Abstract
Species of wild rice (Oryza spp.) possess a wide range of stress tolerance traits that can be potentially utilized in breeding climate-resilient cultivated rice cultivars (Oryza sativa) thereby aiding global food security. In this study, we conducted a greenhouse trial to evaluate the salinity tolerance of six wild rice species, one cultivated rice cultivar (IR64) and one landrace (Pokkali) using a range of electrophysiological, imaging, and whole-plant physiological techniques. Three wild species (O. latifolia, O. officinalis and O. coarctata) were found to possess superior salinity stress tolerance. The underlying mechanisms, however, were strikingly different. Na+ accumulation in leaves of O. latifolia, O. officinalis and O. coarctata were significantly higher than the tolerant landrace, Pokkali. Na+ accumulation in mesophyll cells was only observed in O. coarctata, suggesting that O. officinalis and O. latifolia avoid Na+ accumulation in mesophyll by allocating Na+ to other parts of the leaf. The finding also suggests that O. coarctata might be able to employ Na+ as osmolyte without affecting its growth. Further study of Na+ allocation in leaves will be helpful to understand the mechanisms of Na+ accumulation in these species. In addition, O. coarctata showed Proto Kranz-like leaf anatomy (enlarged bundle sheath cells and lower numbers of mesophyll cells), and higher expression of C4-related genes (e.g., NADPME, PPDK) and was a clear outlier with respect to salinity tolerance among the studied wild and cultivated Oryza species. The unique phylogenetic relationship of O. coarctata with C4 grasses suggests the potential of this species for breeding rice with high photosynthetic rate under salinity stress in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity tolerance is a polygenetic trait that has evolved multiple times in diverse genera (Bromham et al. 2020) due to various modifications in plant physiological and anatomical traits (Chen and Soltis 2020; Munns et al. 2020a; Solis et al. 2020). Phylogenetic analysis shows that salt-tolerant lineages exhibit a ‘tippy’ pattern (occurring on the tips of the phylogeny rather than internally) (Flowers et al. 2010; Bromham et al. 2020). This may suggest a potential loss or gain of salt-tolerant traits in different species during their evolutionary and ecological adaptation to saline conditions (Bromham et al. 2020; Chen and Soltis 2020; Caperta et al. 2020). Salt tolerance also appears to have evolved more frequently in plants with C4 rather than C3 photosynthesis (Bromham and Bennett 2014).
The genus Oryza (Poales, Poaceae) contains 24 species (Ge et al. 2001), with the Asian rice, O. sativa, and African rice, O. glaberrima, being staple crops for over half of the global population (Grieve et al. 2019). Cultivated rice is highly susceptible to salinity stress, and a yield penalty occurs at low salinity levels with electrical conductivities (EC) of 3 dS m− 1 (Munns et al. 2006; Khatun and Flowers 1995; Lutts et al. 1995). In the cultivated rice, O. sativa, salt exclusion from roots, retrieval from the shoots, tissue tolerance (achieved by efficient vacuolar Na+ compartmentation and cytosolic K+ retention), ROS detoxification, and osmotic adjustment are considered to be the main salinity tolerance mechanisms (Malagoli et al. 2008; Nemati et al. 2011; Kavitha et al. 2012; Kobayashi et al. 2017; Lakra et al. 2018; Oda et al. 2018; Liu et al. 2019). Attempts to increase salinity tolerance have mostly focused on traits found in salt-tolerant genotypes such as Pokkali, Nona Bokra and FL468, that have poor reproductive performance resulting in low yields. Salinity tolerance in these lines is mainly achieved by restricting Na+ accumulation in aboveground tissues and by maintaining higher K+ contents (Lutts et al. 1996b, a; Prusty et al. 2018; Gerona et al. 2019). However, development of salinity tolerant lines using these landraces has produced plants that also have poor reproductive traits (Solis et al. 2020), suggesting a negative trade-off between salt sensitivity and yield.
To date, only limited numbers of highly salt-tolerant wild Oryza have been reported. One is halophytic O. coarctata (KKLL genome). Previous findings have demonstrated that salt-tolerant wild rice species employ tolerance mechanisms different from O. sativa. This suggests that salinity tolerance in the genus Oryza may have been acquired in multiple, independent, and recent events similar to the trait’s acquisition in other genera (Bromham et al., 2020). In rice, breeding for salinity tolerance using wild relatives has mostly focused on O. rufipogon and O. nivara, two species that have the same AA genome as cultivated rice (Ganeshan et al. 2016; Wang et al. 2017c). The glycophytic species, O. officinalis (EE), O. latifolia (CCDD), and O. alta (CCDD) were found to have higher leaf Na+ accumulation during salinity treatment (Nakamura et al. 2002; Nishizawa et al. 2015; Prusty et al. 2018; Yichie et al. 2018). Halophytic O. coarctata has unique morphological and anatomical features such as thick and waxy leaves that contain salt glands and a differentiated rhizome that adapted to high salinity in coastal areas (Sengupta and Majumder 2010; Rajakani et al. 2021). However, ion homeostasis of photosynthetically active mesophyll tissue of O. coarctata in response to high salinity remains unclear.
Leaf Na+ exclusion and Na+ sequestration are two main mechanisms of plants in response to Na+ stress (Hanin et al. 2016). Exclusion is mainly mediated by plasma membrane Salt Overly Sensitive1 (SOS1) antiporters that exclude Na+ from mesophyll and retrieval into xylem parenchyma cells via High affinity K+ Transporter1 (HKT1) transporters (Hamamoto et al. 2015). The former is controlled by the SOS signalling pathway, which is consists of SOS3, SOS2 and SOS1 mediated by Ca2+ and ROS signals (Ji et al. 2013). Na+ sequestration mainly carried by Na+/H+ exchanger1 (NHX1) antiporters from the cytosol to the vacuole (Kronzucker and Britto 2011). Sequestered Na+ can be used as a ‘cheap osmoticum’ to increase the salt concentration in vacuoles and thereby enhance water retention in cell when the external concentration of Na+ is high. Moreover, transporters involved in K+ retention are also important for maintaining high cytosolic K+/Na+ ratios (Shabala and Cuin 2008), such as HAK transporters and KEA transporters (Rodríguez-Rosales et al. 2008; Bassil et al. 2011; Shen et al. 2015; Tsujii et al. 2019). The tonoplast and plasma membrane-based proton pumps, V-PPase, V-ATPase and H+-ATPase, are also important for providing the H+ driving force for Na+ transport (Queirós et al. 2009; Zhang et al. 1999). However, the functions of these two salt tolerance mechanisms are not fully explored in wild rice species.
O. coarctata is found in coastal environments that have daily fluctuations in EC between 20 and 40 dS m− 1. O. coarctata is the only Oryza species with a well-developed, putative, Kranz-like anatomy and has enlarged bundle sheath cells associated with a deep leaf furrow on the adaxial side of the leaf and a pair of longitudinally oriented vascular veins on each leaf ridge (Chatterjee et al. 2016; Rajakani et al. 2019). In C4 plants, enlarged bundle sheaths, reduced mesophyll cell/vascular bundle sheath cell size ratios, and a reduced number of mesophyll cells between vascular system in leaves are fundamental structures for C4 photosynthesis (Hatch 1987). These features in O. coarctata may compensate for the increased distance between the upper mesophyll cells and the main vein due to increased leaf thickness and may contribute to the adaptation to extreme, saline environments. The incorporation of C4 photosynthesis into new rice cultivars is suggested as a potential innovative technology towards another Green Revolution to meet future food demand (von Caemmerer et al. 2012). However, the development of C4 rice has mostly relied on the introduction of genes from C4 grasses (e.g., maize and sorghum) as no C4 Oryza species has been discovered so far (Wang et al. 2016c). A C4 wild Oryza species would be ideal to study evolution and function Kranz anatomy, and C4 photosynthesis and would facilitate the development of C4 cultivated rice.
In this study, we evaluated the salinity tolerance of six wild Oryza species, one salt-tolerant landrace and a salt-sensitive cultivar of cultivated rice in the greenhouse. We hypothesized that O. coarctata is a C3-C4 intermediate grass that has salt tolerance linked to the evolution of Kranz leaf anatomy and photosynthesis. Our measurements focused on the response to salinity of typical tolerance indicators (e.g., biomass, Na+/K+ ratio), photosynthesis, ion transport [as assessed by microelectrode ion flux estimation (MIFE)], confocal imaging of reactive oxygen species (ROS) and Na+ concentrations in mesophyll cells, and expression of genes associated with ion transporters and C4-related proteins. Our data suggest that three wild species, O. latifolia, O. officinalis, and O. coarctata, are all highly tolerant of salinity stress; however, this was achieved by employing different mechanisms. All these species accumulated significant amounts of Na+ in the shoot. However, Na+ accumulation in mesophyll cells was only observed in O. coarctata, while O. officinalis and O. latifolia may allocate Na+ to other leaf/shoot tissues. It is concluded that the above traits may be used as a potential target for breeding salt-tolerant rice cultivars.
