Abstract
Background
The possible participation of endogenous islet catecholamines (CAs) in the control of insulin secretion was tested.
Methods
Glucose-induced insulin secretion was measured in the presence of 3-Iodo-L-Tyrosine (MIT), a specific inhibitor of tyrosine-hydroxylase activity, in fresh and precultured islets isolated from normal rats. Incubated islets were also used to measure CAs release in the presence of low and high glucose, and the effect of α2-(yohimbine [Y] and idazoxan [I]) and α1-adrenergic antagonists (prazosin [P] and terazosin [T]) upon insulin secretion elicited by high glucose.
Results
Fresh islets incubated with 16.7 mM glucose released significantly more insulin in the presence of 1 μM MIT (6.66 ± 0.39 vs 5.01 ± 0.43 ng/islet/h, p < 0.02), but did not affect significantly the insulin response to low glucose. A similar enhancing effect of MIT upon insulin secretion was obtained using precultured islets devoid of neural cells, but absolute values were lower than those from fresh islets, suggesting that MIT inhibits islet rather than neural tyrosine hydroxylase. CAs concentration in the incubation media of fresh isolated islets was significantly higher in the presence of 16.7 than 3.3 mM glucose: dopamine 1.67 ± 0.13 vs 0.69 ± 0.13 pg/islet/h, p < 0.001, and noradrenaline 1.25 ± 0.17 vs 0.49 ± 0.04 pg/islet/h, p < 0.02. Y and I enhanced the release of insulin elicited by 16.7 mM glucose while P and T decreased such secretion.
Conclusion
Our results suggest that islet-originated CAs directly modulate insulin release in a paracrine manner.
Similar content being viewed by others
Background
Insulin secretion in response to glucose is modulated by neural [1], hormonal and paracrine [2] factors that bestow great precision to the stimulus: secretion coupling process. Catecholamines (CAs) contribute to this mechanism by exerting a direct and dual effect on the B-cell to induce either inhibition or stimulation of insulin secretion through their interaction with α2 or α1 and β2 adrenergic receptors, respectively [3, 4]. Since islet B-cells have more α2 than α1 and β2 adrenergic receptors, physiological concentrations of CAs will bind mainly to the α2 population receptors and thus inhibit insulin secretion [5–8].
Although the effect of the sympathetic nervous system (SNS) and of circulating CAs on islet physiology has been studied in both normal and pathological states, little is known about the possible participation of endogenously-generated CAs in the control of islet function. Islet cells have been shown to contain enzymes involved both in the synthesis of CAs – tyrosine hydroxylase (TH) [9, 10] and dihydroxyphenylalanine (DOPA) decarboxylase [11, 12] – and in their inactivation – monoamine oxidase [13, 14].
We have recently measured TH activity in normal rat isolated islets, showing an increase in the enzyme's activity, and a decrease of CAs content and insulin release in rats fed only with carbohydrates [15]. In these experiments, comparable TH activity values were measured in islets isolated from either control or solarectomized rats, thus suggesting that the enzyme activity was of islet rather than neural origin.
In order to gain evidence about the possible modulatory role of CAs of endogenous islet origin as paracrine hormonal regulators of insulin secretion, we have currently studied: a) The effect of 3-Iodo-L-Tyrosine (MIT) – a selective drug usually used for the acute inhibition of TH in neuroendocrine tissues [16] – on the insulin secretory response of either fresh or precultured isolated islets to glucose, and b) The effect of specific α2- or α1-adrenergic receptor antagonists upon glucose-induced insulin secretion in isolated islets, and c) The release of endogenous CAs by endocrine islet cells.
Methods
Chemicals and drugs
Collagenase was obtained from Serva Feinbiochemica, (Heidelberg, Germany), while bovine serum albumin, fraction V and other reagents of the purest available grade were purchased from Sigma Chemical Co. (St. Louis, USA).
