Abstract
Background
Cell to cell signaling systems in Gram-negative bacteria rely on small diffusible molecules such as the N-acylhomoserine lactones (AHL). These compounds are involved in the production of antibiotics, exoenzymes, virulence factors and biofilm formation. They belong to the class of furanone derivatives which are frequently found in nature as pheromones, flavor compounds or secondary metabolites. To obtain more information on the relation between molecular structure and quorum sensing, we tested a variety of natural and chemically synthesized furanones for their ability to interfere with the quorum sensing mechanism using a quantitative bioassay with Chromobacterium violaceum CV026 for antagonistic and agonistic action. We were looking at the following questions:
1. Do these compounds affect growth?
2) Do these compounds activate the quorum sensing system of C. violaceum CV026?
3) Do these compounds inhibit violacein formation induced by the addition of the natural inducer N-hexanoylhomoserine lactone (HHL)?
4) Do these compounds enhance violacein formation in presence of HHL?
Results
The naturally produced N-acylhomoserine lactones showed a strong non-linear concentration dependent influence on violacein production in C. violaceum with a maximum at 3.7*10-8 M with HHL. Apart from the N-acylhomoserine lactones only one furanone (emoxyfurane) was found to simulate N-acylhomoserine lactone activity and induce violacein formation. The most effective substances acting negatively both on growth and quorum sensing were analogs and intermediates in synthesis of the butenolides from Streptomyces antibioticus.
Conclusion
As the regulation of many bacterial processes is governed by quorum sensing systems, the finding of natural and synthetic furanones acting as agonists or antagonists suggests an interesting tool to control and handle detrimental AHL induced effects.
Some effects are due to general toxicity; others are explained by a competitive interaction for LuxR proteins. For further experiments it is important to be aware of the fact that quorum sensing active compounds have non-linear effects. Inducers can act as inhibitors and inhibitors might be able to activate or enhance the quorum sensing system depending on chemical structure and concentration levels.
Similar content being viewed by others
Background
Introduction
In Gram-negative bacteria, the best investigated intercellular signaling compounds are the N-acylhomoserine lactones, the action of which are based on the gene products of the luxR gene analogs [1–6]. Different bacterial species may produce different AHL analogs that differ in length of the N-acyl chains, ranging from 4–14 carbons and in the substitution at the 3-position of the side chain [7–9]. A second communication molecule, a furanosyl borate diester, found in Vibrio harvey is suggested to be active in interspecies signaling [10–13]. A genomic database analysis indicates that this interspecies communication is possibly spread throughout the whole group of eubacteria [14, 15]. Butyrolactones (2(3H)-furanones) from Streptomyces species are structurally related to the N-acylhomoserine lactones and act as well in quorum sensing [16, 17].
The AHL communication systems are highly species specific, but crosstalk may disturb proper signaling. AHLs with side chains other than the native ones interfere with signaling in Vibrio fischerii [18, 19]. AHL-signals from Pseudomonas aeruginosa acted on Burkholderia cepacia in a mixed biofilm system but not vice versa [20]. In Pseudomonas sp. diketopiperazines interact with the AHL dependent signaling system [21], and Staphylococcus aureus is sensitive to S. epidermidis quorum signals [22]. In natural bacterial communities several mechanisms have been found to interfere with bacterial signaling. Halogenated furanones from the marine algae Delisea pulchra inhibit quorum sensing mediated by N- acylhomoserine lactones [23–27].
A variety of AHL analogs have been tested for agonist or antagonist activity in quorum sensing. The length of the side chain, the C-3 carbonyl group as well as the ring structure influence binding of the signal molecule to the receptor protein [19, 20, 28]. Furthermore some synthetic furanones as well as structurally related compounds have been shown to interact with quorum sensing [29–32]. Bromoperoxidase in Laminaria digitata forms hypobromous acid which deactivates signaling of 3-oxohexanoylhomoserine lactone by oxidation [33]. Enzymes which degrade N- acylhomoserine lactones are present in Variovorax paradoxus [34], in Bacillus sp. [35, 36]and in other bacteria [37–39]
Many furanones with chemical structures similar to the N- acylhomoserine lactones are produced in nature. Butenolides (2(5H)-furanones) have been isolated from Streptomyces species [28, 40–42] or from Hortonia species [43]. Furanones are also produced by marine green, red or brown algae, by sponges, fungi, and ascidians [44–47] 3(2H)-furanones are sex pheromones from male cockroaches [48] while others are important artificial flavoring compounds in food industry or produced during cooking [49] or fermentation and found in beer and soy products. They occur naturally in pineapples or strawberries [50] and constitute flavoring compounds in cheese and wine [51–54]. Ascorbic acid belongs as well to the group of furanones [50]. Naturally occuring furanones may play a role in inhibiting bacterial infections and biofilm formation on plants which produce these. In recent years the concept to control bacterial infections through their quorum sensing system has gained much interest [55–61]. The availability of natural and synthetic Streptomyces sp. butenolides [28, 29, 62] and many intermediates from synthesis lead us to test these compounds for agonist and antagonist activity in the Chromobacterium test system.