Results
Although salinity stress had a significant, negative effect on the performance of all plants, a large variation in stress tolerance was observed amongst the various rice lines. The relative values of the growth and physiological parameters of these lines were ranked using the homogenous groups assigned by DMRTs (Supplementary Table S2). According to the ranking, overall performance under saline conditions was (from poorest to greatest) as follows: O. longiglumis < IR64 < O. australiensis < O. rufipogon < O. latifolia = O. officinalis < Pokkali < O. coarctata.
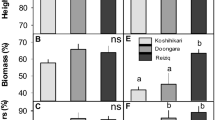
Significant reductions in biomass due to salinity stress were found in O. longiglumis, IR64, O. australiensis, and O. rufipogon (P < 0.05; salinity-sensitive lines) (Supplementary Figs. 1) but not for O. latifolia, Pokkali, and O. officinalis and O. coarctata (salt-tolerant lines); O. coarctata even showed a small (although non-significant) increase in biomass under salinity treatment. Tiller numbers and plant heights were not significantly affected by salinity treatment in any of the species (Supplementary Figs. S1). In terms of photosynthetic properties, total chlorophyll, net CO2 assimilation rates (A), stomata conductance (gs), and transpiration rates (E) of salinity stressed plants were significantly (ANOVA, treatment effect, p < 0.001) reduced compared to those in the control plants. Significant reductions in the total chlorophyll contents, CO2 assimilation rates (A), stomata conductance (gs), and transpiration rates (E) were found in sensitive species- O. longiglumis (except gs and E, P > 0.05), IR64, and O. rufipogon (Figs. 1). Compared to the other rice species, O. coarctata showed unique photosynthetic parameters such as significantly higher A and water use efficiency (WUE) and lower intercellular CO2 concentrations (Ci); these parameters were not affected by the salinity treatment.
Effects of 100 mM NaCl treatment on leaf gas exchange parameters of wild and the cultivated rice after six weeks of salinity stress. (A-C) net CO2 assimilation rate, A; (D-F) stomata conductance, gs; and (G-I) transpiration rate, E. For each species, left bar (red) represents the sensitive species group and right bar (green) represents the tolerant species group. * and ** indicates significant difference found between sensitive and tolerant groups at P < 0.05 and P < 0.01 respectively, using independent sample t-test. The number and dashed line indicate the mean value of each group. The data are means and the error bars indicate the standard errors
Ion transport in leaf mesophyll differ dramatically between O. coarctata and other Oryza species
Steady-state K+ fluxes from leaf mesophyll were positive (net K+ uptake) in salt-sensitive O. australiensis and O. longiglumis and both cultivated O. sativa genotypes (Fig. 2A). The other species showed a small, net K+ effluxes of 0 to − 100 nmol m− 2 s− 1 except for O. coarctata that had much higher net, initial, K+ efflux (−328 nmol m− 2 s− 1). When plants were grown under saline conditions, steady-state mesophyll K+ fluxes were drastically shifted towards negative values (net K+ loss; Fig. 2B) by 200 to 300 nmol m− 2 s− 1 (Fig. 2C). O. coarctata was an exception and showed no statistically significant difference in mesophyll K+ fluxes between control- and salt-grown plants. When O. coarctata was omitted from the analysis, a strong positive (r2 = 0.8) correlation between salinity-induced shift in mesophyll K+ fluxes and relative plant growth under saline conditions was found (Fig. 2C).
Linking salinity tolerance of wild and cultivated rice to net K+ ion fluxes of mesophyll cells (A) after 42 days of 100 mM NaCl treatment. The shift in K+ flux was the flux difference between flux from salinity-stressed and control samples (B). Pearson analysis of shift in K+ flux with relative biomass (C). Data are mean values and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
Net Na+ fluxes were around zero in control-grown plants and slightly negative under saline conditions (Fig. 3A). Salinity-induced shifted in net Na+ fluxes were about −150 nmol m− 2 s− 1 (Fig. 3B) and did not correlate with relative plant performance (r2 = 0.10; not significant; Fig. 3C).
Linking salinity tolerance of wild and cultivated rice to net Na+ ion fluxes of mesophyll cells. Salinity treatment was applied at 100 mM NaCl for 42 days. (A). Shift in K+ flux was the flux difference between flux from salinity stressed and control samples (B). Pearson analysis of shift in Na+ flux with relative biomass (C). Data are mean values and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
Steady-state mesophyll Cl− fluxes ranged between −50 and −200 nmol m− 2 s− 1 (net efflux) in control and increased by 2 to 3-fold under saline conditions (Fig. 4A). Salinity-induced changes in Cl− fluxes were strongest in the salt-sensitive cultivars (Fig. 4B) and correlated positively with plant performance (r2 = 0.59; Fig. 4C). Again, O. coarctata was an exception from this finding.
Linking salinity tolerance of wild and cultivated rice to net Cl− ion fluxes of mesophyll cells. Salinity treatment was applied at 100 mM NaCl for 42 days. (A) Shift in Cl− flux was the flux difference between flux from salinity stressed and control samples. (B) Pearson analysis of shift in Na+ flux with relative biomass. (C) Data are means and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
Salt-tolerant Oryza species had higher ROS production and lower Na+ accumulation in leaf and mesophyll cells
In response to salinity stress, all species had a significant reductions in leaf K+ contents and increases in leaf Na+ contents (Figs. 5, ANOVA, treatment effect, p < 0.001). The sensitive species had the highest reduction in shoot K+ content and increase in shoot Na+ (ANOVA, treatment*species effect, p < 0.001). Few salt-tolerant wild rice (mostly species with C genome) have been reported with higher Na+ accumulation in shoots compared to the salt-tolerant O. sativa lines. This was also observed with our leaf Na+ contents. Na+ contents (Figs. 5D–F) and Na+/K+ ratios (Figs. 5G–I) in the salt-tolerant wild species (O. latifolia, O. officinalis, and O. coarctata) were all significantly higher than the salt-tolerant cultivated genotype Pokkali. Leaf Na+/K+ ratios in the salinity-sensitive O. sativa lines were significantly increased after prolonged salinity stress. Although there were small increases in this ratio for the tolerant lines, the increase was only significant (t-test, P < 0.05) for O. latifolia (Figs. 5G-H). The leaf Na+ (negative; r2 = 0.74, P < 0.05), K+ (positive; r2 = 0.80; P < 0.01) and Na+/K+ ratios (negative; r2 = 0.74, P < 0.05) were strongly correlated with relative biomass (Supplementary Table S3).
Leaf ion contents in six wild and two cultivated rice lines after 6 weeks of 100 mM salinity treatment. (A-C) leaf K+ contents, (D-F) leaf Na+ contents and (G-I) leaf Na+/K+ ratios. Data are mean values and the error bars indicate the standard errors. * and ** indicates significant difference found between sensitive and tolerant groups at P < 0.05 and P < 0.01, respectively, using independent sample t-test. The number and dashed lines indicate the mean values of each group. The data are means and the error bars indicate the standard errors
After six weeks of salinity stress, leaf mesophyll cells accumulated significant amounts of ROS (Figs. 6A–D). The ROS contents in leaf mesophyll cells of most species/cultivars ranged between 13 and 19 (arbitrary) units in control-grown plants with the highest ROS accumulation (26 units) found in IR64 and the lowest (10 units) in O. rufipogon (Fig. 6A). Surprisingly, the salinity tolerant species showed higher ROS accumulation in mesophyll after the salinity stress (Fig. 6B). The analysis indicated that ROS accumulation was significantly correlated to the following salinity tolerance indicators: biomass (positive, r2 = 0.66, P < 0.05), Na+/K+ ratio (negative, r2 = 0.75, P < 0.05), mesophyll Na+ fluorescence (negative, r2 = 0.56, P < 0.05) and net Cl− flux (positive, r2 = 0.79, P < 0.01). O. coarctata produced higher ROS concentrations in mesophyll cells and maintained the largest relative biomass in response to salinity treatment (Fig. 6B).
Linking salinity tolerance of wild and cultivated rice to accumulation of reactive oxygen species (ROS) and Na+ in leaf mesophyll cells after 42 days of 100 mM NaCl treatment: (A & E) ROS & Na+ fluorescence in the mesophyll of control plants; (B & F) relative ROS and Na+ fluorescence in the mesophyll of salinity-stressed and control plants; (C & G) Pearson correlation of relative ROS production and Na+ fluorescence and relative biomass; (D & H) representative image of ROS and Na+ in mesophyll. The scale bars = 5 μm. Data are means and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
To further confirm whether the Na+ was stored in the mesophyll cells of salt-tolerant wild rice species, Na+ accumulation in mesophyll cells was evaluated using confocal imaging. The imaging analysis showed a similar trend to that found in leaf Na+ contents with mesophyll Na+ fluorescence decreasing along with the increase of salt tolerance in those rice species (Figs. 6E–H). The mesophyll Na+ fluorescence was moderately correlated to the leaf Na+ content (positive, r2 = 0.65, P < 0.05) (Supplementary Table S3) and highly correlated to biomass (negative, r2 = 0.81, P < 0.01) (Fig. 6G). However, among the tolerant group, only O. coarctata had significantly higher mesophyll Na+ accumulation. No difference was found between O. latifolia, O. officinalis, and Pokkali.