Animals and islet isolation
Male Wistar rats (180–200 g) were used as a source of islets. They were fed ad libitum and kept under conditions of controlled temperature and lighting (12 h light and 12 h dark). After enzymatic digestion with collagenase, pancreases were repeatedly washed and the islets rapidly hand-picked with siliconized glass pipettes under a dissecting microscope [17].
Islet culture
Isolated islets were cultured at 37°C in a humidified atmosphere for 24 h in plastic Petri dishes (NUNC) with RPMI 1640 supplemented with Hepes (20 mM), CO3HNa (4.1 mM), penicillin, streptomycin (100.000 U/I and 100 mg/l, respectively), 10% newbom calf serum [18], and 3 mM glucose. The pH was adjusted to 7.4. After the first 24-h period, the culture medium was replaced by fresh medium with the same glucose concentration; afterwards, the medium was changed every second day until accomplishing the total one-week culture period.
Islet incubation
Groups of 5 fresh or precultured isolated islets were incubated for 30 min at 37°C in 600 μl Krebs-Ringer-bicarbonate buffer with 1% (w/v) bovine-serum albumin, Trasylol™ (400 IU/ml), and either 3.3 or 16.7 mM glucose, with or without MIT (1 μM) [19]. The medium had been previously gassed with a mixture of 5% CO2:95% O2 (v/v) to adjust the pH to 7.4.
Separately, groups of 5 fresh isolated islets were incubated for 60 min in the presence of α2-(yohimbine [Y, 0.1 and 1 μM] or idazoxan [I, 1 μM]) and α1- (prazosin [P, 0.1 and 0.5 μM] or terazosin [T, 0.1 and 0.5 μM]) adrenergic antagonists. In all cases, aliquots from the medium were obtained at the end of the incubation period and kept frozen until insulin determination by radioimmunoassay [20].
CAs quantitation
Another group of islets was incubated for 30 min in the presence of 3.3 and 16.7 mM glucose. Following that incubation period, CAs were measured in the incubation media after partial purification by batch alumina extraction, separated by reverse-phase high-pressure liquid chromatography on a 4.6 X 250-mm Zorbax Rp C18 column (DuPont), and quantified amperometrically with a triple-electrode system (ESA, Bedford, NA) by measuring the current produced upon exposure of the column effluent to first oxidizing and then reducing potentials in series [21].
Data analysis
For the statistical evaluation of the data, we employed both variance analysis and the paired Student t-test.
Results
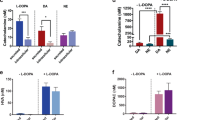
The addition of 1 μM MIT to the incubation medium of fresh isolated islets enhanced significantly the release of insulin elicited by 16.7 mM glucose (Fig. 1, upper pannel; p < 0.02). Such effect was not observed in the presence of a low glucose concentration. Similarly, MIT enhanced significantly the release of insulin elicited by high glucose in precultured islets (Fig. 1, lower pannel; p < 0.05). In this case, however, the absolute insulin concentration values measured in the incubation media were lower than those measured in the experiments performed with fresh islets.
Effect of MIT upon insulin secretion elicited by 16.7 mM glucose in fresh (upper pannel) and precultured (lower pannel) isolated islets of Langerhans. Each bar represents the mean ± SEM and refers to 3 separate experiments and 12 individual determinations. P values were <0.02 and <0.05 for fresh and precultured islets, respectively.
The assessment of CAs in the incubation media from freshly isolated islets showed a glucose-induced increase in the concentration of dopamine and noradenaline (3.3 vs 16.7 mM glucose): dopamine 0.69 ± 0.13 (n = 4) vs 1.67 ± 0.13 (n = 6) pg/islet/h; p < 0.001, and noradrenaline 0.49 ± 0.04 (n = 3) vs 1.25 ± 0.17 (n = 3) pg/islet/h; p < 0.02.
