Abstract
Background
Therapeutic regulation of PPARδ activity using selective agonists has been proposed for various disorders. However, the consequences of altered peroxisome proliferator-activated receptor delta (PPARδ) activity in the context of intestinal tumourigenesis remain somewhat unclear. Contradictory evidence suggesting PPARδ either attenuates or potentiates intestinal neoplasia. To further investigate the PPARδ dependency of intestinal tumourigenesis, we have analysed the consequences of PPARδ deficiency upon intestinal neoplasia occurring in mice with impaired mismatch DNA repair.
Methods
Mice deficient for both PPARδ and the mismatch repair gene Mlh1 were produced and the incidence and severity of intestinal neoplasia recorded.
Results
No significant differences between the control genotypes and the double mutant genotypes were recorded indicating that deficiency of PPARδ does not modify impaired mismatch repair induced neoplasia.
Conclusion
In contrast with the previously observed acceleration of intestinal neoplasia in the context of the Apc Min/+ mouse, PPARδ deficiency does not alter the phenotype of mismatch repair deficiency. This data supports the notion that PPARδ is not required for adenoma formation and indicate that any pro-tumourigenic effect of PPARδ inactivation may be highly context dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Peroxisome proliferator-activated receptors (PPARs) are lipid-activated transcription factors exerting several functions in development and metabolism. There are 3 major PPAR isoforms; α,β/δ and γ and each has distinct agonist binding properties and different regulation of expression resulting in distinct distributions [1]. The roles for PPARδ appear diverse and are not fully characterised, but include the regulation of lipid uptake, metabolism, and regulation of proliferation and differentiation within many different cell types. Consequently, the therapeutic regulation of PPARδ activity using selective agonists has been proposed for many varied disorders including: lung cancer [2], experimental autoimmune encephalomyelitis [3], skin disorders such as psoriasis and cancer [4], type 2 diabetes [5], metabolic syndrome [6] and dyslipidemias [7, 8].
Although a great deal of evidence exists to show that PPARδ is potentially important in intestinal tumourigenesis, it is currently unclear whether PPARδ attenuates or potentiates this condition. Several studies have shown that activation of PPARδ or increased PPARδ levels are associated with increased intestinal neoplasia in a variety of tissues [9–13]. Also, two studies have shown that PPARδ deficiency suppressed or had no role upon tumourigenesis [14, 15]. Taken together these analyses suggest that PPARδ potentiates colon carcinogenesis. However, two independent approaches have recently suggested that PPARδ expression in vivo is not up-regulated in intestinal adenomas. Firstly a study of matched human tumour and normal intestinal tissues found reduced levels of PPARδ expression in the tumours [16]. Second several studies using the classical mouse model of intestinal neoplasia (the ApcMin mouse), found either no change, or reduced PPARδ expression in colonic adenomas compared to normal tissues in this model [17–20], while a recent publication has shown that ligand activation of PPARδ attenuates chemically induced colon carcinogenesis [20]. Furthermore, it has been demonstrated that PPARδ deficiency does not suppress intestinal tumourigensis in Apc Min/+ mice, but indeed promotes some aspects of intestinal neoplasia [17, 20, 21]. These data suggest that PPARδ attenuates colon carcinogenesis.
Thus, given the obvious disparity within the published literature, we have utilized another mouse neoplasia model to further investigate the role of PPARδ in intestinal tumourigenesis. Mice possessing null mutations in the mismatch repair (MMR) gene Mlh1 are prone to develop different types of neoplasia and present with lymphomas and intestinal tumours but show increased mutation in all tissues examined [22]. Likewise, germline mutations in the human MLH1 gene are involved in Hereditary non-polyposis colorectal cancer [23]. We have inter-crossed PPARδ null mice [24] to the mismatch repair defective Mlh1 null mice [25] and produced cohorts for the different combinations of the genotypes in order to investigate the consequences of impaired MMR induced tumourigenesis in the context of PPARδ deficiency.
Methods
Mice were generated from sixth generation C57BL 6 backcrossed mice. All experiments were performed according to UK Home Office regulations. Inter-crossing the PPARδ null mice (PPARδ-/-) to the mismatch repair defective Mlh1 null mice (Mlh1 -/-) produced cohorts containing a minimum of 16 animals for each genotype combination of interest (Mlh1 -/- PPARδ+/+ , Mlh1 -/- PPARδ+/- , Mlh1 -/- PPARδ-/-). Littermates were genotyped by PCR on DNA from tail biopsy and allowed to age and monitored for signs of intestinal tumours. Animals were harvested when they displayed overt symptoms of disease, and tumour burden was ascertained upon dissection.