Salinity stress-induced expression of ion transporter genes reveals stronger ion homeostasis ability in salinity tolerant Oryza species
We then evaluated the expression of ten salinity tolerance-related genes of two tolerant wild species (O. latifolia, O. coarctata), and salt-tolerant (Pokkali) and salt-sensitive (IR64) lines of cultivated rice. sativa. The selected genes included Na+ transporters (NHX1, SOS1, HKT1;4), K+ transporters (high affinity potassium transporters [HAK1], HAK5), proton pumps (plasma membrane H+-ATPase [AHA1], V-type H+ATPase subunit C [VHA-C], and vacuolar PPase [VPPase]), and Ca2+ signaling proteins (SOS2, SOS3). Two-way ANOVA sindicated a significant difference of expression of each gene between species in response to the prolonged salinity treatment (Two-way ANOVA, main effects and species×treatment effects all < 0.001). Expression of the SOS signaling genes- SOS2and SOS3, were upregulated in all species except SOS3 expression in salt-sensitive IR64 (Figs. 7A-B). Relative to the control, Na+ and K+ transporter and proton pump genes were mostly upregulated in the salinity-tolerant wild rice species, O. coarctata, O. latifolia, and the cultivated rice, Pokkali (Figs. 7C-E). OlSOS1 was the only transporter gene downregulated in the salt-tolerant species. The salt-sensitive IR64 had the highest numbers of genes (OsNHX1, OsHKT1;4 & OsVPPase) downregulated in response to prolonged salinity treatment (Fig. 7K).
Expression of salinity tolerance-related genes in the leaf of wild and cultivated rice after 42 days of 100 mM NaCl treatment. (A) SOS2, (B) SOS3, (C) SOS1, (D) NHX1, (E) HKT1;4, (F) AHA1, (G) VHA-C, (H) VPPase, (I) HAK1 and (J) HAK5. (K) Fold change of genes after salinity treatment is relative to the control. Data are mean of 3 technical replicates (each containing at least 3 technical replicates) and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
Expression of the vacuolar Na+ compartmentation-related transporter, NHX1, was significantly higher in both wild salt-tolerant species (Fig. 7K). On the other hand, the Na+ exclusion-related transporter, SOS1, was significantly higher in salt-tolerant O. sativa. Correspondingly, the expression of the tonoplast proto pumps, VHA-C and VPPase (only O. coarctata significantly upregulated), was also higher in the wild tolerant species. This finding supported the importance of Na+ compartmentation in both wild species, which had shown higher leaf Na+ content and mesophyll Na+ compared to the salt-tolerant cultivated rice Pokkali. Na+ accumulation in O. coarctata’s mesophyll was further evaluated in response to direct 50 mM and 100 mM NaCl treatments on exposed mesophyll tissue with the epidermal layer removed for 2 h and in response to 1 week of 100 mM NaCl hydroponic solution (Supplementary Fig. 2). Consistently, the results showed significant Na+ accumulation in the vacuole based on the area and shape of the Na+ fluorescence in the cell. The Na+ fluorescence in the mesophyll cell chloroplasts in response to 1 week of 100 mM NaCl hydroponic solution was surprisingly low compared to the control. The increase in vacuole Na+ fluorescence of mesophyll cells with 100 mM NaCl treatment was 5 times higher than mesophyll with 50 mM NaCl treatment. The result suggested that Na+ compartmentation is the main strategy of salinity defense in mesophyll cells.
K+ inward transporters, HAK1 & HAK5, were overall upregulated in all species. The expressions were significantly higher in O. coarctata and Pokkali. The fold-change of HAK1 expression was higher than the fold-change of HAK5 in all tolerant species. The fold-change HAK1 was correlated to the leaf K+ content (Figs. 5A-C) and biomass (Supplementary Fig. 1), which is highest in O.coarctata, followed by Pokkali, O. latifolia then IR64.
O. coarctata has a proto Kranz-like anatomy, higher photosynthetic rate, and highly expressed C4 photosynthesis-related genes
O. coarctata showed significant photosynthetic capacity in the control and salinity stress in the greenhouse trial (Fig. 1). To evaluate whether O. coarctata is a C4 or a C3-C4 intermediate species, we first constructed a phylogenic tree of a C4-related gene, phosphoenolpyruvate carboxylase (PEPC) , for a group of C3 and C4 plants. The phylogeny showed that this gene in O. coarctata is phylogenetically closer to those in C4 grasses such as Setaria, maize, and Sorghum compared to other C3 species in the Oryza genus (Fig. 8A).
Phylogenetic, morphological and photosynthetic evidence of O. coarctata being a C3-C4 intermediate. (A) Maximum likelihood tree showing the phylogenetic relationship of PEPC, a C4 key enzyme, in C3 (red) and C4 (blue) species. The percentages of trees from 1000 bootstrap replications in which the associated taxa clustered together are shown next to the branches. (B-E) Leaf gas exchange characteristics of O. coarctata compared to other Oryza species used in this study. (F) Photosynthesis dependence on intracellular CO2 curve fitting a C4 type photosynthesis model species. (G) Leaf ultrastructure showing the presence of plasmodesmata connecting mesophylle and bundle sheath cells. (H) Shoot and leaf morphology of O. sativa and O. coarctata, salt secretion on leaf surface of O. coarctata, and Na+ localization from leaf cross section of O. coarctata. Epi- epidermis, MC- mesophyll cells, BSC- bundle sheath cells, X- xylem, P- phloem, BC-bulliform cells, ST- sclerenchymatous thickening and SG- salt glands. (I) Na+ accumulation and chlorophyll fluorescence in a leaf in response to high salinity stress in O. coarctata.. Curve was fitted with a 3-parameters exponential rise to maximum [Net CO2 assimilation = −1.191 + 4.628 × (1-exp(− 4.533 × CO2 concentration) with a calculated CO2
Overall, O. coarctata had significantly (P < 0.01) higher total chlorophyll contents, CO2 assimilation rates (A), WUEs, and significantly lower intracellular CO2 concentrations (Ci) compared to the six other Oryza species (Figs. 8B–E). The A/Ci curves indicate that O. coarctata was highly responsive to increasing CO2 concentration with CO2 compensation points at 64.5 μmol CO2 m− 2 s− 1 (3-parameter exponential curve) (Fig. 8F) and 50.4 μmol CO2 m− 2 s− 1 with a linear fitting of the first 6 points. The photosynthetic characteristics showed the O. coarctata may not be a C4 rice.
Leaf anatomy was investigated to confirm if O. coarctata possesses proto-Kranz or Kranz anatomy. Under transmission electron microscopy (TEM), plasmodesmata connections between mesophyll and vascular bundle sheath cells were observed (Fig. 8G). The cross-sections of O. coarctata leaves showed larger vascular bundle sheath cells relative to mesophyll cells, higher numbers of chloroplasts in the vascular bundles, and lower numbers of mesophyll cells between veins. The leaves have distinct adaxial surfaces that have a high density of ridges and furrows (Fig. 8H). Each ridge contains a vascular bundle system, and salt glands for salt exclusion are found on the surface of the furrows. The number of mesophyll cells between each adjacent vascular vein is significantly lower in O. coarctata than reported in O. sativa (Chatterjee et al. 2016). Confocal imaging analysis showed a strong Na+ signal in the epidermal layer, salt glands, leaf veins, and mesophyll cells (Fig. 8I).
Further, we compared the expression of five C4 photosynthesis-related genes in O. coarctata, IR64, and Pokkali in the control and salinity treatments (Fig. 9). The results indicate that expression of these genes was significantly higher (up to 800-fold for ribulose-bisphosphate carboxylase [RBCL]) in both control and salinity stressed O. coarctata as compared to those in O. sativa. PEPC was the only gene upregulated in O. coarctata after the salinity stress while pyruvate phosphate dikinase (PPDK), RBCL, and ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (RBCS) were significantly downregulated after salinity stress.
Molecular evidence for O. coarctata being a C3-C4 intermediate. Effect of salinity on the following C4 photosynthesis-related genes in O. coarctata, Pokkali and IR64: (A), NADP-dependent malic enzyme (NADPME), (B) PEPC, (C) PPDK, (D) RBCL, and (E) RBCS. Data are means and the error bars indicate the standard errors. Different lowercase letters indicate significant differences at P < 0.05 for each Duncan group
Discussion
More than 10,000 years ago, ancient humans began to consume grains of O. rufipogon that grew in the marshes throughout Asia (Sweeney and McCouch 2007). Continuous domestication for desirable traits has slowly caused O. rufipogon to evolve into O. sativa, a staple crop for billions of people worldwide (Kovach et al. 2007; Sweeney and McCouch 2007). However, after almost 8000 years of cultivation, O. sativa is estimated to only have conserved 20% of the genetic diversity of wild Oryza (Zong et al. 2007; Palmgren et al. 2015). This narrow genetic diversity is the major constraint on breeding salinity-tolerant rice lines (Waziri et al. 2016; Chen et al. 2020). Thus, investigating gene expression, ion homeostasis, Na+ transport between the vascular system and mesophyll tissue in salt-tolerant, wild rice species may enable the identification of new mechanisms that contribute to salt tolerance.