Results
Bioassay with C. violaceum CV026 for N-acylhomoserine lactone and furanone activity
As an example, the full activity test for compound 3.31 from the list of furanones given in Figure 1 is shown in Figure 2. The compound was tested alone and in the presence of increasing concentrations of HHL. The concentration of compound 3.31 decreased horizontally from column 1 to 12, the HHL concentration vertically from A to F, each concentration was tested in triplicates. The experiment showed that compound 3.31 acted as an inhibitor of violacein production. Besides this, the compound was toxic, as estimated by the decrease in turbidity as indicator of growth (compare the growth control in H7 to H9 with H10 to H12 in the presence of compound 3.31).
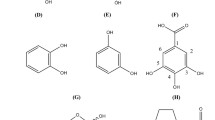
Structures of the tested furanones. Synthesis of furanones related to Streptomyces antibioticus metabolites and chemical structure of the compounds tested. A) general scheme for the synthesis of furanones related to Streptomyces antibioticus metabolites; reagents: a: Lithium diisopropylamide, b: BBr3, c: NaBH4 B) synthesized compounds (for details [29]).1.01: 5-hydroxy-4-methylfuran-2(5H)-one a), 3.01: 5-hydroxy-3-[(1R)-1-hydroxyethyl]-4-methylfuran-2(5H)-one b), 3.02: 5-hydroxy-3-[(1S)-1-hydroxyethyl]-4-methylfuran-2(5H)-one b), 3.11: 5-hydroxy-3-[(1R)-1-hydroxypropyl]-4-methylfuran-2(5H)-one b), 3.12: 5-hydroxy-3-[(1S)-1-hydroxypropyl]-4-methylfuran-2(5H)-one b), 3.21: 5-hydroxy-3-[(1R,2S)-1-hydroxy-2-methylbutyl]-4-methylfuran-2(5H)-one b), 3.22: 5-hydroxy-3-[(1S,2S)-1-hydroxy-2-methylbutyl]-4-methylfuran-2(5H)-one b), 3.31: 5-hydroxy-3-[(1R)-1-hydroxy-2,2-dimethylpropyl]-4-methylfuran-2(5H)-one b), 3.32: 5-hydroxy-3-[(1S)-1-hydroxy-2,2-dimethylpropyl]-4-methylfuran-2(5H)-one b), 3.41: 5-benzyloxy-3-bromo-4-methylfuran-2(5H)-one b), [63] 3.51: 5-hydroxy-3-(1-hydroxydecyl)-4-methylfuran-2(5H)-oned), 4.01: 3-[(1R)-1-hydroxyethyl]-4-methylfuran-2(5H)-one, 4.02: 3-[(1S)-1-hydroxyethyl]-4-methylfuran-2(5H)-one, 4.11: 3-[(1R)-1-hydroxypropyl]-4-methylfuran-2(5H)-one, 4.12: 3-[(1S)-1-hydroxypropyl]-4-methylfuran-2(5H)-one, 4.21: 5-hydroxy-3-[(1R,2S)-1-hydroxy-2-methylbutyl]-4-methylfuran-2(5H)-one, 4.22: 5-hydroxy-3-[(1S,2S)-1-hydroxy-2-methylbutyl]-4-methylfuran-2(5H)-one, 4.31: 3-[(1R)-1-hydroxy-2,2-dimethylpropyl]-4-methylfuran-2(5H)-one, 4.32: 3-[(1S)-1-hydroxy-2,2-dimethylpropyl]-4-methylfuran-2(5H)-one, 4.51: 3-(1-hydroxydecyl)-4-methylfuran-2(5H)-one a). a) Racemate b) Mixture of the two C(5)-epimers c) Mixture of the two C(1')-epimers d) Mixture of all four diastereoisomersC) commercially available flavoring compounds. 11: sotolone, 3-hydroxy-4,5-dimethylfuran-2(5H)-one; 12: emoxyfurane or EMF, 5-ethyl-3-hydroxy-4-methylfuran-2(5H)-one; 13: dihydroactinolide, 5,6,7,7a-4,4,7a-trimethyltetrahydrobenzofuran-2(4H)-one; 14: methyltetrahydrofuranone, 2-methyldihydrofuran-3(2H)-one;, 15: norfuraneol, 4-hydroxy-5-methylfuran-3(2H)-one; 16: DMHF or furaneol, 4-hydroxy-2,5-dimethylfuran-3(2H)-one; 17: pineapple ketone acteate, 2,5-dimethyl-4-oxo-4,5-dihydrofuran-3-yl acetate; 18: HF or homofuraneol; 5-ethyl-4-hydroxy-2-methylfuran-3(2H)-one; 19: methyl furyl butanal, 3-(5-methyl-2-furyl)butanal; 20: L-ascorbic acid, (5R)-3,4-dihydroxy-5-[(1S)-1,2-dihydroxyethyl]furan-2(5H)-one; 21: (5Z)-4-bromo-5-(bromomethylene)-3-butylfuran-2(5H)-one; 22: (5Z)-4-bromo-5-(bromomethylene)-3 [(1R)-1-hydroxybutyl]furan-2(5H)-one; 23: AHL, principal structure of N-acyl-L-homoserine lactones with N-decanoyl-L-homoserine lactone as example.