Results and discussion
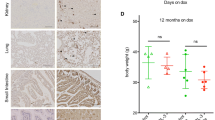
Through inter-crossing the PPARδ null mice (PPARδ-/-) to the mismatch repair defective Mlh1 null mice (Mlh1 -/-) we produced cohorts containing a minimum of 16 animals for each genotype combination of interest and examined survival, intestinal adenoma multiplicity and tumour size at death for each of the cohorts (Figure 1). We find that, although the mean age at death of Mlh1 -/- PPARδ-/- mice was 248.1 days compared to 203.5 days in controls, this was not statistically different (Figure 1a, p = 0.34 Log-Rank test). Furthermore, the predisposition to lymphomagenesis was not significantly altered between the Mlh1 -/- PPARδ+/+ and Mlh1 -/- PPARδ-/- cohorts (Figure 1b). The possibility remains that there may be subtle effects of PPARδ deficiency that lie below the detection threshold of the present study, a possibility that would be resolved by a substantially increased cohort analysis.
Survival of and tumour multiplicity within each of the cohort. Survival of (A), tumour type (B), intestinal adenoma number (C) and size (D) in Mlh1 -/- PPARδ+/+, Mlh1 -/- PPARδ+/-, and Mlh1 -/- PPARδ-/- mice. KaplañMeier plot (A) showing the age of each animal at the time of death: Solid line, Mlh1 -/- PPARδ+/+ (n = 20); dashed line, Mlh1 -/- PPARδ+/- (n = 20); broken line, Mlh1 -/- PPARδ-/- (n = 16). Pie charts (B) indicate the type identified at death in each mouse. Grey slice, mice with lymphoma; white slice, mice with akanthoma; striped slice, mice with dysplastic cysts; black slice, mice with Hemangiosarcomas. Box plots showing the number (combining small and large intestine) (C) or size (D) of intestinal adenomas per mouse at death. Intestinal preparations were collected from animals, fixed and tumours in the small and large intestine were counted and sized (expressed as width by length). Boxes encompass the first quartile (at bottom) to the third quartile (at top) of the data set; the horizontal boxed line represents the median; the asterisks represent outliers.
Contrary to the finding from the APCMin/+ PPARδ-/- intercross, which indicated that PPARδ deficiency promotes some aspects of intestinal neoplasia [17, 20, 21], no significant differences were discovered between either number or size of intestinal adenomas in the Mlh1 -/- PPARδ+/+ and Mlh1 -/- PPARδ-/- cohorts (Figure 1c, d). This was confirmed in both the small intestinal and large intestinal tumours (P > 0.1, Mann Whitney U test), although notably the group sizes in both these analyses were small.
To assess the nature of the Mlh1 -/- PPARδ+/+ and Mlh1 -/- PPARδ-/- aberrant intestinal tissues, β-catenin immunohisochemistry was performed and again no differences were observed between the two genotypes (data not shown). Up-regulation of β-catenin in large intestinal adenomas was observed in both genotypes. Furthermore in the small intestine, in addition to lesions displaying increased β-catenin levels, it was also possible to identify a small subset of lesions that maintain normal levels of β-catenin, a phenomenon previously described within defective MMR intestine [26]. Thus, the induction of dysplastic lesions and the deregulation of β-catenin in the intestine occurring as a consequence of defective MMR is not dependent upon PPARδ status and has no requirement for PPARδ.
We therefore find that loss of PPARδ does not alter tumour incidence or morphology of tumourigenesis on the Mlh1 -/- background. Given that MMR deficiency is considered to lead to a mutator phenotype which thereby increases the rate of mutation of elements of the Wnt pathway, our findings argue that PPARδ deficiency does not enhance or diminish such a mutator phenotype. By implication, the increased adenoma formation seen in the Apc Min/+ PPARδ-/- mutants may arise through PPARδ dependent modification of the frequency of gene conversion events that are known to underlie the majority of polyp formation in the Apc Min/+ mouse [27]. Alternatively, subsequent to the gene conversion events, PPARδ deficiency may alter the resulting cellular signalling/gene expression pathways which permit cell survival and tumour progression. Our data again apparently contradict the observed acceleration of adenoma formation following agonist activation of PPARδ[11], as this predicts reduced adenoma burden in the absence of PPARδ. However, PPARδ deficiency (as assessed through the PPARδ null mutation) may not necessarily be functionally opposite of ectopic PPARδ activation as it is possible that any alteration in the levels of PPARδ activity, whether a reduction or increase, have different consequences dependant on the genetic background or indeed tissue being studied. In terms of evaluating the potential deleterious pro-tumourigenic effect of PPARδ deficiency, our data suggests that this only accelerates adenoma formation in certain defined genetic settings (e.g. Apc Min/+), and does not generally enhance adenoma formation per se. This genetic dependency may reflect differences in the mutational events occurring in the Mlh1 and Apc mutant backgrounds. By analogy, any potential danger in the therapeutic use of PPARδ agonists to activate PPARδ may critically depend upon subtle changes in the underlying genetic predisposition.