Salt-tolerant wild rice exhibits distinct leaf tissue tolerance to Na+ and K+ transport compared to O. sativa
Salt-tolerant O. sativa generally employs Na+ exclusion to avoid high Na+ accumulation in aboveground tissues. This trait likely results from artificial selection for Na+ exclusion and may account for the poor tissue tolerance to Na+ in this rice species (Lutts et al. 1996b, a; Prusty et al. 2018; Gerona et al. 2019). In this study, we evaluated the performance of six wild Oryza species with two lines of cultivated rice after prolonged stress at a moderate salinity level of 10 dS m− 1. We found O. coarctata, O. officinalis, and O. latifolia were highly tolerant of salinity stress. However, only the halophytic O. coarctata showed high Na+ accumulation in mesophyll cells when grown under salt stress among salt-tolerant species (Figs. 6), suggesting that the other salt-tolerant wild rice species, O. officinalis, and O. latifolia, avoid Na+ accumulation in mesophyll cells.
SOS2 and SOS3 were first identified as key components of the salt overly sensitive pathway to exclude Na+ by activating SOS1 (Ji et al. 2013). Other than SOS1, the Ca2+-activated SOS2/SOS3 complex was also reported to activate/upregulate tonoplast Ca2+ exchangers (CAXs), the internal membrane Na+ antiporters, NHX1, NHX2 and NHX4, and the plasma membrane and tonoplast proton pumps,- AHA, V-ATPase, and VPPase (Huertas et al. 2012; Che-Othman et al. 2017; Yang et al. 2019). Expression of these transporter genes (SOS1, NHX1, AHA, VHA-C, and VPPase) was upregulated in the halophytic O. coarctata and mostly upregulated in salt-tolerant Pokkali and O. latifolia. SOS1, HKT1;4, and NHX1 are important Na+ transporters responsible for Na+ exclusion, compartmentation, and retrieval (Wu et al. 2018). The expression of SOS1 in O. coarctata and O. latifolia was consistently low and was not affected by six weeks of salt treatment (Figs. 7C, K). Leaf Na+ contents in both species were relatively higher than Pokkali (Figs. 5D-F), suggesting that Na+ exclusion may not be a main defense mechanism for salt tolerance in mesophyll cells of these wild rice species. In addition, O. coarctata and O. latifolia had significantly higher NHX1 expression after salinity stress compared to the control and the two cultivated rice lines (Figs. 7D, K). O. coarctata’s mesophyll showed a preference to compartmentalise Na+ in the vacuole and regulate apoplastic Na+ after the salinity treatment (Supplementary Figs. 2), indicating a key role for NHX1 and HKT1;4 in the leaf salt tolerance of O. coarctata. O. latifolia is a member of O. officinalis complex, which is well known for its resistance to various biotic and abiotic stresses (Shenton et al. 2020). In this study, mesophyll Na+ in O. latifolia was surprisingly low. Given the high leaf Na+ content found in O. latifolia, higher relative mesophyll Na+ fluorescence was expected compared to Pokkali. Na+ homeostasis strategies in leaves of salt-tolerant O. latifolia require further investigation.
We previously reported a significant, positive correlation between net K+ flux and salinity tolerance in O. sativa (Yong et al. 2020). Here, except for O. coarctata, we extended this finding to wild Oryza species (Figs. 2). Our results show a significant, strong, and positive correlation between net K+ flux and biomass (Fig. 2E). In addition, net K+ flux was also significantly and positively correlated with mesophyll Na+, leaf Na+ contents, and leaf Na+/K+ ratios (Supplementary Table S3). K+ is the macronutrient responsible for the coordination of more than 50 enzymes in plants (Shabala and Cuin 2008). Stress-induced K+ leakage from the tissue was reported in plants due to salinity (Liu et al. 2017a), hypoxia (Wang et al. 2017a), drought (Mak et al. 2014), and high light (Babla et al. 2020). This enables the operation of the GORK channels as a master switch of the cell metabolism, thus adjusting intracellular K+ homeostasis to altered environmental conditions (Adem et al. 2020). Also, the K+ efflux may be conducted by non-selective cation channels (Zepeda-Jazo et al. 2008). These ion channels may be key factors involved in K+ leakage in the sensitive wild rice species in our study. Alternatively, K+ can be actively transported into cells through HAK transporters, such as some key members (OsHAK1, OsHAK5, and OsHAK21) of this gene family in rice (Mangano et al. 2008; Chen et al. 2015; Shen et al. 2015; Nieves-Cordones et al. 2017; Feng et al. 2020). HAK1 and HAK5 were upregulated in both O. sativa and wild species. The HAK1 expression matched the results of relative biomass and K+ content of four species. HAK5 expression was also highest in the most tolerant species O. coarctata and Pokkali but lowest in O. latifolia.
O. coarctata is an outlier of salt tolerance among Oryza species
The gas exchange required for photosynthesis cannot be maintained without balancing ions, organic compounds, and water contents being maintained within species-specific tissue tolerances under salinity stress (Shabala and Cuin 2008; Mishra et al. 2020; Munns et al. 2020b). In cultivated rice, photosynthetic activities are negatively correlated with the magnitude and duration of salinity stress (Yeo et al. 1985; Radanielson et al. 2018). In this study, O. coarctata showed a significantly greater relative biomass after salinity stress as compared to the other species (Figs. S1). In comparison to the other Oryza species, O. coarctata exhibited the highest A, WUE, and total chlorophyll contents in both the control and the salt-treated plants; none of these parameters was significantly affected by salinity stress (Figs. 1 and Supplementary Figs. S1), indicating remarkable adaption of photosynthetic traits to the saline condition in this species. In addition, across the six key physiological parameters that were significantly correlated with relative biomass (Figs. 2 and 4), O. coarctata was a consistent outlier compared to the studied glycophytic species, and its salinity tolerance did not show links with net K+ and Cl− efflux nor to ROS production and Na+ fluorescence. Thus, salinity tolerance of O. coarctata must be determined by other mechanisms.
Confocal imaging of Na+ of O. coarctata revealed Na+ fluorescence in the salt glands, the vascular tissues, and in epidermal tissues is higher than that in mesophyll tissues (Fig. 6F). Therefore, most of the Na+ may be transferred through the plant to the salt glands without passing through mesophyll cells, the major cell type in which photosynthesis occurs. This was validated by direct treatment of the mesophyll tissue of O. coarctata with the epidermis removed with 50 mM and 100 mM NaCl. The directly stressed mesophyll showed obvious accumulation of Na+ around or within the chloroplasts (Supplementary Figs. S2). This was not found in leaf samples with intact epidermis from plants subjected to one month of salinity treatment in hydroponic solution. Thus, O. coarctata increases Na+ accumulation around the mesophyll tissue thereby maintaining photosynthesis (Figs. 1); this has also been found in other plant species such as chickpea (Kotula et al. 2019; Kotula et al. 2020).
Linking high photosynthetic capacity to high salt tolerance in O. coarctata
There are increasing numbers of reports on the potential for the genetic engineering of C4 photosynthesis into C3 crops such as rice to improve their productivity and stress tolerance (Wang et al. 2017b; Ermakova et al. 2020). Evolutionarily, the transition from C3 to C4 photosynthesis was closely related to ambient CO2 concentration and temperature (Sage 2004; Edwards et al. 2010), which may have been a stepwise conversion of cellular biochemistry and a few key anatomical structures (Wang et al., 2017a). Three C3-C4 intermediate types, proto-Kranz, C2-C2+ photosynthesis, and C4-like photosynthesis, have been proposed based on the mesophyll cell numbers between veins, the size of bundle sheath cells, the amount and coordination of chloroplasts and mitochondria in bundle sheath cells, and Rubisco and PEPC regulation (Sage et al. 2014). In grasses, no C4 plant is reported so far in the BEP (Bambusoideae, Ehrhartoideae, Pooideae) clade; the genus, Oryza, belongs to this clade (Grass Phylogeny Working Group II, 2012). However, many members of BEP clade are reported as having C4-like leaf structure, and O. coarctata is currently classified in this group (Christin et al. 2013). In addition, C4 plants usually have thin leaves so that the mesophyll and bundle sheath cells can coordinate closely to assure efficiently C4 photosynthesis (Ghannoum et al. 2005). O. coarctata has developed a minor vein on top of the major vein that shortens the distance between mesophyll cells on the adaxial side and vascular system, but this feature is not found in other Oryza species (Chatterjee et al. 2016). The A/Ci curve and CO2 compensation point indicate that the photosynthetic activities of O. coarctata are responsive to fluctuating CO2 concentration without having true C4 photosynthesis (Ku et al. 1983; Ueno et al. 2007; Schlüter et al. 2017; Monson and Jaeger 1991; Vogan and Sage 2012; Yorimitsu et al. 2019).
In C4 photosynthesis, NADP-ME, PEPC, and PPDK are responsible for oxaloacetate and phosphoenolpyruvate conversion in mesophyll cells and CO2 release in bundle sheath cells during the C4 cycle (Hibberd and Covshoff 2010). In C4 plants, Rubisco is responsible for the conversion of carbon into organic acid, and the two subunits of this enzyme, RbcL, and RbcS, are localized in chloroplasts and the nucleus, respectively (Berry et al. 2016). In this study, the expression of all C4 photosynthesis-related genes (NADPME, PEPC, PPDK, RbcL, and RbcS) was significantly higher in O. coarctata compared to IR64 and Pokkali. Although O. coarctata does not possess true C4 photosynthesis, higher gene expression, photosynthetic rates, and WUE as well as the unique morphology will be useful to understand the salinity tolerance mechanism in this halophytic wild rice.
