Abstract
Background
Neurally adjusted ventilatory assist (NAVA) delivers pressure in proportion to diaphragm electrical activity (Eadi). However, each patient responds differently to NAVA levels. This study aims to examine the matching between tidal volume (Vt) and patients’ inspiratory demand (Eadi), and to investigate patient-specific response to various NAVA levels in non-invasively ventilated patients.
Methods
12 patients were ventilated non-invasively with NAVA using three different NAVA levels. NAVA100 was set according to the manufacturer’s recommendation to have similar peak airway pressure as during pressure support. NAVA level was then adjusted ±50% (NAVA50, NAVA150). Airway pressure, flow and Eadi were recorded for 15 minutes at each NAVA level. The matching of Vt and integral of Eadi (ʃEadi) were assessed at the different NAVA levels. A metric, Range90, was defined as the 5-95% range of Vt/ʃEadi ratio to assess matching for each NAVA level. Smaller Range90 values indicated better matching of supply to demand.
Results
Patients ventilated at NAVA50 had the lowest Range90 with median 25.6 uVs/ml [Interquartile range (IQR): 15.4-70.4], suggesting that, globally, NAVA50 provided better matching between ʃEadi and Vt than NAVA100 and NAVA150. However, on a per-patient basis, 4 patients had the lowest Range90 values in NAVA100, 1 patient at NAVA150 and 7 patients at NAVA50. Robust coefficient of variation for ʃEadi and Vt were not different between NAVA levels.
Conclusions
The patient-specific matching between ʃEadi and Vt was variable, indicating that to obtain the best possible matching, NAVA level setting should be patient specific. The Range90 concept presented to evaluate Vt/ʃEadi is a physiologic metric that could help in individual titration of NAVA level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Non-invasive ventilation (NIV) is widely used in cases of acute respiratory failure [1] and for patients who are considered at risk of post-extubation respiratory failure [2]. However, NIV is usually delivered in pressure support (PS) mode despite the poor synchronization observed in intensive care unit (ICU) patients [3]. In comparison to PS, neurally adjusted ventilatory assist (NAVA) ventilation improves patient-ventilator interaction during invasive and NIV [4–7], and have shown to increase respiratory variability in comparison to PS [8].
NAVA triggers and cycles off the ventilator based on the patient’s diaphragmatic electrical activity (Eadi). The amount of pressure delivered by the ventilator is proportional to the Eadi amplitude [9]. Clinicians can adapt the amount of assist delivered with NAVA by selecting a NAVA level corresponding to a proportionality factor between instantaneously recorded Eadi and delivered pressure. Currently, there is limited information about how to correctly set patient-specific NAVA levels [10–14]. Additionally, implementing the best described method at the bedside [11] is difficult, potentially limiting the daily use of NAVA. The best way to adapt NAVA level on a day-to-day basis for individual patients is also unknown. Moreover, it is likely that each patient responds differently to various NAVA levels, complicating NAVA selection even further.
The aims of this study were two-fold. The primary goal was to investigate the matching between patient-specific inspiratory demand (ʃEadi) with ventilatory supply, tidal volume (Vt) at different NAVA levels during NIV. A second goal was the development of a new physiological approach for titrating NAVA level setting to the individual patient in a consistent fashion.
Materials and methods
This study analyses Eadi-time, flow-time signal and derived parameters during NIV at three different NAVA levels. This study was conducted at the University Hospital of Liege (Liege, Belgium) and Cliniques Universitaires Saint-Luc (Brussels, Belgium). The Ethics Committees of both participating hospitals approved the study protocol and use of the data.
Patients
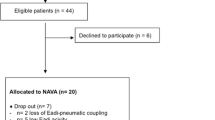
The study cohort consisted of 12 non-invasively ventilated ICU patients. Patients were included in the study if they required NIV because of acute respiratory failure, or at risk of developing respiratory failure after extubation. Specific exclusion criteria were: 1) Severe hypoxemia requiring FiO2 > 0.6; 2) hemodynamic instability; 3) patient with a hiatal hernia or other oesophageal problem; upper gastrointestinal bleeding or any other contraindication to the insertion of a naso-gastric tube; 4) poor short term prognosis; and 5) age < 18 years. A summary of the patient demographic information with clinical diagnosis is shown in Table 1.
