Abstract
Background
Malaria is the third most prevalent cause of infectious disease in the world. Resistance of the parasite to classical drugs makes the discovery of new and effective drugs more urgent. The oxidized derivative of hydroxy-cis terpenone (OHCT) is a synthetic molecule that is not toxic to cultured human liver cells at concentrations as high as 60 μM and inhibits activity of cytochrome P450s that metabolize many drugs.
Methods
OHCT activity against chloroquine-sensitive and -resistant strains of Plasmodium falciparum, and a P. falciparum clone that is partially resistant to artemisinin was assayed in vitro.
Results
OHCT at nanomolar concentrations was effective against all intraerythrocytic stages of P. falciparum and exhibited activity in vitro against both chloroquine-sensitive and -resistant strains of P. falciparum as well as a P. falciparum clone that is partially resistant to artemisinin. Moreover, OHCT exhibited potent activity against gametocytes, the form that is transmitted by mosquitoes and essential for the spread of malaria.
Conclusion
OHCT displays strong growth inhibitory activity against all stages of P. falciparum and no evidence of toxicity to human cells in culture. It is easily synthesized and has the potential for inhibiting metabolism of drugs used in combination therapies.
Similar content being viewed by others
Background
Anti-malarial drug resistance is the major challenge to reducing mortality caused by Plasmodium falciparum infection. Parasite resistance has caused some of the least expensive, traditional anti-malarial drugs to be ineffective. Because there is concern that resistance will emerge against the current first-line drugs, such as the artemisinin-based combination therapy, there is currently great interest in discovering the next generation of anti-malarial drugs.
Terpenes isolated from the roots of several plant families have a broad range of biological activities, including anti-microbial and anti-plasmodial activity [1, 2]. It has been shown that a number of terpenes and terpene derivatives isolated from a variety of sources ranging from plants to marine fungi kill P. falciparum parasites [3–6]. Synthetic cis-terpenones, including the oxidized derivative of hydroxy-cis terpenone (OHCT), are synthetic analogues of natural terpene quinone methides that have a broad spectrum of biological activities [7].
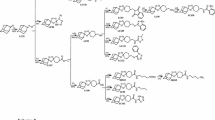
Synthesis of HCT and OHCT was described previously [7, 8]. It was previously shown that OHCT protects human liver cells against aflatoxin and inhibits activity of liver microsomal cytochrome P450 3A4 [9]. This enzyme not only activates toxins, such as aflatoxin [10], but also contributes to the degradation of anti-malarials, such as artemisinin, thus suggesting that OHCT might increase the half-life of current anti-malarials [11]. Based on the anti-plasmodial effects of terpenes [1, 2], the anti-malarial effects of OHCT were investigated.
Methods
Culture of Plasmodium falciparum
Four clones of P. falciparum, the chloroquine-sensitive clone HB3, the two chloroquine-resistant clones, FCR3-Gambia and Dd2Nm-Indochina, and the laboratory-induced artemisinin-resistant 7G6R, were cultured by a method modified from that of Trager and Jensen [12] in a 5% CO2 atmosphere at 37°C.
In vitro selection of artemisinin-resistant parasite lines
The Plasmodium falciparum 7G8 clone was used for the selection of artemisinin (ART) resistance. Drug resistance selection experiments were performed as previously described [13]. After the parasitaemia reached 2–3%, frozen stocks of ART-selected parasites were prepared with Glycerolyte.
Anti-malarial in vitro activity
Chloroquine diphosphate, artemisinin (Sigma Aldrich), and OHCT were dissolved in medium (RPMI 1640), ethanol and DMSO, respectively. Compounds were further diluted in medium to give a final concentration of 0.1% ethanol or DMSO. Solutions were checked to determine that precipitation did not occur under these conditions. Parasite growth was estimated by microscopic observation of Giemsa-stained blood smears and the parasite lactate dehydrogenase (pLDH) activity [14]. Effective concentrations that prevented survival of parasites were determined. Unless indicated, all results presented are the means of at least three independent experiments, and each experiment was performed in duplicate. Assays were performed for 48 h at concentrations ranging from 25 nM-50 μM.
Production of Plasmodium falciparum gametocytes
The P. falciparum Dd2Nm clone was cultured in medium supplemented with 0.5% Albumax II, and O+ human erythrocytes. The culture was treated with PIGPA solution (50 mM hydrogen phosphate and 5 mM adenine in 0.9% (w/v) NaCl, pH7.2 and 50 mg/L of hypoxanthine) as previously described [15]. Gametocytes were cultured in 2% erythrocyte suspension and started with 1% parasitaemia, containing mostly ring stage trophozoite after synchronization with sorbitol treatment. The medium was replaced on days 4, 6 and 8. After sorbitol treatments on day 9, 10 and 11, the number of asexual parasites was reduced to 99%. For sorbitol treatment, 2.5 volumes of 5% (w/v) sorbitol were added once a day. Pure gametocyte cultures of P. falciparum Dd2Nm clone were achieved on day 11 with an average number of gametocytes of 315 and 282 per 8,500 erythrocytes. These gametocytes that were used in the drug studies were in the range of 19% stage I, 21% stage II, 39% stage III and 21% stage IV.