Bioassay for quorum sensing using C. violaceum CV026 demonstrating effects of compound 3.31 on violacein formation. Concentrations of compound 3.31 in rows A to F: Columns 1 to 3 = 10-2 M, columns 4 to 6 = 10-3 M, columns 7 to 9 = 10-4 M, columns 10 to 12 = 10-5 M. HHL concentrations: row A 10-6 M, row B 3.3*10-7 M, row C 1.1*10-7 M, row D 3.7*10-8 M, row E 1.2*10-8 M, row F 4.1*10-9 M. Rows G and H: calibration for HHL, G1-G3 10-6 M, G4-G6 10-7 M, G7-G9 10-8 M, G10-G12 10-9 M, H1-H3 10-10 M, H4-H6 10-11 M. H7-H9 is a growth control of the mutant CV026 lacking HHL, wells H10-H12 contain compound 3.31 at 10-2 M to observe growth inhibition.
The data from the experiment given in Figure 2 is plotted in Figure 3. The control lacking compound 3.31 gave the typical response to increasing concentrations of the native AHL. The maximal violacein production was reached within one decade in concentration and the maximum dye development lay in all experiments around 3.7*10-8 M. Violacein formation did not saturate at higher concentrations of the signaling compound but clearly decreased from 3.7*10-8 M HHL to 10-6 M by about 40%. Increasing concentrations of compound 3.31 resulted in decreased violacein production at all HHL concentrations tested. At 10-3 M of 3.31 dye formation was practically fully blocked and the values below zero at the concentration of 10-2 M indicated growth inhibition due to the toxicity of the compound.
Quantitative presentation of the bioassay showing effects of compound 3.31 on violacein production in C. violaceum CVO 26 at various HHL concentrations. Quantitative presentation of data from Figure 2 showing effects of compound 3.31 on violacein production in C. violaceum CVO 26 at various HHL concentrations. A value of 1 is equal to the violacein production induced by 3.7*10-8 M HHL. Values are the mean of 3 determinations, the standard deviation was between 0.01 and 0.09 (error bars indicated). Note that the x-axis is logarithmic.
Induction and inhibition of violacein production in C. violaceum CV026 by different N-acylhomoserine lactones
Antagonism towards AHL effects on violacein production or inhibition of quorum sensing is often followed by using the wild type C. violaceum. However, observing these effects with CV026 in the presence of defined concentrations of the native inducer HHL allows to better understand the interaction between the compound to be tested and the native N- acylhomoserine lactone at the cellular level. Inhibition or activation of violacein production is then related to the dye development achieved with defined concentrations of HHL.
Figure 4 summarizes the activating effect of different N- acylhomoserine lactones on violacein formation. Activation was observed at 3.7*10-8 M for side chain lengths from C6 to C8, while concentrations of 10-6 M or 10-4 M were necessary to induce violacein formation for side chain lengths of C4 and C10 to C14. None of the tested AHL inhibited growth at the highest concentration of 10-4 M (data not shown). As demonstrated in Figure 4, DHL (C10 side chain) stimulated dye formation at concentrations higher than 10-6 M, dDHL (C12 side chain) only at 10-4 M.
Induction of violacein production in C. violaceum CV026 by N -acylhomoserine lactones and compound 12 (10 -4 M). Induction of violacein production was observed by adding different N- acylhomoserine lactones to cultures of C. violaceum CV026. The experiments where conducted according to the well assay described in the methods section. A value of 1 is equal to the violacein production induced by 3.7*10-8 M HHL, 0 = no violacein formed. Values are the mean of 3 determinations, the standard deviation was between 0.01 and 0.09 (error bars indicated).
Violacein was optimally induced by 3.7*10-8 M HHL, this value was again set to 1 in Figure 5. The addition of any other N- acylhomoserine lactone except C4 to cells supplied with 3.7*10-8 M HHL acted inhibitory. As already indicated in Figure 2, any increase in concentration of the native HHL itself reduced the amount of violacein produced and the inhibition increased with chain length. At a concentration of 10-6 M, compounds with C4 to C8 acyl side chains inhibited stronger than at the lower concentration (3.7*10-8 M). Interestingly, C10 to C14 AHLs were more effective inhibitors at the low concentration of 3.7*10-8 M compared to 10-6 M.
Inhibition of violacein production by C. violaceum CV026 by N -acylhomoserine lactones and compound 12 (10 -4 M). Inhibition of violacein production was observed by adding different N- acylhomoserine lactones and HHL at 3.7*10-8 M HHL to cultures of C. violaceum CV026. The experiments where conducted according to the well assay described in the methods section. A value of 1 is equal to the violacein production induced by 3.7*10-8 M HHL alone (no inhibition), 0 = no violacein formed (complete inhibition). Values are the mean of 3 determinations, the standard deviation was between 0.01 and 0.09 (error bars indicated).