Ventilator and delivered ventilation
All patients were ventilated with Servo-I ventilators (Maquet, Solna, Sweden) equipped with a commercially available NAVA module and software version 5.0. NIV was delivered through oronasal facemasks (Vygon SA, Ecouen, France) tightly attached in order to minimise the occurrence of leaks.
Study protocol and recordings
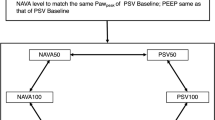
After written informed consent was obtained, the patient’s standard nasogastric tube was replaced by NAVA tube. For each patient, 15 minutes of continuous recording (~200-300 breaths) was carried out at NAVA100. This NAVA100 level was set in order to have similar peak airway pressure (P in ) as in PS mode using the previsualization system included with the ventilator. Two additional NAVA levels, denoted NAVA50 and NAVA150, that modified the initial NAVA level by ±50%, were used with an additional 15 minutes of breathing and continuous recordings.
Eadi, airway pressure and flow signals were acquired from the Servo-I ventilator, sampled at 100Hz using Servo-tracker V4.0 software (Maquet, Solna, Sweden). Positive end-expiratory pressure (PEEP), FiO2 and inspiratory trigger settings were maintained constant across each NAVA level for a given patient.
Data analysis
Signal processing
The sign of the flow signal defined ventilator pressurisation. Pressurisation was defined to begin with a positive flow signal and end with a negative signal. Inspiratory tidal volume (Vt) for each breath was calculated by integrating the flow signal between the pressurisation beginning and end points. The length of time between these two points was termed the pressurisation time or inspiratory time (Ti). Breaths with Vt < 50 ml were discarded from analysis. This selection was made through post hoc analysis of the Vt distribution, suggesting that breaths with Vt < 50 ml likely corresponded to measurement artefacts.
The Eadi signal was integrated over the period Ti to obtain ʃEadi, representing patient inspiratory demand [8]. This approach did not account for the delay between the beginning of patient’s neural inspiration (reflected by the initial increase in Eadi signal) and the beginning of ventilator’s pressurization. However, this trigger delay is very low under NAVA [4], and did not significantly influence these results. By definition, the inspiration end point corresponded to the time when Eadi signal was decreased to 70% of the maximum Eadi signal as set in the NAVA software.
Range90 assessment of matching
In this study, ʃEadi was used to represent the intensity of the electrical activity during patient’s inspiration and thus, is the representation of the intensity of the patient’s inspiration effort. The resulting inspiratory Vt, corresponded to the supply delivered by the ventilator according to the patient’s demand. Figure 1 shows an example patient with demand, ʃEadi and corresponding ventilatory supply, Vt. Thus, Vt/ʃEadi is the ratio of outcome ventilator supply to patient demand (defined as Neuroventilatory efficiency [15]), and was assessed for each breath. The width of the 5-95th percentile range of Vt/ʃEadi as shown in Equation (1) was calculated for each patient and NAVA level to enable analysis. This width was termed ‘Range90’ and defines a patient-specific metric characterizing the overall ‘matching’ between ventilator supply and patient demand.
If Vt for each breath were equally matched to the ʃEadi, then the Vt/ʃEadi ratio would be a constant. In contrast, a larger range of the Vt/ʃEadi ratio indicates an inability to consistently match Vt and ʃEadi. Thus, a smaller value of Range90 indicates consistently better matching of Vt to ʃEadi. Patients with larger values of Range90 have a higher incidence of inconsistent Vt/ʃEadi breaths, which is a lesser ability to match Vt and ʃEadi, regardless of the patient-specific ʃEadi. Matching, as captured by Range90, is thus the ability to match the variability of ventilator supply (Vt) to the variability of patient’s demand (ʃEadi).