Methods and materials
Greenhouse trial
Six wild rice species (O. longiglumis Jansen, O. australiensis Domin, O. rufipogon Griff., O. latifolia Desv., O. officinalis Wall. ex Watt and O. coarctata Roxb.), the O. sativa subspecies indica cultivar, IR64, and the landrace, Pokkali, were grown in a greenhouse at Western Sydney University, Hawkesbury Campus (33.62 °S, 150.75 °E). With the exception of O. coarctata, plants of the other species were raised from seed in sand for 14 days and then transferred to 9 L buckets filled with a loamy sandy soil. The remaining wild rice, O. coarctata, was prepared through vegetative propagation by separating newly emerged tillers with two leaves from parent plants. The young tillers were placed in Yoshida’s medium (Yoshida et al. 1971) for 14 days and transferred to the 9 L buckets containing the loamy sandy soil. The times of sowing and vegetative propagation of the wild and cultivated rice species were synchronized based on previous work to ensure that the plants were in the same growth stage during the measurements. The greenhouse was maintained with controlled day/night temperatures of 29 °C/24 °C and supplemental HPS lights with a 14/10 h day/night cycle. The relative humidity was maintained at 70–80%.
For each treatment and wild rice species/cultivated rice line, four replicates (4 buckets with 2 plants per bucket) were used for the different measurements. Water levels were maintained up to a height of 30 mm above the soil by daily watering with tap water. The initial EC of the soil was 0.25 dS m− 1. Incremental salinity treatments using NaCl commenced ~ 2 months after sowing with increments of 2 dS m− 1 per day until a final salinity of 10 dS m− 1 was reached. The final salinity treatment was maintained for six weeks before the collection of leaf samples for assessments. The EC values were monitored and maintained at the desired level throughout the growing season. After 42 days of salinity stress, plant height, tiller number and shoot biomass were measured. The 1st and 2nd fully expanded leaves were used for measurement of gas exchange, ion flux of mesophyll tissue, imaging of Na+ and ROS in mesophyll cells, chlorophyll content, nutrient analysis, and real-time PCR.
Hydroponic trials
O. coarctata seedlings were prepared by vegetative propagation by separating freshly emerged tillers that had produced two leaves from their parent plants. The young tillers were placed in conical flasks containing a hydroponic solution consisting of Yoshida’s medium. Each flask was covered with aluminum foil to minimize exposure of the roots to sunlight. The solution was renewed every 3 days. After 2 weeks of growth, an incremental salinity stress treatment was applied through the subsequent addition to the medium of 25, 50, and 100 mM NaCl at 3-day intervals. After 1 month of salinity stress, Na+ localization in leaf tissue was measured using confocal imaging. The epidermal layer of the leaves was removed by using scalpel. For the 2 h salinity treatment, the exposed mesophyll tissue was submerged in the buffer solution with 50 or 100 mM NaCl.
Gas exchange measurement
In the greenhouse trial, gas exchange was measured on the first, fully-expanded leaves using a LI-6400XT infrared gas analyser (LI-COR, Lincoln, USA) following Liu et al. (2017b); three leaves were randomly selected for measurement. In each measurement, the leaf was held in the chamber for 3–5 min before the reading was taken. The chamber conditions were as follows: flow rate, 500 mL min− 1, reference CO2 400 μmol m− 2 s− 1; block temperature, 30 °C; light fluorescence, 1000 μmol m− 2 s− 1. The chamber reference humidity was maintained at ~ 60%.
For A/Ci curve measurements, the chamber conditions were set as follows: flow rate, 500 mL min− 1, block temperature, 30 °C; light fluorescence, 1000 μmol m− 2 s− 1, and reference CO2, 425 μmol m− 2 s− 1. Prior to the measurement, the selected leaf was held in the chamber for 20 min. Net CO2 assimilation rate was recorded at each of the following incremental ambient CO2 concentrations: 425, 600, 800, 1000, 1250, 1500, 1750 and 2000 μmol m− 2 s− 1. The readings at lower ambient CO2 concentrations of 350, 250, 150, 100 and 50 μmol m− 2 s− 1 were taken after 20 min adaptation at 425 μmol m− 2 s− 1 following the 2000 μmol m− 2 s− 1 treatment. The CO2 compensation point was measured from an A/Ci curve according to Laisk (1977).
Chlorophyll content
Chlorophyll contents were determined as per Lichtenthaler and Buschmann (2001) with slight modifications. Ten milligrams of homogenised leaf samples were placed in Eppendorf tubes containing 1 mL 80% acetone and incubated in the dark overnight. Absorbance values were obtained at 470, 649, and 664 nm using a spectrophotometer.
Leaf K+ and Na+ contents
K+ and Na+ contents in leaves were measured using a flame photometer (Jenway PFP7, John Morris, Australia) using a modified method based on Chen et al. (2007). The 1st and 2nd fully expanded leaves of each plant were harvested and oven-dried for 2 days. The dried leaf samples were finely ground and mixed using a Retsch Mixer Miller 400. Each sample (~ 50 mg) was digested in 4 mL of concentrated HNO3 (69%) in a boiling water bath until the sample solution was clear. The solutions were then diluted with 100 mL MilliQ water before measurement.
Ion flux measurements
As leaf photochemistry is ultimately linked to ionic conditions in leaf mesophyll (Pottosin and Shabala 2016; Pan et al. 2020), steady-state net K+, Na+, Cl− and Ca2+ fluxes were measured from leaf mesophyll cells of 1st fully expanded leaves using MIFE. Microelectrode preparation and mesophyll isolation protocols were as per Shabala et al. (2012). Prior to the measurement, leaf samples (20 × 20 mm2) were clamped in Perspex measuring chambers and submerged with standard MIFE solution (0.5 mM KCl, 0.1 mM CaCl2) and allowed to adapt to the new ionic environment for at least 1 h. Steady-state ion fluxes were then measured for ~ 5 min from each sample; there were 4–6 replicates for each combination of species and treatment. Net ion fluxes were calculated using MIFEFLUX software based on the ion concentration gradient recorded between two positions (see Shabala et al. (1997) for a theory and details).
Confocal microscopy
The production of ROS and Na+ accumulation in mesophyll cells were measured using confocal microscopy according to Wang et al. (2016a). 5-(and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) was employed to monitor cellular oxidative stress, and CoroNa Green AM was employed to localize Na+ distribution in leaf mesophyll tissues. The epidermal layer of the 1st fully expanded leaf was removed by using scalpel prior to incubation in a buffer solution (10 mM KCl, 5 mM Ca2+-MES, pH 6.1) with 20 μM of CM-H2DCFDA or 50 μM CoroNa Green for 1 h in the dark. ROS and Na+ were measured using an upright laser scanning confocal microscope (Leica SP5, Germany) with 50× objective lens [laser power: 10%, excitation laser: 488 nm (20%), emission range: 505–550, filter: TP488/543/633]. Chloroplasts were localized by detecting auto-fluorescence of chlorophyll at an emission range between 680 and 720. ROS and Na+ fluorescence was then quantified on a cell-by-cell basis.
Quantitative real-time PCR
Transcripts of the following C4 pathway-related genes were quantified using total RNA extracted from the first fully expanded leaf samples collected at 42 days after sowing: NADPME, (PEPC, PPDK, RBCL and RBCS and salinity tolerance related genes NHX1, VHA-C, VPPase, HKT1;4, SOS1, SOS2, SOS3, -ATPaseAHA1, HAK1and HAK5 (Liu et al. 2014; Wang et al. 2016b). Reverse transcription was performed as per the manufacturer’s instructions (Bioline, Australia). Quantitative real-time PCR (qPCR) was performed using a Quantinova SYBR Green Kit (QIAGEN, USA) in a Rotor-Gene 3000 quantitative PCR thermoscycler (QIAGEN, USA). Relative gene expression was calculated using the comparative threshold cycle (Ct) 2-∆∆Ct method (Livak et al. 2001); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and elongation factor 1-alpha (EF1A) were used as the internal reference genes. The experiments were conducted with three biological replicates and three technical replicates. The primer pairs are listed in Supplementary Table S1.
Bioinformatic analysis
Chloroplast genome sequences from Oryza and other plant species were retrieved from public databases and were used for phylogenetic analysis of the genetic variation in cultivated and wild rice; the chloroplast genomes of five eudicot species were used as the outgroup. The sequences of PEPC, a C4 key enzyme in C3 and C4 species used in this study, were retrieved from the Michigan State University Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu/index.shtml), NCBI Protein and Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and Ensembl Plants (http://plants.ensembl.org/index.html). These sequences were aligned using CLUSTAL W (http://www.clustal.org/clustal2/). The phylogenetic relationships among the sequences were inferred using maximum likelihood in MEGA X (Kumar et al. 2018). The percentages of trees from 1000 bootstrap replications in which the associated taxa clustered together are shown next to the branches.