Conclusion
In summary, we show that PPARδ deficiency does not alter either lymphomagenesis or adenoma formation in mice with defective MMR. This data again support the notion that PPARδ is not required for adenoma formation and indicate that any pro-tumourigenic effect of inactivation may be highly context dependent. Thus, in the context of a defective MMR environment, PPARδ agonism is unlikely to be pro tumourigenic.
References
Kliewer SA, Xu HE, Lambert MH, Willson TM: Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res. 2001, 56: 239-63. 10.1210/rp.56.1.239.
Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, Senba H, Suzuki K, Asano R, Yamada K, Yano T: Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005, 579: 3829-3836. 10.1016/j.febslet.2005.06.004.
Polak PE, Kalinin S, Dello Russo C, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL: Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005, 168: 65-67. 10.1016/j.jneuroim.2005.07.006.
Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM: PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006, 13 (1): 53-60. 10.1038/sj.cdd.4401713.
Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA: Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta. 2005, 1740: 313-317. Review,
Berger JP, Akiyama TE, Meinke PT: PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005, 26: 244-251. 10.1016/j.tips.2005.03.003. Review,
Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM: Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003, 113: 159-170. 10.1016/S0092-8674(03)00269-1.
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q: Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004, 10: 1245-1250. 10.1038/nm1116.
He TC, Chan TA, Vogelstein B, Kinzler KW: PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999, 99: 335-345. 10.1016/S0092-8674(00)81664-5.
Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN: Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci USA. 2000, 97: 13275-13280. 10.1073/pnas.97.24.13275.
Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN: Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004, 10: 245-247. 10.1038/nm993.
Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN: Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004, 64: 3162-3170. 10.1158/0008-5472.CAN-03-2760.
Roy HK, Karolski WJ, Ratashak A: Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int J Cancer. 2001, 92: 609-615. 10.1002/ijc.1226.
Park BH, Vogelstein B, Kinzler KW: Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA. 2001, 98: 2598-2603. 10.1073/pnas.051630998.
Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM: Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA. 2002, 99: 303-308. 10.1073/pnas.012610299.
Notterman DA, Alon U, Sierk AJ, Levine AJ: Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001, 61: 3124-3130.
Reed KR, Sansom OJ, Hayes AJ, Gescher AJ, Winton DJ, Peters JM, Clarke AR: PPARdelta status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004, 23: 8992-8996. 10.1038/sj.onc.1208143.
Orner GA, Dashwood W-M, Blum CA, Diaz GD, Dashwood RH: Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003, 24: 263-267. 10.1093/carcin/24.2.263.
Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, Moretto J, Mendes F, Smith AP, Bennington JL, Moore D, Lee NM: Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004, 64: 3694-3700. 10.1158/0008-5472.CAN-03-3264.
Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM: Ligand activation of peroxisome proliferator-activated receptor beta inhibits colon carcinogenesis. Cancer Res. 2006, 66: 4394-4401. 10.1158/0008-5472.CAN-05-4277.
Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM: Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nat Med. 2004, 10: 481-483. 10.1038/nm1026.
Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronner CE, Zheng B, Gordon M, Reneker J, Arnheim N, Shibata D, Bradley A, Liskay RM: Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat Genet. 1998, 18: 276-279. 10.1038/ng0398-276.
Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A, Tannergård P, Bollag RJ, Godwin AR, Ward DC, Nordensk M, Fishel R, Kolodner R, Liskay M: Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994, 368: 258-261. 10.1038/368258a0.
Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ: Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol. 2000, 20: 5119-5128. 10.1128/MCB.20.14.5119-5128.2000.
Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM: Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nature Genet. 1996, 13: 336-342. 10.1038/ng0796-336.
Rungtiva Kongkanuntn, Vivien Bubb, Owen Sansom, Andrew Wyllie, David Harrison, Alan Clarke: Dysregulated expression of -catenin marks early neoplastic change in Apc mutant mice, but not all lesions arising in Msh2 deficient mice. Oncogene. 1999, 18: 7219-7225. 10.1038/sj.onc.1203181.
Haigis KM, Dove WF: A Robertsonian translocation suppresses a somatic recombination pathway to loss of heterozygosity. Nat Genet. 2002, 33: 33-9. 10.1038/ng1055.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/6/113/prepub
Acknowledgements
We thank Derek Scarborough and Mark Bishop for technical assistance and Michael Liskay for Mlh1 mutant mice. This work was supported by a programme grant from Cancer Research UK, a grant from the CR-UK New Targets Initiative and the Wales Gene Park.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
KRR, OJS and AJH participated in the animal studies; KRR and OJS carried out the statistical analysis and data presentation; ARC, JMP and AJG conceived the study, and participated in its design and coordination. KRR drafted the manuscript and all authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Reed, K.R., Sansom, O.J., Hayes, A.J. et al. PPARδ status and mismatch repair mediated neoplasia in the mouse intestine. BMC Cancer 6, 113 (2006). https://doi.org/10.1186/1471-2407-6-113
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-6-113