Effects of 32 natural and synthetic furanone compounds on growth, and on induction, inhibition and enhancement of violacein production by C. violaceum
The complete set of furanone analogs and derivatives illustrated in Figure 1 were tested for activation and inhibition of the quorum sensing system of C. violaceum CV026, as demonstrated before for compound 3.31 in Figure 2. For comparison with the natural inducers, the long chain DHL (compound 23) was included as test compound. The data are summarized in Table 1. Synthesized furanones or intermediates from synthesis, which were not soluble in water, were not tested for biological activities and are not listed in Table 1. Activation of quorum sensing gives the effect on violacein production at 10-4 M in the absence of HHL (Column 1); 0 means no violacein compared to the blank, 1 is equal to the effect of 3.7*10-8 M HHL. Activation of the CV026 violacein formation was negative for all furanones, except for #12, which resulted in a slight stimulation. Effects on growth are seen in the change in turbidity at 10-2 M (Column 2); 1 is equal to the control with no additions. Some furanones were toxic at 10-2 M. Turbidity dropped to 40 to 60 % of the control in the presence of 10-2 M of the compounds 3.31, 3.32, 4.31, 4.32, 3.51, 13, 18, 19 or 22. Some compounds induced an increase in absorption compared to the control (3.01, 3.02, 12, DHL). It may be that these were degraded by CV026 and stimulated growth by acting as additional carbon source. For two activators, #12 and the long chain DHL, this increase in absorption was partially due to the simultaneous induction of violacein formation as the assays turned purple. Inhibition was tested at a compound concentration of 10-4 M in the presence of either 3.7*10-8 M HHL (column 3) or 10-6 M HHL (column 4); setting the effect of these concentrations to 1 in the absence of the added compound. All furanones including compound DHL (see also Fig. 5) inhibited violacein formation at 10-4 M in the presence of optimum HHL concentration (column 3). The inhibition ranged from a few percent for #19, 15, 8 and 11, to 70 to 80% for #3.01, 4.01, 3.11, 3.31 and 12. Interestingly, the same experiment at an elevated HHL concentration of 10-6 M (column 4) resulted in different inhibitory effects for some furanones; it could both be larger (e.g. #3.22, 4.22, or 3.02) or smaller (e.g. #3.41, 3.01, or 4.51) compared to the ones shown in column 3. Enhanced expression gives the effect on violacein production by 10-5 M furanones at a suboptimal HHL concentration of 4.6*10-9 M, (Column 5); 1 is equal to the control lacking an addition. These data indicate whether a compound acted cooperatively with HHL present at a suboptimal concentration (4.6*10-9 M) and enhanced expression of violacein. Obviously quite a few furanones acted synergistically with limiting HHL and stimulated violacein formation.
Discussion
Most compounds tested interacted in some way with growth or violacein production in C. violaceum. Some of them clearly inhibited growth at 10-2 M (Table 1, column 2), but none showed negative effects on growth at 10-4 M (data not shown). It is therefore assumed that the effects on violacein production observed at 10-4 M furanone concentration are not due to inhibition of growth processes, but rather based on interferences with the signaling system of quorum sensing. Several compounds clearly stimulated growth, suggesting that these may become metabolized and act as growth substrates.
Only one single furanone, compound 12, was found to simulate N- acylhomoserine lactone activity and induce violacein formation, furthermore, it stimulated growth (Table 1, column 1 and 2). In the presence of superoptimal concentrations of HHL, addition of compound 12 lowered violacein production, while increasing violacein production at suboptimal HHL (Table 1, columns 3 to 5), which is the behavior expected for a member of the N- acylhomoserine lactone family. Compound 12 thus acts in C. violaceum CV026 similar to long chain AHLs; it inhibits violacein formation in the presence of optimal HHL, but induces it in the absence of HHL with increasing concentrations. Furthermore, the inhibition is partially relieved by increasing the concentration of HHL.
Halogenated furanones from Delisea pulchra are well described as antagonists to N- acylhomoserine lactones [24]; our data show that compound 22 inhibited violacein formation stronger than compound 21, confirming the reported results with E. coli (pSB403) [24]. Furthermore, #22 was toxic on growth at 10-2 M and did not enhance expression in the presence of suboptimal HHL concentrations (Table 1, columns 2 and 5).
The most effective substances acting negatively both on growth and quorum sensing were compounds 3.31, 3.32, 3.51, 4.31 and 4.32, analogs of the natural products from Streptomyces antibioticus. Growth was inhibited by 40 to 60 % and violacein formation dropped in some cases to 20 %.
The different tests of combinations of furanones with the natural HHL at various concentrations made it difficult to simply group the compounds into agonists and antagonists of quorum sensing. Although except for #12 none of the furanones exhibited an activating effect on violacein formation, about half of them stimulated violacein formation in the presence of suboptimal HHL (Table 1, column 5). This suggests some cooperative action for binding to the receptor site. Another interaction, possibly as well a competition for this site, is seen when the inhibitory effects of the furanones are compared at two different HHL concentrations (Table 1, columns 3 and 4). With some furanones the inhibition decreases with increasing concentration of HHL, e.g. with compounds 3.01, 3.41, and 4.51, while with others the inhibition is increased, as e.g. clearly seen with 3.02 or 18.
Both cooperative and antagonistic effects are also observed with N- acylhomoserine lactones having C4 to C14 side chains with respect to the action of HHL, the natural inducer of CV026. AHLs act in both ways depending on the concentration. The short side chain AHLs (BHL to OHL) induced at low concentrations but induction became repressed with increasing concentrations. In contrast, long chain AHLs acted in the opposite way as demonstrated by McClean et al. [9] with less quantitative diffusion plate tests. As these compounds activated the quorum sensing system at higher concentrations, long chain AHLs strongly stimulated also violacein formation in the presence of suboptimal HHL concentrations, as shown by DHL in Table 1.