Addition of selective α2- and α1- CA receptor antagonists to the medium significantly affected the insulin released by islets incubated with glucose: α2-antagonists Y (0.1 and 1 μM) and I (1 μM) enhanced significantly the release of insulin in response to 16.7 mM glucose (p < 0.05,0.001, and 0.001, respectively; Fig. 2, upper pannel), while α1-antagonists P and T (0.1 and 0.5 μM) decreased it significantly (P, p < 0.02 and 0.005, and T, p < 0.001, 0.001, respectively; Fig. 2, lower pannel). Excepting I, the effect of the antagonists was dose-dependent: insulin release increased 82, 171 and 895 % in the presence of 0.1 and 1 μM Y and 1 μM I, respectively, while it decreased 40 and 49% with 0.1 and 0.5 μM P, and 51 and 45% in the presence of T at the same concentrations.
Effect of α2- (Y and I, upper pannel) and α1- (P and T, lower pannel) adrenergic antagonists upon insulin secretion in isolated islets of Langerhans incubated in the presence of 16.7 mM glucose. Each bar represents the mean ± SEM and refers to 3 separate experiments and 10 individual determinations. The concentrations of Y, I, P, and T are shown under each bar. P values: a vs b, p < 0.05; a vs c and d, p < 0.001; e vs f, p < 0.02; e vs g, p < 0.005; e vs h and i, p < 0.001.
Discussion
In our experiments, MIT addition to the incubation medium induced a significant increase in glucose-induced insulin release, either from fresh or precultured isolated islets. Incubation of different tissues with MIT, for even shorter periods than the one we used, blocks significantly the activity of TH, the first limiting step enzyme in CA biosynthesis [16]. The presence of immunochemically demonstrable TH in α and B islet cells has been reported in fetal-mouse pancreases [9], and in the B-cells of several adult rodents (Sprague Dawley rats, two strains of mice, and pigmented guinea pigs) [10]. Further, we have measured TH activity in the endocrine pancreas of normal adult rats, recording comparable TH activity values in islets isolated from either control or solarectomized rats (330 ± 40 vs 300 ± 80 pmol/mg protein/h), indicating that the TH activity measured was of islet rather than neural origin [15]. We could then assume that as in other tissues, TH inhibition by MIT decreases CA production rate in the islets, and consequently its availability [22]. Following this reasoning, it could be argued that, at least in our model, CAs synthesis (or availability) participates in the regulation of insulin secretion.
Our cultured islets would be devoid of adrenergic innervation since sympathetic neurons maintained in a culture medium with a Ca2+ concentration above 2 mM (condition employed in our islet culture) do not survive more than 6–8 days [23]. Since the blocking effect of MIT was also observed using cultured islets, we can assume that islet rather than neural CAs biosynthesis is involved in this modulatory process of insulin secretion. We cannot however completely exclude the possibility that MIT could affect insulin secretion either directly or by modifying other processes than CAs biosynthesis.
There is considerable evidence in the literature for the possible presence of CAs biosynthesis in islet tissue: a) We have recently reported the immunocytochemical identification of DOPA decarboxylase in glucagon-containing cells of normal adult rat islets [12]; b) Oomori et al. have reported the presence of dopamine beta-hydroxylase immunoreactivity in rat pancreatic cells which – according to their features – were neither neural nor endocrine cells, and suggested that these cells may release norepinephrine (NE) to adequate stimuli [24]; c) Lundquist et al. detected dopamine in islets obtained from chemically-sympathectomized mice by means of high-performance liquid chromatography [25]; and d) Falck and Hellman showed that B-cells from guinea-pig islets stored monoamines [26, 27] despite their failure to observe sympathetic nerves in the endocrine pancreas of those animals.