Data analysis
Independent sample t-tests, ANOVA, Duncan’s multiple range tests (DMRTs), Pearson’s correlation was performed using IBM SPSS Statistics, Version 24 (IBM Corp. Release 2020). O. coarctata outperformed the other Oryza species in this study with respect to salinity tolerance resulting outlying data; therefore, it was excluded from the correlation analyses. The relative values of the parameters from the control and salinity treatments were calculated to evaluate the salinity sensitivity of the species/cultivars. The ranking was based on the homogenous groups identified by DMRT of the parameters listed in Supplemental Table S2. The ranking of the homogenous groups for each parameter started from 1 as the most sensitive group and increased based on the numbers of homogenous groups identified by DMRT analyses. Relative values that occurred in multiple groups were averaged (e.g., if the species was included in groups 1 and 2 it was then ranked as 1.5). The average score of all parameters was used for the final ranking.
Availability of data and materials
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
- CM-H2DCFDA:
-
5-(and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- NADPME:
-
NADP-dependent malic enzyme
- PEPC:
-
phosphoenolpyruvate carboxylase
- PPDK:
-
pyruvate phosphate dikinase
- RBCL:
-
ribulose-bisphosphate carboxylase
- RBCS:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- EF1A:
-
elongation factor 1-alpha
- HAK:
-
high affinity potassium transporter
- SOS:
-
salt overly sensitive
- NHX:
-
sodium/hydrogen exchanger
- VHA-C:
-
V-type H+ATPase subunit C
References
Adem GD, Chen G, Shabala L, Chen Z-H, Shabala S (2020) GORK channel: a master switch of plant metabolism? Trends Plant Sci 25(5):434–445. https://doi.org/10.1016/j.tplants.2019.12.012
Babla MH, Tissue DT, Cazzonelli CI, Chen Z-H (2020) Effect of high light on canopy-level photosynthesis and leaf mesophyll ion flux in tomato. Planta 252(5):80. https://doi.org/10.1007/s00425-020-03493-0
Bassil E, Tajima H, Liang Y-C, M-a O, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23(9):3482–3497. https://doi.org/10.1105/tpc.111.089581
Berry JO, Mure CM, Yerramsetty P (2016) Regulation of rubisco gene expression in C4 plants. Curr Opin Plant Biol 31:23–28. https://doi.org/10.1016/j.pbi.2016.03.004
Bromham L, Bennett TH (2014) Salt tolerance evolves more frequently in C4 grass lineages. J Evol Biol 27(3):653–659. https://doi.org/10.1111/jeb.12320
Bromham L, Hua X, Cardillo M (2020) Macroevolutionary and macroecological approaches to understanding the evolution of stress tolerance in plants. Plant Cell Environ 43(12):2832–2846. https://doi.org/10.1111/pce.13857
Caperta AD, Róis AS, Teixeira G, Garcia-Caparros P, Flowers TJ (2020) Secretory structures in plants: lessons from the Plumbaginaceae on their origin, evolution and roles in stress tolerance. Plant Cell Environ 43(12):2912–2931. https://doi.org/10.1111/pce.13825
Chatterjee J, Dionora J, Elmido-Mabilangan A, Wanchana S, Thakur V, Bandyopadhyay A, Brar DS, Quick WP (2016) The evolutionary basis of naturally diverse rice leaves anatomy. PLoS ONE 11(10):e0164532. https://doi.org/10.1371/journal.pone.0164532
Chen G, Hu Q, Luo L, Yang T, Zhang S, Hu Y, Yu L, Xu G (2015) Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ 38(12):2747–2765. https://doi.org/10.1111/pce.12585
Chen T, Zhu Y, Chen K, Shen C, Zhao X, Shabala S, Shabala L, Meinke H, Venkataraman G, Chen ZH (2020) Identification of new QTL for salt tolerance from rice variety Pokkali. J Agron Crop Sci 206(2):202–213. https://doi.org/10.1111/jac.12387
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58(15–16):4245–4255. https://doi.org/10.1093/jxb/erm284
Chen Z, Soltis DE (2020) Evolution of environmental stress responses in plants. Plant, Cell and Environment, pp 2827–2831. https://doi.org/10.1111/pce.13922
Che-Othman MH, Millar AH, Taylor NL (2017) Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant Cell Environ 40(12):2875–2905. https://doi.org/10.1111/pce.13034
Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci 110(4):1381–1386. https://doi.org/10.1073/pnas.1216777110
Edwards EJ, Osborne CP, Stromberg CA, Smith SA, Consortium CG (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328:587–591
Ermakova M, Danila FR, Furbank RT, von Caemmerer S (2020) On the road to C4 rice: advances and perspectives. Plant J 101(4):940–950. https://doi.org/10.1111/tpj.14562
Feng X, Liu W, Qiu CW, Zeng F, Wang Y, Zhang G, Chen ZH, Wu F (2020) HvAKT2 and HvHAK1 confer drought tolerance in barley through enhanced leaf mesophyll H+ homoeostasis. Plant Biotechnol J 18(8):1683–1696. https://doi.org/10.1111/pbi.13332
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37(7):604–612. https://doi.org/10.1071/FP09269
Ganeshan P, Jain A, Parmar B, Rao A, Sreenu K, Mishra P, Mesapogu S, Subrahmanyam D, Ram T, Sarla N (2016) Identification of salt-tolerant rice lines among interspecific BILs developed by crossing Oryza sativa x O. rufipogon and O. sativa x O. nivara. Aust J Crop Sci 10(2):220
Ge S, Sang T, Lu B-R, Hong D-Y (2001) Phylogeny of the genus Oryza as revealed by molecular approaches. Rice Genetics IV. World Scientific, pp 89–105 https://doi.org/10.1142/9789812814296_0006
Gerona MEB, Deocampo MP, Egdane JA, Ismail AM, Dionisio-Sese ML (2019) Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci 26(4):207–219. https://doi.org/10.1016/j.rsci.2019.05.001
Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S (2005) Faster rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol 137(2):638–650. https://doi.org/10.1104/pp.104.054759
Grieve BD, Duckett T, Collison M, Boyd L, West J, Yin H, Arvin F, Pearson S (2019) The challenges posed by global broadacre crops in delivering smart Agri-robotic solutions: a fundamental rethink is required. Glob Food Secur 23:116–124. https://doi.org/10.1016/j.gfs.2019.04.011
Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder JI, Uozumi N (2015) HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol 32:113–120. https://doi.org/10.1016/j.copbio.2014.11.025
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787. https://doi.org/10.3389/fpls.2016.01787
Hatch MD (1987) C4 photosynthesis: a unique elend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta (BBA) - Rev Bioenerg 895(2):81–106. https://doi.org/10.1016/S0304-4173(87)80009-5
Hibberd JM, Covshoff S (2010) The regulation of gene expression required for C4 photosynthesis. Annu Rev Plant Biol 61(1):181–207. https://doi.org/10.1146/annurev-arplant-042809-112238
Huertas R, Olias R, Eljakaoui Z, Gálvez FJ, Li J, De Morales PA, Belver A, Rodríguez-Rosales MP (2012) Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 35(8):1467–1482. https://doi.org/10.1111/j.1365-3040.2012.02504.x
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant 6(2):275–286. https://doi.org/10.1093/mp/sst017
Kavitha P, Miller A, Mathew M, Maathuis FJ (2012) Rice cultivars with differing salt tolerance contain similar cation channels in their root cells. J Exp Bot 63(8):3289–3296. https://doi.org/10.1093/jxb/ers052
Khatun S, Flowers T (1995) Effects of salinity on seed set in rice. Plant Cell Environ 18(1):61–67. https://doi.org/10.1111/j.1365-3040.1995.tb00544.x
Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T (2017) OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 91(4):657–670. https://doi.org/10.1111/tpj.13595
Kotula L, Clode PL, Jimenez JDLC, Colmer TD (2019) Salinity tolerance in chickpea is associated with the ability to ‘exclude’ Na from leaf mesophyll cells. J Exp Bot 70(18):4991–5002. https://doi.org/10.1093/jxb/erz241
Kotula L, Garcia Caparros P, Zörb C, Colmer TD, Flowers TJ (2020) Improving crop salt tolerance using transgenic approaches: an update and physiological analysis. Plant Cell Environ 43(12):2932–2956. https://doi.org/10.1111/pce.13865
Kovach MJ, Sweeney MT, McCouch SR (2007) New insights into the history of rice domestication. Trends Genet 23(11):578–587. https://doi.org/10.1016/j.tig.2007.08.012
Kronzucker HJ, Britto DT (2011) Sodium transport in plants: a critical review. New Phytol 189(1):54–81. https://doi.org/10.1111/j.1469-8137.2010.03540.x
Ku MS, Monson RK, Littlejohn RO, Nakamoto H, Fisher DB, Edwards GE (1983) Photosynthetic characteristics of C3-C4 intermediate Flaveria species: I. leaf anatomy, photosynthetic responses to O2 and CO2, and activities of key enzymes in the C3 and C4 pathways. Plant Physiol 71(4):944–948. https://doi.org/10.1104/pp.71.4.944