The screening of chemically related compounds for activity with the bacterial quorum sensing system should give some information on structure-function relationships. In each of the three pairs of enantiomers, 3.01/3.02, 4.01/4.02, and 3.11/3.12, the two enantiomers not surprisingly showed a quite different behavior. While all are non-toxic on growth, only one isomer of each pair showed a positive cooperative effect in combination with suboptimal concentrations of HHL (Table 1, column 5). Similarly a 60-fold difference between the L and D form of OHHL has been noted by McClean et al. [9]. However, this is not true for the other two enantiomeric pairs, 3.31/3.32 and 4.31/4.32, where no significant difference in the activity spectrum was observed.
Compounds 3.21 and 3.22, as well as 4.21 and 4.22 constitute pairs of diastereoisomers differing only by an OH-group at the furanone ring. While in the first pair the compound with the R-configuration at the OH bearing carbon atom of the side chain (3.21) was much more stimulating than the one with the S-configuration (3.22), the situation was reversed with the second pair, the S-form (4.22) being more active. The opposite was seen with compounds 3.01/3.02 and 4.01/4.02, as well as with the pairs 3.11/3.12 and 4.11/4.12, where in the presence of the OH-group the R-form acted as inhibitor.
One is tempted to correlate some of the findings with the length of the side chains of the furanones [63]: compounds with small side chains (3.01/3.02, 4.01/4.02, 3.11/3.12 and 4.11/4.12) gave more response than the branched and thus sterically more demanding compounds (3.31/3.32, 4.31/4.32, 3.51 and 4.51).
Recently the high-resolution structure of the TraR protein has been published [64]. Binding studies with point mutations in the TraR protein confirm the specific amino acids involved in the binding of the AHL [65]. The furanone ring of the inhibitory compound 3.31 could fit into the model of the R protein. Hydrogen bonds with Trp57 and Tyr53 would be possible, however, the link to the amide-N of Asp70 would not occur. Furthermore the hydrophobic part of compound 3.31 would be at another site than for a N- acylhomoserine lactone bound to the R protein.
Most of the commercially available products inhibited the HHL induced violacein production (Table 1, columns 3 and 4); exceptions are #11, 14 and 17 where the effect was negligible. Enhanced expression at suboptimal HHL concentration was strongly observed with #11 and 12 (the latter compound differing only in having an ethyl instead of a methyl side chain). Compounds 13, 14 and 16 showed similar stimulating effects, #13 in spite of its more complex structure. Within the structurally related group #14 to #18 the addition of compounds 14 and 16 stimulated dye formation, while #15, 17 and 18 produced no or only a slight inhibitory effect. Interestingly, ascorbic acid (#20) behaved like a long chain member of the AHL family, inhibiting violacein formation at optimal and higher concentrations of HHL, but stimulating at suboptimal ones.
Interaction of various chemical compounds especially N- acylhomoserine lactone analogs [18, 56] with AHL signaling have been investigated before. Also the azalide antibiotic azithromycin inhibits quorum sensing in Pseudomonas [30], as well as metabolites from a marine bryozoan [66] or plants [67], but so far it was not possible to propose a general concept of the interaction of the compounds with the receptor protein.
Conclusions
From the results described it is difficult to find a general structure-function relationship. All furanone compounds tested, including the bacterial N- acylhomoserine lactones, showed strong concentration dependent activities. Some effects are due to general toxicity; others may be explained by a competitive interaction for LuxR protein. Even interaction with a (hypothetic) second autoinducer system as found in Vibrio harveii [10] might be possible. It must be emphasized that when compounds interacting with the quorum sensing system are studied, it is crucial to observe these effects over a broad range of concentrations, since depending on the concentration applied, also natural inducers act as inhibitors while inhibitors are able to activate the system.
Methods
Bacterial strains and culture conditions
C. violaceum CV026 (a mini-Tn5 mutant) served as indicator organism for quorum sensing by quantifying violacein synthesis [9]. In this mutant the AHL regulated phenotypes are specifically repressed [68]. The organism was grown on 0.5% yeast extract and 1% tryptone. For each experiment a fresh over night culture (27°C without shaking) with an OD660 of approximately 1.2 was used.
Signaling compounds
A series of 30 furanone derivatives was synthesized using furanone 1 and various aliphatic aldehydes 2 (Figure 1). Deprotection and reduction gave compounds 3 and 4, respectively [62]. Compounds 3 and 4 are analogs to natural butenolides that were recently isolated form Streptomyces antibioticus [28]. All compounds tested are compiled in Figure 1. Substances derived from 1, 3, and 4 were synthesized as reported by Grossmann [29]. Eight commercially available furanones were obtained from Givaudan S.A., Dübendorf, Switzerland (11–19, Figure 1). Two halogenated furanones (21, 22) were provided by Staffan Kjelleberg (University of New South Wales, Sidney, Australia). Synthetic N- acylhomoserine lactones were obtained from Fluka (Buchs, Switzerland).