CAs inhibit glucose-induced insulin secretion by interacting with α2-adrenoceptors in B-cells, but could stimulate insulin secretion by two differents actions: activation of B-cell α1 and β2 adrenoceptors [28], and a direct action on α-cells, mediated by α2 and β2 adrenoceptors, which stimulate glucagon secretion [29, 30]. We have currently observed that high glucose induced an increase in the release of CAs from normal rat isolated islets to the incubation media. We have also found that different α2- and α1-adrenergic antagonists added to the incubation media significantly enhanced and decreased, respectively, the secretion of insulin elicited by glucose in a dose-dependent manner. The adrenoceptor blockade by Y increased the secretion of insulin by 82 and 172% while I enhanced such secretion by 895%. Conversely, P and T diminished insulin secretion by 40–49% and 51–45%, respectively, with the doses currently employed. As shown by other authors, the uneven effect of adrenergic antagonists could be ascribed to the relative abundance or activity of α2-adrenoceptors in B-cells, as compared with α1- and β2-adrenoceptors [5–8]. On the other hand, Schuit et al. showed that P was ineffective in both a and B-cells [31]; such discrepancy with our results can be attributed to the method used in each case.
Since we did not add CAs to the incubation media, it could be assumed that the effect of the antagonists was due to an interference with the action of CAs, elaborated and co-released with insulin by endocrine islet cells. The results presented here as well our previous report on the glucose-induced release of CAs by isolated rat islets [32] support this assumption. On account of these results, therefore, we postulate that islet-originated CAs could directly modulate the release of insulin in a paracrine hormonal manner.
References
Woods SC, Porte D: Neural control of the endocrine pancreas. Physiol Rev. 1974, 54: 596-619.
Pipeleers DG, Schuit FC, in't Veld PA, Maes E, Hooghe-Peters EL, van de Winkel M, Gepts W: Interplay of nutrients an hormones in the regulation of insulin release. Endocrinology. 1985, 117: 824-833.
Skoglund G, Lundquist I, Ahren B: Effects of alpha 1- and alpha 2-adrenoceptor stimulation and blockade on plasma insulin levels in the mouse. Pancreas. 1986, 1: 415-420.
Ahren B, Taborsky GJ, Porte D: Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986, 29: 827-836.
Ahren B, Lundquist I, Jarhult J: Effects of alpha 1-, alpha 2- and beta-adrenoceptor blockers on insulin secretion in the rat. Acta Endocrinol (Copenh). 1984, 105: 78-82.
Nakaki T, Nakadata T, Ishii K, Kato R: Postsynaptic alpha 2-adrenergic receptors in isolated rat islets of Langerhans: inhibition of insulin release and cyclic 3':5'-adenosine monophosphate accumulation. J Pharmacol Exp Ther. 1981, 216: 607-612.
Lacey RJ, Chan SL, Cable HC, James RF, Perrett CW, Scarpello JH, Morgan NG: Expression of alpha 2- and beta-adrenoreceptor subtypes in human islets of Langerhans. J Endocrinol. 1996, 148: 531-543.
Ahren B: Autonomic regulation of islet hormone secretion – implications for health and disease. Diabetologia. 2000, 43: 393-410. 10.1007/s001250051322.
Teitelman G, Alpert S, Polak JM, Martínez A, Hanahan D: Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon, and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993, 118: 1031-1039.
Iturriza FC, Thibault J: Immunohistochemical investigation of tyrosine-hydroxylase in the islets of Langerhans of adult mice, rats, and guinea pigs. Neuroendocrinology. 1993, 57: 476-480.
Teitelman G, Lee JK, Alpert S: Expression of cell type-specific markers during pancreatic development in the mouse: implications for pancreatic cell lineages. Cell Tissue Res. 1987, 250: 435-439.
Borelli MI, Villar MJ, Orezzoli A, Gagliardino JJ: Presence of DOPA decarboxylase and its localisation in adult rat pancreatic islet cells. Diabetes Metab. 1997, 23: 161-163.
Stenström A, Panagiotidis G, Lundquist I: Monoamine oxidase (MAO) in pancreatic islets of the mouse: some characteristics and the effect of chemical sympathectomy. Diabetes Res. 1989, 11: 81-84.
Stenström A, Lundquist I: Monoamine oxidase (MAO) A and B in pancreatic islets from the mouse. Biogenic Amines. 1990, 6: 547-555.