Gametocytocidal effects of OHCT against P. falciparum in vitro
Following sorbitol treatment, an aliquot of 150 μL of a 2.0% erythrocyte suspension containing gametocytes was transferred to a 96-well plate containing 5 μL of drugs in each well at a concentration ranging from 50 μM-25 nM for another 48 h. Thin blood films were prepared and gametocytes were counted per 7,000–10,000 erythrocytes. The effect of each drug concentration was assessed in two independent experiments in duplicate. The gametocytocidal action of each drug was recorded and the IC50 and IC90 were determined.
Time of OHCT action on the erythrocytic life cycle
Plasmodium falciparum cultures were synchronized with 5% sorbitol and the Percoll-Sorbitol method. Dilutions of OHCT, chloroquine, and artemisinin were prepared. After synchronization, the parasites were plated at the ring stage in 96-well plates. Cultures were treated with OHCT or vehicle for 8 hours, and parasite viability was measured by the parasite lactate dehydrogenase (pLDH) activity [14]. The cultures were centrifuged at 10,000 g (Fisher scientific), washed in incomplete media and replaced with fresh drug every hour to remove dead parasites.
Results
Anti-malarial activity of OHCT
The inhibitory concentration of OHCT was determined by incubating the four P. falciparum clones with OHCT, chloroquine and artemisinin, respectively. As shown in Table 1, OHCT inhibited survival of all P. falciparum clones examined with an IC50 ranging from 3.9 – 93 nM, and its IC90 was 17 – 523 nM. OHCT and chloroquine exhibited similar potency against clone Dd2Nm. Although OHCT had a lower IC50 than chloroquine against the chloroquine-resistant clone FCR3, it was less potent than chloroquine against the chloroquine-sensitive clone HB3. Despite selection of 7G8R parasites that could survive in artemisinin, the resistant parasites that were thawed and cultured for the drug assay were consistently killed by artemisinin at low nanomolar concentrations. It should be noted that induced resistance to artemisinin in this clone is reported to be unstable [13]. OHCT at low nanomolar concentrations also inhibited survival of the artemisinin resistant 7G8R clone. Thus, OHCT is effective against all P. falciparum clones examined and might work well in combination with established anti-malarials, such as artemisinin and chloroquine.
OHCT at 1 – 16 μM kills most parasites within 24 hours (data not shown). The time course of action of 1 μM OHCT was investigated with the P. falciparum clone Dd2. As shown in Table 2, the parasitaemia level decreased by 50% following treatment with OHCT for 8 h, suggesting OHCT has a fast mechanism of action.
The P. falciparum clone, Dd2Nm, produces gametocytes in culture. When OHCT was tested against gametocyte cultures of P. falciparum Dd2Nm, decreased survival with an IC50 17-fold lower than the IC50 of chloroquine with stage I and stage II gametocytes and 26-fold lower than that of chloroquine with stage III and IV gametocytes was observed (Table 3 and Figure 1). The IC90 of OHCT actions on gametocytes was 9-fold lower than that of chloroquine (Table 3). These data indicate that P. falciparum gametocytes are more sensitive to the action of OHCT than chloroquine.
Effect of OHCT on developmental stages of P. falciparum gametocytes. Stage III gametocytes are resistant to chloroquine (A) whereas they are sensitive to OHCT (B). Gametocytes and dead parasites are shown by arrow. Representative Giemsa stain of P. falciparum (Dd2Nm) culture treated with chloroquine (A) and OHCT (B) at 0.5 μM for 48 h (1000×).
Discussion
In this paper, the in vitro anti-malarial activity of OHCT on all blood stages of chloroquine-resistant and artemisinin-resistant P. falciparum clones, including the mosquito-transmissible gametocytes, is reported. OHCT displayed a significant inhibitory activity with an IC50 in the range of 3.9 – 93 nM depending on the parasite clone tested. OHCT is very active against the gametocyte stages of P. falciparum. Unlike chloroquine OHCT killed stage III gametocytes [16]. OHCT, like artemisinin, rapidly kills the early ring stage in vitro, suggesting that it could be used in combination with a slow-acting drug, such as chloroquine. The previous demonstrations that OHCT inhibits human liver cytochrome P450 3A4 [8, 9] further suggests that it may reduce metabolism of anti-malarial compounds, such as artemisinin, that are metabolized by this enzyme [11], thus increasing the half-life and rendering them more effective. The inhibition of human liver cytochrome P450 3A4 by OHCT was shown to be mixed competitive inhibition indicating that OHCT was binding at a site different from the active site [9]. Mixed enzymatic inhibition takes place by alteration in the conformation of cytochrome P450 3A4. Thus, OHCT would not be metabolized by cytochrome P450 3A4.