Bioassay
Experiments were conducted in 96 well flat bottom plastic microplates (Sarstedt Inc, USA). With the mutant CV026, violacein formation is dependent upon the external addition of N- hexanoylhomoserine lactone (HHL). Activation of quorum sensing by furanones was tested directly by addition of the compounds to the wells. As control, a calibration curve with HHL was obtained separately in each experiment. To study inhibition using CV026, N- hexanoylhomoserine lactone at optimal induction concentration (3.7*10-8 M HHL) plus furanones were added to each assay in four dilution steps. To the wells that were used for growth control neither furanones nor N- hexanoylhomoserine lactone were added. For toxicity control furanones only were added. Turbidity at 660 nm was used for growth control. The final concentrations used are given in the results. Then, 100 μl of freshly grown CV026 were transferred into each well and the microplates incubated on a lab plate-shaker (0.01 Hz) for 16 hours at 27°C to allow induction of violacein formation. The plates were then dried at 60°C until all medium had evaporated (around 6 hours or overnight). The violacein was resolubilized by adding 100 μl of DMSO to each well and the plates incubated on a lab shaker for two hours. The absorbance of each well was measured with an automatic Anthos 2001 96 well plate reader (Anthos Labtec Instruments, Austria) at a fixed wavelength of 590 nm. The amount of violacein produced in the individual experiments was calibrated with respect to the HHL calibration series present on each microplate. Each measurement was done in triplicate.
Abbreviations
- BHL = N- butanoylhomoserine lactone:
-
HHL = N- hexanoylhomoserine lactone, HPHSL = N- heptanoylhomoserine lactone, OHL = N- octanoylhomoserine lactone, DHL = N- decanoylhomoserine lactone, dDHL = N- dodecanoylhomoserine lactone, tDHL = N- tetradecanoylhomoserine lactone.
References
Cha CP, Gao YC, Chen PD, Shaw D, Farrand SK: Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Molec Plant-Microbe Interact. 1998, 11: 1119-1129.
Fuqua C, Parsek MR, Greenberg EP: Regulation of gene expression by cell to cell communication: Acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001, 35: 439-68. 10.1146/annurev.genet.35.102401.090913.
ASM Press, Swift S, Williams P, Stewart GSAB: N-acylhomoserine lactones and quorum sensing in proteobacteria. In: Cell-cell signaling in bacteria. Edited by: Dunny GM, Williams SC. 1999, ASM Press, 291-313.
Parsek MR, Val DL, Hanzelka BL, Cronan Jr, Greenberg EP: Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci U S A. 1999, 96: 4360-4365. 10.1073/pnas.96.8.4360.
Parsek MR, Greenberg EP: Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci U S A. 2000, 97: 8789-87893. 10.1073/pnas.97.16.8789.
Gray KM, Garey JR: The evolution of bacterial LuxI and LuxR quorum sensing regulators. Microbiology. 2001, 147: 2379-2387.
ASM Press, Fuqua C, A Eberhard: Signal generation in audoinduction systems: Synthesis of acylated homoserine lactones by Lux-I proteins. In: Cell-cell signaling in bacteria. Edited by: Dunny GM, Williams SC. 1999, ASM Press, 211-231.
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GSAB, Williams P: Quorum sensing and Chromobacterium violaceum :Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997, 143: 3703-3711.
Shaw PD, Ping G, Daly SL, Cha C, Cronan Jr, Rinehart KL, Farrand SK: Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci U S A. 1997, 94: 6036-6041. 10.1073/pnas.94.12.6036.
Miller MB, Bassler BL: Quorum sensing in bacteria. Annu Rev Microbiol. 2001, 55: 165-199. 10.1146/annurev.micro.55.1.165.
Schauder S, Shokat K, Surette MG, Bassler BL: The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001, 41: 463-76. 10.1046/j.1365-2958.2001.02532.x.
Surette MG, Miller MB, Bassler BL: Quorum sensing in Escherichia coli, Salmonella typhimurium and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A. 1999, 96: 1639-44. 10.1073/pnas.96.4.1639.
Chen X, Schauder S, Potier N, Van Dorsselaer, Pelczer I, Bassler BL, Hughson FM: Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002, 415: 545-9. 10.1038/415545a.
Bassler BL: How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999, 2: 582-7. 10.1016/S1369-5274(99)00025-9.
Sperandio V, Torres AG, Giron JA, Kaper JB: Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2001, 183: 5187-5197. 10.1128/JB.183.17.5187-5197.2001.
Kinoshita H, Ipposhi H, Okamoto S, Nakano H, Nihira T, Yamada Y: Butyrolactone autoregulator receptor protein (BarA) as a transcriptional regulator in Streptomyces virginiae. J Bacteriol. 1997, 179: 6986-6993.
England RR, Hobbs G, Bainton NJ, Roberts DM: Microbial signaling and communication,. In: Symposia of the society for general mocrobiology. Edited by: Robers D. 1999, Cambridge University Press, Cambridge, 71-85.
Schaefer AL, Hanzelka BL, Eberhard A, Greenberg EP: Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol. 1996, 178: 2897-2901.
Eberhard A, Widrig CA, McBath P, Schineller JB: Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch Microbiol. 1986, 146: 35-40.
Riedel K, Hentzer M, Geisenberger O, Huber B, Steidle A, Wu H, Hoiby N, Givskov M, Molin S, Eberl L: N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001, 147: 3249-62.
Holden MT, Ram CS, de Nys R, Stead P, Bainton NJ, Hill PJ, Manefield M, Kumar N, Labatte M, England D, Rice S, Givskov M, Salmond P, Stewart GSAB, Bycroft W, Kjelleberg S, Williams P: Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol. 1999, 33: 1254-66. 10.1046/j.1365-2958.1999.01577.x.
Otto M, Echner H, Voelter W, Gotz F: Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 2001, 69: 1957-60. 10.1128/IAI.69.3.1957-1960.2001.