Kumar V, Khare T (2015) Individual and additive effects of Na+ and cl− ions on rice under salinity stress. Arch Agron Soil Sci 61(3):381–395. https://doi.org/10.1080/03650340.2014.936400
Laisk AK (1977) Kinetics of photosynthesis and photorespiration of C3 in plants. FAO
Lakra N, Kaur C, Anwar K, Singla-Pareek SL, Pareek A (2018) Proteomics of contrasting rice genotypes: identification of potential targets for raising crops for saline environment. Plant Cell Environ 41(5):947–969. https://doi.org/10.1111/pce.12946
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1(1):F4.3.1–F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Liu J, Shabala S, Shabala L, Zhou M, Meinke H, Venkataraman G, Chen Z, Zeng F, Zhao Q (2019) Tissue-specific regulation of Na+ and K+ transporters explains genotypic differences in salinity stress tolerance in rice. Front Plant Sci 10:1361. https://doi.org/10.3389/fpls.2019.01361
Liu X, Cai S, Wang G, Wang F, Dong F, Mak M, Holford P, Ji J, Salih A, Zhou M (2017a) Halophytic NHXs confer salt tolerance by altering cytosolic and vacuolar K+ and Na+ in Arabidopsis root cell. Plant Growth Regul 82(2):333–351. https://doi.org/10.1007/s10725-017-0262-7
Liu X, Fan Y, Mak M, Babla M, Holford P, Wang F, Chen G, Scott G, Wang G, Shabala S, Zhou M, Chen Z-H (2017b) QTLs for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genomics 18(1):9. https://doi.org/10.1186/s12864-016-3380-0
Liu X, Mak M, Babla M, Wang F, Chen G, Veljanoski F, Wang G, Shabala S, Zhou M, Chen Z-H (2014) Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front Plant Sci 5:634. https://doi.org/10.3389/fpls.2014.00634
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of Rice ( Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78(3):389–398. https://doi.org/10.1006/anbo.1996.0134
Mak M, Babla M, Xu S-C, O’Carrigan A, Liu X-H, Gong Y-M, Holford P, Chen Z-H (2014) Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ Exp Bot 98:1–12. https://doi.org/10.1016/j.envexpbot.2013.10.003
Malagoli P, Britto DT, Schulze LM, Kronzucker HJ (2008) Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance. J Exp Bot 59(15):4109–4117. https://doi.org/10.1093/jxb/ern249
Mangano S, Silberstein S, Santa-María GE (2008) Point mutations in the barley HvHAK1 potassium transporter lead to improved K+-nutrition and enhanced resistance to salt stress. FEBS Lett 582(28):3922–3928. https://doi.org/10.1016/j.febslet.2008.10.036
Mishra M, Wungrampha S, Kumar G, Singla-Pareek SL, Pareek A (2020) How do rice seedlings of landrace Pokkali survive in saline fields after transplantation? Physiology, biochemistry, and photosynthesis. Photosynth Res 150(1-3):117–135. https://doi.org/10.1007/s11120-020-00771-6
Monson RK, Jaeger CH (1991) Photosynthetic characteristics of C3-C4 intermediate Flaveria floridana (Asteraceae) in natural habitats: evidence of advantages to C3-C4 photosynthesis at high leaf temperatures. Am J Bot 78(6):795–800. https://doi.org/10.2307/2445070
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt CS, Chen Z-H, Foster KJ, Gilliham M, Henderson SW, Jenkins CLD, Kronzucker HJ, Miklavcic SJ, Plett D, Roy SJ, Shabala S, Shelden MC, Soole KL, Taylor NL, Tester M, Wege S, Wegner LH, Tyerman SD (2020a) Energy costs of salt tolerance in crop plants. New Phytol 225(3):1072–1090. https://doi.org/10.1111/nph.15864
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57(5):1025–1043. https://doi.org/10.1093/jxb/erj100
Munns R, Passioura JB, Colmer TD, Byrt CS (2020b) Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol 225(3):1091–1096. https://doi.org/10.1111/nph.15862
Nakamura I, Murayama S, Tobita S, Bong BB, Yanagihara S, Ishimine Y, Kawamitsu Y (2002) Effect of NaCl on the photosynthesis, water relations and free proline accumulation in the wild Oryza species. Plant Prod Sci 5(4):305–310. https://doi.org/10.1626/pps.5.305
Nemati I, Moradi F, Gholizadeh S, Esmaeili M, Bihamta M (2011) The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ 57(1):26–33. https://doi.org/10.17221/71/2010-PSE
Nieves-Cordones M, Mohamed S, Tanoi K, Kobayashi NI, Takagi K, Vernet A, Guiderdoni E, Périn C, Sentenac H, Véry AA (2017) Production of low-Cs+ rice plants by inactivation of the K+ transporter OsHAK1 with the CRISPR-Cas system. Plant J 92(1):43–56. https://doi.org/10.1111/tpj.13632
Nishizawa Y, Nakamura I, Hossain M, Akamine H, Zheng S (2015) Dry matter production and photosynthetic ability of Oryza officinalis wall ex Watt under salinity stress condition. Jpn J Crop Sci 84(1):49–55. https://doi.org/10.1626/jcs.84.49
Oda Y, Kobayashi NI, Tanoi K, Ma JF, Itou Y, Katsuhara M, Itou T, Horie T (2018) T-DNA tagging-based gain-of-function of OsHKT1;4 reinforces Na exclusion from leaves and stems but triggers Na toxicity in roots of rice under salt stress. Int J Mol Sci 19(1). https://doi.org/10.3390/ijms19010235
Palmgren MG, Edenbrandt AK, Vedel SE, Andersen MM, Landes X, Østerberg JT, Falhof J, Olsen LI, Christensen SB, Sandøe P (2015) Are we ready for back-to-nature crop breeding? Trends Plant Sci 20(3):155–164. https://doi.org/10.1016/j.tplants.2014.11.003
Pan T, Liu MM, Kreslavski VD, Zharmukhamedov SK, Nie CR, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S (2021) Nonstomatal limitation of photosynthesis by soil salinity. Crit Rev Environ Sci Technol 51(8):791–825
Pottosin I, Shabala S (2016) Transport across chloroplast membranes: optimizing photosynthesis for adverse environmental conditions. Mol Plant 9(3):356–370
Prusty MR, Kim S-R, Vinarao R, Entila F, Egdane J, Diaz MGQ, Jena KK (2018) Newly identified wild rice accessions conferring high salt tolerance might use a tissue tolerance mechanism in leaf. Front. Plant Sci 9(417). https://doi.org/10.3389/fpls.2018.00417
Queirós F, Fontes N, Silva P, Almeida D, Maeshima M, Gerós H, Fidalgo F (2009) Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J Exp Bot 60(4):1363–1374. https://doi.org/10.1093/jxb/erp011
Radanielson AM, Angeles O, Li T, Ismail AM, Gaydon DS (2018) Describing the physiological responses of different rice genotypes to salt stress using sigmoid and piecewise linear functions. Field Crop Res 220:46–56. https://doi.org/10.1016/j.fcr.2017.05.001
Rajakani R, Sellamuthu G, Ishikawa T, Ahmed HA, Bharathan S, Kumari K, Shabala L, Zhou M, Chen ZH, Shabala S, Venkataraman G (2021) Reduced apoplastic barriers in tissues of shoot-proximal rhizomes of Oryza coarctata are associated with Na+ sequestration. J Exp Bot (in press). https://doi.org/10.1093/jxb/erab440
Rajakani R, Sellamuthu G, Saravanakumar V, Kannappan S, Shabala L, Meinke H, Chen Z, Zhou M, Parida A, Shabala S (2019) Microhair on the adaxial leaf surface of salt secreting halophytic Oryza coarctata Roxb. Show distinct morphotypes: isolation for molecular and functional analysis. Plant Sci 285:248–257. https://doi.org/10.1016/j.plantsci.2019.05.004
Rodríguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179(2):366–377. https://doi.org/10.1111/j.1469-8137.2008.02461.x
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161(2):341–370. https://doi.org/10.1111/j.1469-8137.2004.00974.x
Sage RF, Khoshravesh R, Sage TL (2014) From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. J Exp Bot 65(13):3341–3356. https://doi.org/10.1093/jxb/eru180
Schlüter U, Bräutigam A, Gowik U, Melzer M, Christin P-A, Kurz S, Mettler-Altmann T, Weber AP (2017) Photosynthesis in C3–C4 intermediate Moricandia species. J Exp Bot 68(2):191–206. https://doi.org/10.1093/jxb/erw391
Sengupta S, Majumder AL (2010) Porteresia coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant Cell Environ 33(4):526–542. https://doi.org/10.1111/j.1365-3040.2009.02054.x
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669. https://doi.org/10.1111/j.1399-3054.2007.01008.x
Shabala S, Cuin TA, Shabala L, Newman I (2012) Quantifying kinetics of net ion fluxes from plant tissues by non-invasive microelectrode measuring MIFE technique. Plant Salt Tolerance:119–134. https://doi.org/10.1007/978-1-61779-986-0_7 Springer
Shabala S, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113(1):111–118. https://doi.org/10.1104/pp.113.1.111
Shen Y, Shen L, Shen Z, Jing W, Ge H, Zhao J, Zhang W (2015) The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ 38(12):2766–2779. https://doi.org/10.1111/pce.12586