OHCT is a small hydrophobic organic molecule that does not resemble any structure of known anti-malarials. In addition, OHCT and its precursor exhibit no cytotoxicity at concentrations of 10–60 μM in either human liver cells [8, 9] or human lung cells (unpublished data). Both compounds are able to protect human liver cells against aflatoxin B1-induced toxicity [7–9]. Therefore, it is conceivable that the nanomolar anti-malarial activity of OHCT may due to a unique mechanism yet to be identified, which is currently under investigation. It is also conceivable that in combination with other known anti-malarials, OHCT would be effective in vivo against the drug resistant forms of the parasite.
Conclusion
This study indicates that OHCT displays strong anti-malarial activity in the nanomolar range against P. falciparum. In addition, OHCT is particularly effective on the gametocyte stage. Previous studies suggest OHCT has the potential for inhibiting metabolism of drugs used in anti-malarial combination therapies and at μM concentrations exhibits no toxicity to human cells in culture.
References
Mandal D, Panda N, Kumar S, Banerjee S, Mandal NB, Sahu NP: A triterpenoid saponin possessing antileishmanial activity from the leaves of Careya arborea. Phytochemistry. 2006, 67: 183-190.
Portet B, Fabre N, Roumy V, Gornitzka H, Bourdy G, Chevalley S, Sauvain M, Valentin A, Moulis C: Activity-guided isolation of antiplasmodial dihydrochalcones and flavanones from Piper hostmannianum var. berbicense. Phytochemistry. 2007, 68: 1312-1320.
Riel M, Kyle D, Milhous W: Efficacy of scopadulcic acid A against Plasmodium falciparum in vitro. J Nat Prod. 2002, 65: 614-615.
Benoit-Vical F, Imbert C, Bonfils J, Sauvaire Y: Antiplasmodial and antifungal activities of iridal, a plant triterpenoid. Phytochemistry. 2003, 62: 747-751.
Ziegler H, Jensen T, Christensen J, Staerk D, Hägerstrand H, Sittie A, Olsen C, Staalsø T, Ekpe P, Jaroszewski J: Possible artefacts in the in vitro determination of antimalarial activity of natural products that incorporate into lipid bilayer: apparent antiplasmodial activity of dehydroabietinol, a constituent of Hyptis suaveolens. Planta Med. 2002, 68: 547-549.
Kuo P, Damu A, Lee K, Wu T: Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg Med Chem. 2004, 12: 537-44.
Zhou Q: Natural Diterpene and Triterpene Quinone Methides: Structures, Synthesis and Biological Potentials. Quinone Methides. Edited by: Rokita SE. 2009, New York: John Wiley & Son, 269-295.
Zhou Q, Xie H, Zhang L, Stewart JK, Gu XX, Ryan JJ: cis-Terpenones as an effective chemopreventive agent against aflatoxin B1-induced cytotoxicity and TCDD-induced P450 1A/B activity in HepG2 cells. Chem Res Toxicol. 2006, 19: 1415-1419.
Zhou Q, Zhang L, Tombes RM, Stewart JK: Mixed inhibition of P450 3A4 as a chemoprotective mechanism against aflatoxin B1-induced cytotoxicity with cis-terpenones. Chem Res Toxicol. 2008, 21: 732-738.
Guengerich FP: Cytochrome P450 oxidations in the generation of reactive electrophiles: epoxidation and related reactions. Arch Biochem Biophys. 2003, 409: 59-71.
Svensson US, Ashton M: Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999, 48: 528-535.
Trager W, Jensen JB: Human malaria parasites in continuous culture. Science. 1976, 193: 673-675.
Hunt P, Afonso A, Creasey A, Culleton R, Sidhu AB, Logan J, Valderramos SG, McNae I, Cheesman S, do Rosario V, Carter R, Fidock DA, Cravo P: Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol Microbiol. 2007, 65: 27-40.
Druilhe P, Moreno L, Blanc C, Brasseur PH, Jacquier P: A calorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2001, 64: 233-241.
Fairlamb AH, Warhurst DC, Peters W: An improved technique for the cultivation of Plasmodium falciparum in vitro without daily medium change. Ann Trop Med Parasitol. 1985, 79: 379-384.
Benoit-Vical F, Lelièvre J, Berry A, Deymier C, Dechy-Cabaret O, Cazelles J, Loup C, Robert A, Magnaval JF, Meunier B: Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob Agents Chemother. 2007, 51 (4): 1463-1472.
Acknowledgements
This study was supported by Jeffress Memorial Trust Grants J-849 and J-923.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DCGM – Designed and performed in vitro assays; MB and OK assisted with in vitro assays; JKS – Contributed to over-all design of the study, performed data analyses; QZ – Designed and synthesized oxidized hydroxy cis-terpenone. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mayer, D.G., Bruce, M., Kochurova, O. et al. Antimalarial activity of a cis-terpenone. Malar J 8, 139 (2009). https://doi.org/10.1186/1475-2875-8-139
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-8-139