Rasmussen TB, Manefield M, Andersen JB, Eberl L, Anthoni U, Christophersen C, Steinberg P, Kjelleberg S, Givskov M: How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology. 2000, 146: 3237-3244.
Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S: Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999, 145: 283-291.
Steinberg PD, Schneider R, Kjelleberg S:Chemical defenses of seaweeds against microbial colonization.Biodegradation. 1997, 8: 211-220. 10.1023/A:1008236901790.
Manefield M, Harris L, Rice SA, de Nys R, Kjelleberg S: Inhibition of luminescence and virulence in the Black Tiger Prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl Environ Microbiol. 2000, 66: 2079-2084. 10.1128/AEM.66.5.2079-2084.2000.
Gram L, de Nys R, Maximilien R, Givskov M, Steinberg P, Kjelleberg S: Inhibitory effects of secondary metabolites from the red algae Delisea pulchra on swarming motility of Proteus mirabilis. Appl Environ Microbiol. 1996, 62: 4284-4287.
Braun D, Pauli N, Séquin U, Zähner H: New butenolides from the photoconductivity screening of Streptomyces antibioticus (Waksman and Woodruff) Waksman and Henrici 1948. FEMS Microbiol Lett. 1995, 126: 37-42. 10.1016/0378-1097(94)00523-T.
Grossmann G, Poncioni M, Bornand M, Jolivet B, Neuburger M, Séquin U: Bioactive butenolides from Streptomyces antibioticus. TÜ99: absolute configurations and synthesis of analogs. Tetrahedron. 2003, 59: 3237-3251. 10.1016/S0040-4020(03)00483-6.
Tateda K, Comte R, Pechere JC, Köhler T, Yamaguchi K, Van Delden C: Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrol Agents Chemother. 2001, 45: 1930-1933. 10.1128/AAC.45.6.1930-1933.2001.
Gao M, Teplitski M, Robinson JB, Bauer WD: Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol Plant Microbe Interact. 2003, 16: 827-834.
Kline T, Bowman J, Iglewski BH, de Kievit T, Kakai Y, Passador L: Novel synthetic analogs of the Pseudomonas autoinducer. Bioorg Med Chem Lett. 1999, 9: 3447-52. 10.1016/S0960-894X(99)00626-5.
Borchardt SA, Allain EJ, Michels JJ, Stearns GW, Kelly RF, McCoy WF: Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials. Appl Environ Microbiol. 2001, 67: 3174-3179. 10.1128/AEM.67.7.3174-3179.2001.
Leadbetter JR, Greenberg EP: Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000, 182: 6921-6926. 10.1128/JB.182.24.6921-6926.2000.
Dong YH, Xu JL, Li XZ, Zhang LH: AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci U S A. 2000, 97: 3526-3531. 10.1073/pnas.060023897.
Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH: Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001, 411: 813-817. 10.1038/35081101.
Park SY, Lee SJ, Oh TK, Oh JW, Koo BT, Yum DY, Lee JK: AhlD, an N-acylhomoserine lactonase in Arthrobacter sp. and predicted homologous in other bacteria. Microbiology. 2003, 149: 1541-1550. 10.1099/mic.0.26269-0.
Uroz S, D'Angelo-Picard C, Carlier A, Elasri M, Sicot C, Petit A, Oger P, Faure D, Dessaux Y: Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology. 2003, 149: 1981-1989. 10.1099/mic.0.26375-0.
Taga ME, Bassler BL: Chemical communication among bacteria. Proc Natl Acad Sci U S A. 2003, 2: 14549-14554. 10.1073/pnas.1934514100.
Mukku VJ, Speitling M, Laatsch H, Helmke E: New butenolides from two marine streptomycetes. J Nat Prod. 2000, 63: 1570-1572. 10.1021/np0001676.
Cho KW, Lee HS, Rho JR, Kim TS, Mo SJ, Shin J: New lactone-containing metabolites from a marine-derived bacterium of the genus streptomyces. J Nat Prod. 2001, 64: 664-667. 10.1021/np000599g.
Guo YW, Gavagnin M, Mollo E, Trivellone E, Cimino G: Three new butenolide lipids from the caribbean gorgonian pterogorgia anceps. J Nat Prod. 1999, 62: 1194-1196. 10.1021/np9901231.
Ratnayake R, Karunaratne V, Ratnayake BBM, Kumar V, MacLeod JK, Simmonds P: Two new lactones with mosquito larvicidal activity from three Hortonia species. J Nat Prod. 2001, 64: 376-378. 10.1021/np000371t.
Faulkner DJ: Marine natural products. Nat Prod Rep. 2001, 18: 1-49. 10.1039/b006897g.
Amagata T, Usami Y, Minoura K, Ito T, Numata A: Cytotoxic substances produced by a fungal strain from a sponge: physico-chemical properties and structures. J Antibiot (Tokyo). 1998, 51: 33-40.
Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S: Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996, 178: 6618-6622.
Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, De Nys R: Do marine natural products interfere with prokaryotic AHL regulatory systems?. Aquatic Microbial Ecology. 1997, 13: 85-93.
