thesis in Environmental Science, Swedish Univeristy of Agricultural Sciences, Department of Soil and Environment 2016:04. https://stud.epsilon.slu.se/8955/
Rockström J, Karlberg L, Wani SP, Barron J, Hatibu N, Oweis T, Bruggeman A, Farahani J, Qiang Z (2010) Managing water in rainfed agriculture – the need for a paradigm shift. Agric Water Manage 97:543–550. https://doi.org/10.1016/j.agwat
Roobroeck D, Hood-Nowotny R, Nakubulwa D, Tumuhairwe J-B, Mwanjalolo MJG, Ndawula I, Vanlauwe B (2019) Biophysical potential of crop residues for biochar carbon sequestration, and co-benefits, in Uganda. Ecol Appl 29(8):e01984. https://doi.org/10.1002/eap.1984
Schmidt H-P, Kammann C, Hagemann N, Leifeld J, Bucheli TD, Sánchez Monedero MA, Cayuela M (2021) Biochar in agriculture – a systematic review of 26 global meta-analyses. GCB Bioenergy 13:1708–1730. https://doi.org/10.1111/gcbb.12889
Schulz H, Glaser B (2012) Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J Plant Nutr Soil Sc 175:410–422. https://doi.org/10.1002/jpln.201100143
Sundberg C, Karltun E, Gitau J, Kätterer T, Kimutai G, Mahmoud Y, Njenga M, Nyberg G, Röing de Novina K, Roobroeck D, Sieber P (2020) Biochar from cookstoves reduces greenhouse gas emissions from smallholder farms in Africa. Mitig Adapt Strateg Glob Chang 25:953–967. https://doi.org/10.1007/s11027-020-09920-7
Tan Z, Lin CSK, Ji X, Rainey TJ (2017) Returning biochar to fields: a review. Appl Soil Ecol 116:1–11. https://doi.org/10.1016/j.apsoil.2017.03.017
Tittonell P, Corbeels M, Van Wijk MT, Vanlauwe B, Giller KE (2008) Combining organic and mineral fertilizers for integrated soil fertility management in smallholder farming systems of Kenya: explorations using the crop-soil model FIELD. Agron J 100:1511–1526. https://doi.org/10.2134/agronj2007.0355
Tittonell P, Vanlauwe B, Misiko M, Giller KE (2011) Targeting resources within diverse, heterogeneous and dynamic farming systems: towards a ‘Uniquely African green revolution’. In: Innovations as key to the green revolution in Africa: exploring the scientific facts. Springer, Dordrecht, pp 747–758
Van Deynze A, Zamora P, Delaux PM, Heitmann C, Jayaraman D, Rajasekar S, Graham D, Maeda J, Gibson D, Schwartz KD, Berry AM, Bhatnagar S, Jospin G, Darling A, Jeannotte R, Lopez J, Weimer BC, Eisen JA, Shapiro H-Y et al (2018) Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol 16(8):e2006352. https://doi.org/10.1371/journal.pbio.2006352
Van Ittersum MK, Van Bussel LGJ, Wolf J, Grassini P, Van Wart J, Guilpart N, Cassman KG (2016) Can sub-Saharan Africa feed itself? Proc Natl Acad Sci USA 113:14964–14969. https://doi.org/10.1073/pnas.1610359113
Vanlauwe B, Coyne D, Gockowski J, Hauser S, Huising J, Masso C, Nziguheba G, Schut M, Van Asten P (2014) Sustainable intensification and the African smallholder farmer. Curr Opin Environ Sustain 8:15–22. https://doi.org/10.1016/j.cosust.2014.06.001
Wang Z, Zong H, Zheng H, Liu G, Chen L, Xing B (2015) Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 138:576e583–576e583. https://doi.org/10.1016/j.chemosphere.2015.06.084
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523. https://doi.org/10.1111/gcbb.12266
Wardle DA, Nilsson M-C, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320(5876):629. https://doi.org/10.1126/science.1154960
Woolf D, Lehmann J, Lee DR (2016) Optimal bioenergy power generation for climate change mitigation with or without carbon sequestration. Nature Com 7:13160. https://doi.org/10.1038/ncomms13160
Xu R, Zhao A, Yuan J, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sedim 12:494–502. https://doi.org/10.1007/s11368-012-0483-3
Ye L, Camps-Arbestain M, Shen Q, Lehmann J, Singh B, Sabir M (2020) Biochar effects on crop yields with and without fertilizer: a meta-analysis of field studies using separate controls. Soil Use Manage 36:2–18. https://doi.org/10.1111/sum.12546
Acknowledgements
We gratefully acknowledge the valuable contributions from the smallholder farmers at the study sites. We also thank the students who were involved in field work, especially Helene Pühringer.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. The work was supported by the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (FORMAS), through grant number 942-2015-1648.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.R.N., D.R., E.K., G.N., C.S., and T.K.; methodology: K.R.N., D.R., E.K., G.N., G.K., and T.K.; investigation: D.R., K.R.N., and G.K.; resources: T.K., D.R., C.S., and K.R.N.; writing: T.K., K.R.N., G.K., E.K., G.N., C.S., and D.R.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kätterer, T., Roobroeck, D., Kimutai, G. et al. Maize grain yield responses to realistic biochar application rates on smallholder farms in Kenya. Agron. Sustain. Dev. 42, 63 (2022). https://doi.org/10.1007/s13593-022-00793-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-022-00793-5