Shenton M, Kobayashi M, Terashima S, Ohyanagi H, Copetti D, Hernández-Hernández T, Zhang J, Ohmido N, Fujita M, Toyoda A (2020) Evolution and diversity of the wild rice Oryza officinalis complex, across continents, genome types, and ploidy levels. Genome Biol Evol 12(4):413–428. https://doi.org/10.1093/gbe/evaa037
Solis CA, Yong MT, Vinarao R, Jena K, Holford P, Shabala L, Zhou M, Shabala S, Chen Z-H (2020) Back to the wild: on a quest for donors toward salinity tolerant rice. Front Plant Sci 11:323. https://doi.org/10.3389/fpls.2020.00323
Sweeney M, McCouch S (2007) The complex history of the domestication of rice. Ann Bot 100(5):951–957. https://doi.org/10.1093/aob/mcm128
Tsujii M, Kera K, Hamamoto S, Kuromori T, Shikanai T, Uozumi N (2019) Evidence for potassium transport activity of Arabidopsis KEA1-KEA6. Sci Rep 9(1):10040. https://doi.org/10.1038/s41598-019-46463-7
Ueno O, Bang SW, Wada Y, Kobayashi N, Kaneko R, Kaneko Y, Matsuzawa Y (2007) Inheritance of C3–C4 intermediate photosynthesis in reciprocal hybrids between Moricandia arvensis (C3–C4) and Brassica oleracea (C3) that differ in their genome constitution. Plant Prod Sci 10(1):68–79. https://doi.org/10.1626/pps.10.68
Vogan PJ, Sage RF (2012) Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C3, C3–C4 intermediate, and C4 species from three evolutionary lineages of C4 photosynthesis. Oecologia 169(2):341–352. https://doi.org/10.1007/s00442-011-2201-z
von Caemmerer S, Quick WP, Furbank RT (2012) The development of C4 rice: current progress and future challenges. Science 336(6089):1671–1672. https://doi.org/10.1126/science.1220177
Wang F, Chen Z-H, Liu X, Colmer TD, Shabala L, Salih A, Zhou M, Shabala S (2016a) Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J Exp Bot 68(12):3191–3204. https://doi.org/10.1093/jxb/erw378
Wang F, Chen Z-H, Liu X, Colmer TD, Zhou M, Shabala S (2016b) Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J Exp Bot 67(12):3747–3762. https://doi.org/10.1093/jxb/erw034
Wang F, Chen Z-H, Shabala S (2017a) Hypoxia sensing in plants: on a quest for ion channels as putative oxygen sensors. Plant Cell Physiol 58(7):1126–1142. https://doi.org/10.1093/pcp/pcx079
Wang P, Khoshravesh R, Karki S, Tapia R, Balahadia CP, Bandyopadhyay A, Quick WP, Furbank R, Sage TL, Langdale JA (2017b) Re-creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Curr Biol 27(21):3278–3287. e3276. https://doi.org/10.1016/j.cub.2017.09.040
Wang P, Vlad D, Langdale JA (2016c) Finding the genes to build C4 rice. Curr Opin Plant Biol 31:44–50. https://doi.org/10.1016/j.pbi.2016.03.012
Wang S, Cao M, Ma X, Chen W, Zhao J, Sun C, Tan L, Liu F (2017c) Integrated RNA sequencing and QTL mapping to identify candidate genes from Oryza rufipogon associated with salt tolerance at the seedling stage. Front Plant Sci 8:1427. https://doi.org/10.3389/fpls.2017.01427
Waziri A, Kumar P, Purty R (2016) Saltol QTL and their role in salinity tolerance in rice. Austin J Biotechnol Bioeng 3(3):1067
Wu H, Zhang X, Giraldo JP, Shabala S (2018) It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 431(1):1–17. https://doi.org/10.1007/s11104-018-3770-y
Yang Z, Wang C, Xue Y, Liu X, Chen S, Song C, Yang Y, Guo Y (2019) Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat Commun 10(1):1–12. https://doi.org/10.1038/s41467-019-09181-2
Yeo A, Caporn S, Flowers T (1985) The effect of salinity upon photosynthesis in rice (Oryza sativa L.): gas exchange by individual leaves in relation to their salt content. J Exp Bot 36(8):1240–1248. https://doi.org/10.1093/jxb/36.8.1240
Yichie Y, Brien C, Berger B, Roberts TH, Atwell BJ (2018) Salinity tolerance in Australian wild Oryza species varies widely and matches that observed in O. sativa. Rice 11(1):66. https://doi.org/10.1186/s12284-018-0257-7
Yong M-T, Solis CA, Rabbi B, Huda S, Liu R, Zhou M, Shabala L, Venkataraman G, Shabala S, Chen Z-H (2020) Leaf mesophyll K+ and cl− fluxes and reactive oxygen species production predict rice salt tolerance at reproductive stage in greenhouse and field conditions. Plant Growth Regul 92(1):53–64. https://doi.org/10.1007/s10725-020-00619-y
Yorimitsu Y, Kadosono A, Hatakeyama Y, Yabiku T, Ueno O (2019) Transition from C3 to proto-Kranz to C3–C4 intermediate type in the genus Chenopodium (Chenopodiaceae). J Plant Res 132(6):839–855. https://doi.org/10.1007/s10265-019-01135-5
Yoshida S, Forno DA, Cock JH (1971) Laboratory manual for physiological studies of rice. Laboratory Manual for Physiological Studies of Rice, Manila
Zepeda-Jazo I, Shabala S, Chen Z, Pottosin II (2008) Na+-K+ transport in roots under salt stress. Plant Signal Behav 3(6):401–403. https://doi.org/10.4161/psb.3.6.5429
Zhang JS, Xie C, Li ZY, Chen SY (1999) Expression of the plasma membrane H+-ATPase gene in response to salt stress in a rice salt-tolerant mutant and its original variety. Theor Appl Genet 99(6):1006–1011. https://doi.org/10.1007/s001220051408
Zong Y, Chen Z, Innes JB, Chen C, Wang Z, Wang H (2007) Fire and flood management of coastal swamp enabled first rice paddy cultivation in East China. Nature 449(7161):459–462. https://doi.org/10.1038/nature06135
Acknowledgments
We would like to thank Dr. Rong Liu (WSU Confocal Bio-imaging Facility), Linda Westmoreland, Renee Smith, Craig Barton, Sharleen Hamersma, and Rosie Freeman for excellent technical support. The wild rice O. coarctata was obtained from the M. S. Swaminathan Research Foundation (MSSRF), India. Other wild rice species and cultivars were obtained from the International Rice Research Institute, Philippines respectively.
Funding
Sergey Shabala acknowledges support from the Department of Industry, Science, Energy and Resources (project AISRF48490), China National Distinguished Expert Project (WQ20174400441), grant 31961143001 for Joint Research Projects between Pakistan Science Foundation and National Natural Science Foundation, and Chinese National Natural Science Foundation (Project 31870249). Zhong-Hua Chen was supported by Australian Research Council (DE140101143; FT210100366) and by Hort Innovation Australia Projects (VG17003, LP18000). Gayatri Venkataraman acknowledges support from the Department of Biotechnology, GOI (BT/Indo-Aus/09/03/2015). Sergey Shabala also acknowledges the support provided by the National Biodiversity Authority (NBA), India Government in assisting with O. coarctata material transfer (Appl.no.1712).
Author information
Authors and Affiliations
Contributions
ZHC and SS designed the research. MTY, CAS, SA, GO, and RS conducted the experiments. MTY, CAS, PH and ZHC contributed to the data analysis. MTY and ZHC wrote the manuscript with contributions from all authors. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SS is a member of the editorial board but was not involved in the journal’s review or any decisions related to this submission.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig S1.
Effect of salinity on biomass, plant height, tiller numbers and total chlorophyll of cultivated and wild rice species after 42 days of 100 mM NaCl. Mean ± SE (n = 4). Different lowercase letters indicate significant differences at P < 0.05. Fig. S2. Confocal imaging of Na+ fluorescence in mesophyll cells of O. coarctata in response to Control, 2 h of direct exposure to 50 mM NaCl, 2 h of direct exposure to 100 mM NaCl and 1 week of 100 mM NaCl treatment through root. The mesophyll tissue without epidermal layer was directly exposed to salinity solution. Yellow colour represents Na+ signal and red colour represents chlorophyll signal. Data are mean ± SE (n = 5). Different lowercase letters indicate significant differences at P < 0.05 for each Duncan Group
Additional file 2: Table S1.
The primers for analysis of gene expression related to salinity tolerance and C4 photosynthesis in cultivated and wild rice species. Table S2. Salinity tolerance scores (high values indicate tolerance, low values sensitivity), based on the ranking of homogeneous groups assigned by Duncan’s Multiple Range tests, of cultivated and wild rice species based on analyses of physiological parameters. Table S3. Correlation analysis among the physiological parameters and gene expression in the cultivated and wild rice species
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yong, MT., Solis, C.A., Amatoury, S. et al. Proto Kranz-like leaf traits and cellular ionic regulation are associated with salinity tolerance in a halophytic wild rice. Stress Biology 2, 8 (2022). https://doi.org/10.1007/s44154-021-00016-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-021-00016-z