Gagliardino JJ, Borelli MI, Rubio M: Possible role of endogenous islet catecholamines in the paracrine control of insulin secretion. Diabetologia. 1996, 39 (Suppl 1): A123-
Smythe GA, Bradshaw JE: Different acute effects of the tyrosine hydroxylase inhibitors alpha-methyl-p-tyrosine and 3-yodo-L-tyrosine on hypothalamic noradrenaline activity and adrenocorticotrophin release in the rat. Aust J Biol Sci. 1983, 36: 519-523.
Lacy PE, Kostianovsky M: Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967, 16: 35-39.
Brunstedt J, Nielsen JH: Direct long-term effect of hydrocortisone on insulin and glucagon release from mouse pancreatic islets in tissue culture. Acta Endocrinol (Copenh). 1981, 96: 498-504.
Gagliardino JJ, Nierle C, Pfeiffer EF: The effect of serotonin on in vitro insulin secretion and biosynthesis in mice. Diabetologia. 1974, 10: 411-414.
Herbert V, Lau KS, Gottlieb CW, Bleicher SJ: Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965, 25: 1375-1384.
Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ: Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986, 32: 2030-2033.
Levitt M, Spector S, Sjoerdsma A, Udenfriend S: Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea pig heart. J Pharmacol Exp Ther. 1965, 148: 1-8.
Wakade TD, Przywara DA, Kulkarni JS, Wakade AR: Morphological and transmitter release properties are changed when sympathetic neurons are cultured in low Ca2+ culture medium. Neuroscience. 1995, 67: 967-976. 10.1016/0306-4522(95)00097-3.
Oomori Y, luchi H, Ishikawa K, Satoh Y, Ono K: Immunocytochemical study of tyrosine hydroxylase and dopamine-beta-hydroxylase immunoreactivities in the rat pancreas. Histochemistry. 1994, 101: 313-323.
Lundquist I, Ahren B, Hansson C, Hakanson R: Monoamines in pancreatic islets of guinea pig, hamster, rat, and mouse determined by high performance liquid chromatography. Pancreas. 1989, 4: 662-667.
Falck B, Hellman B: A fluorescent reaction for monoamines in the insulin producing cells of the guinea pig. Ada Endocrinol (Copenh). 1964, 1: 133-138.
Falck B, Hellman B: Evidence for the presence of biogenic amines in pancreatic islets. Experientia. 1963, 19: 139-140.
Ahrén B, Lundquist I: Effects of selective and non-selective beta-adrenergic agents on insulin secretion in vivo. J Pharmacol. 1981, 71: 93-104. 10.1016/0014-2999(81)90390-3.
Chan SL, Perrett CW, Morgan NG: Differential expression of alpha 2-adrenoceptor subtypes in purified rat pancreatic islet A- and B-cells. Cell Signal. 1997, 9: 71-78. 10.1016/S0898-6568(96)00096-4.
Lacey RJ, Berrow NS, Scarpello JH, Morgan NG: Selective stimulation of glucagon secretion by beta2-adrenoceptors in isolated islets of Langerhans of the rat. Br J Pharmacol. 1991, 103: 1824-1828.
Schuit FC, Pipeleers DG: Differences in adrenergic recognition by pancreatic A and B cells. Science. 1986, 232: 875-877.
Borelli MI, Armando I, Barontini M, Gagliardino JJ: Effect of islet catecholamine release on insulin secretion. Diabetologia. 1997, 40(Supl. 1): A107-
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6823/1/1/prepub
Acknowledgements
This work was partially supported with funds provided by CONICET from Argentina. The authors thank Dr I Armando and Dr M Barontini (CEDIE – Centro de Investigaciones Endocrinológicas, CONICET) for catecholamine determinations, A Díaz for technical assistance, and A Di Maggio for careful secretarial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Borelli, M.I., Gagliardino, J.J. Possible modulatory effect of endogenous islet catecholamines on insulin secretion. BMC Endocr Disord 1, 1 (2001). https://doi.org/10.1186/1472-6823-1-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6823-1-1