Farine JP, Le Quere JL, Duffy J, Semon E, Brossut R: 4-Hydroxy-5-methyl-3(2H)-furanone and 4-hydroxy-2,5-dimethyl-3(2H)-furanone, two components of the male sex pheromone of Eurycotis floridana (Walker) (Insecta, Blattidae, Polyzosteriinae). Biosci Biotech Biochem. 1993, 57: 2026-2030.
Kim KW, Lee SB: Inhibitory effect of maillard reaction products on growth of the aerobic marine hyperthermophilic archaeon Aeropyrum pernix. Appl Environm Microbiol. 2003, 69: 4325-4328. 10.1128/AEM.69.7.4325-4328.2003.
Slaughter CJ: The naturally occurring furanones: formation and function from pheromone to food. Biol Rev Camb Philos Soc. 1999, 74: 259-276. 10.1017/S0006323199005332.
Suriyaphan O, Drake MA, Chen XQ, Cadwallader KR: Characteristic aroma components of British Farmhouse Cheddar cheese. J Agric Food Chem. 2001, 49: 1382-1387. 10.1021/jf001121l.
Rychlik M, Warmke R, Grosch W: Ripening of Emmental cheese wrapped in foil with and without addition of Lactobacillus casei subsp. casei. III. Analysis of character impact flavour compounds. Lebensmittel Wissenschaft Technologie. 1997, 30: 471-478. 10.1006/fstl.1996.0209.
Rapp A, Engel L: Detection and determination of furaneol (2,5-dimethyl-4-hydroxy-3-furanone) in wines from Vitis vinifera varieties. Vitis. 1995, 34: 71-72.
Masuda M, Okawa E, Nishimura K, Yunome H: Identification of 4,5-dimethyl-3-hydroxy-2(5H)-furanone (sotolone) and ethyl 9-hydroxynonanoate in botrytised wine and evaluation of the roles of compounds characteristic of it. Agric Biol Chem. 1984, 48: 2707-2710.
Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Hoiby N, Kjelleberg S, Givskov M: Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002, 148: 87-102.
Smith KM, Bu Y, Suga H: Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem Biol. 2003, 10: 81-89. 10.1016/S1074-5521(03)00002-4.
Hentzer M, Eberl L, Nielsen J, Givskov M: Quorum sensing : a novel target for the treatment of biofilm infections. Bio Drugs. 2003, 17: 241-50.
Donabedian H: Quorum sensing and its relevance to infectious diseases. J Infect. 2003, 46: 207-14. 10.1053/jinf.2002.1120.
Camara M, Williams P, Hardman A: Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect Dis. 2002, 2: 667-76. 10.1016/S1473-3099(02)00447-4.
Suga H, Smith KM: Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr Opin Chem Biol. 2003, 7: 586-91. 10.1016/j.cbpa.2003.08.001.
Smith KM, Bu Y, Suga H: Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem Biol. 2003, 10: 563-71. 10.1016/S1074-5521(03)00107-8.
Grossmann G, Séquin U: Synthetic access to biologically active butenolides from Streptomyces antibioticus. Synlett. 2001, 278-280.
Hjelmgaard T, Persson T, Rasmussen TB, Givskov M, Nielsen J: Synthesis of furanone-based natural product analogues with quorum sensing antagonist activity. Bioorg Med Chem. 2003, 11: 3261-3271. 10.1016/S0968-0896(03)00295-5.
Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak : A Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002, 417: 971-974. 10.1038/nature00833.
Luo ZQ, Smyth AJ, Gao P, Qin Y, Farrand SK: Mutational analysis of TraR. Correlating function with molecular structure of a quorum-sensing transcriptional activator. J Biol Chem. 2003, 278: 13173-13182. 10.1074/jbc.M210035200.
Peters L, König GM, Wright AD, Pukall R, Stackebrandt E, Eberl L, Riedel K: Secondary metabolites of Flustra foliacea and their onfluence of bacteria. Appl Environm Microbiol. 2003, 69: 3469-3475. 10.1128/AEM.69.6.3469-3475.2003.
Fray RG, Throup JP, Daykin M, Wallace A, Williams P, Stewart GSAB, Grierson D: Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat Biotechnol. 1999, 17: 1017-1020. 10.1038/13717.
John Wiley & Sons Ltd, Throup J, Winson MA, Bainton NJ, Bycroft BW, Williams P, Stewart GSAB: Signaling in bacteria beyond luminescence. In: Bioluminescence and chemiluminescence: Fundamental and applied aspects. Edited by: Campell A, Kricka L, Stanley P. 1995, John Wiley & Sons Ltd, 89-92.
Acknowledgements
C. violaceum (CV026) was obtained from B. Laue (University of Nottingham, United Kingdom). We thank Staffan Kjelleberg (University of New South Wales, Australia) for the furanones from Delisea pulchra and Givaudan SA (Dübendorf, Switzerland) for the flavouring furanones. Financial support by the Bundesamt für Bildung und Wissenschaft (Switzerland) (COST 520) and the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 20-63344.00) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
All bioassays were conducted by DM under the supervision of HB. RB and US assessed the structure-function relationship of the furanones and RB with DM drafted the manuscript. JG performed all synthesis of the natural analogs of Streptomyces antibioticus furanones. US supervised the work of JG and provided the structures and the sterical comparison of the furanones.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Martinelli, D., Grossmann, G., Séquin, U. et al. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum . BMC Microbiol 4, 25 (2004). https://doi.org/10.1186/1471-2180-4-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-4-25