Thus, the ratio of Vt/ʃEadi for each breath and the analysis of its distribution (Range90) over a given set of NAVA settings for a single patient enable a fair comparison between different NAVA levels. This simple metric could be calculated in real-time, for example by implementing dedicated software in the ventilator, to monitor patient-specific response to different NAVA levels. Hence, it may provide a simple solution to guide and titrate NAVA level. The detail and application of Range90 metric is reported elsewhere [16] and can also be found the Additional file 1 provided in the manuscript.
Statistical and correlation analysis
For each patient at each NAVA level, median [IQR (Interquartile range)] of ʃEadi, Peak inspiratory pressure (P in ), Vt, and Ti were calculated. The distributions of ʃEadi, P in , Vt and Ti at 3 different NAVA levels were compared using the non-parametric Wilcoxon rank-sum test as they were not normally distributed. A Pearson’s correlation analysis was carried out for Vt with ʃEadi at different NAVA levels. Robust coefficient of variation (CVR = median absolute deviation/ median) was calculated for variability analysis in each parameter.
Results
Table 2 summarizes patients’ ʃEadi, P in , Vt, and Ti at each NAVA level (median of medians with interquartile range [IQR]). Overall, NAVA50 had a higher ʃEadi = 18.5 uVs [IQR: 10.3-23.9] with lower Vt = 525 ml [IQR: 473–573] corresponding to a relatively low P in = 15.9 cmH 2 O [IQR: 12.7-17.6]. In contrast, NAVA150 had lower ʃEadi (11.8 uVs [IQR: 7.8-17.9]) and higher Vt (630 ml [IQR: 478-721]) with P in = 22.6 cmH 2 O [19.7-27.3]. Ti for NAVA50 was slightly higher at 0.88 s [IQR: 0.72-1.06] compared to other levels.
Table 3 shows the Range90 values at different NAVA levels and Table 4 presents Pearson’s correlation coefficients between ʃEadi and the corresponding Vt. Comparing NAVA ventilation at different NAVA levels in Table 3, the Range90 value for the entire cohort was the smallest for NAVA50, with a median of 25.6 uVs [IQR: 15.4-70.4]. This result suggested that NAVA50 provided better matching for the cohort in general, compared to NAVA100 and NAVA150 where Range90 values were 31.7 uVs [IQR: 18.0-90.9] and 36.4 uVs [IQR: 18.9-109.8], respectively.
Figures 2 and 3 present the Vt-ʃEadi scatter plot and Vt/ʃEadi cumulative distribution plot for Patients 2, 7 and 10. These particular patients had minimum Range90 values (best matching) at each different NAVA level. Each Figure section highlights the patient-specific supply and demand for the specific NAVA level.
Table 5 shows the robust coefficients of variation for patients’ ʃEadi, P in , Vt and Ti at each NAVA level (p > 0.05 for all tested parameters). Further details relating to Tables 2 and 5 can be found in the Additional file 1 provided with the manuscript.
Discussion
Overall, these results (ʃEadi, P in , Vt and Ti trend) show that patients behaved in general as expected from other studies. More importantly, they also show that NAVA level was highly patient-specific due to significant inter-patient variability. The top and middle panes of Figure 2 (Patient 2 and 7) provide two examples where NAVA50 was associated with lower Vt and higher ʃEadi and NAVA150 was associated with higher Vt and lower ʃEadi. The results for NAVA100 were located between those of NAVA50 and NAVA150.
Schmidt et al. [8] and Patroniti et al. [17] showed that a higher NAVA level resulted in a lower Eadi magnitude with higher Vt. Higher NAVA level delivers higher pressure, proportional to the level settings, possibly resulting in higher ventilator supply, Vt. Thus, the Eadi signal that represents the patient-specific demand may decrease. Hence, the overall results in our study match other published results [7, 11, 13, 17–19].
No significant intra-patient difference between NAVA levels was found in the variability of ʃEadi, P in , Vt, and Ti. This result indicates that ventilation at different NAVA levels results in similar variability [7, 11, 13, 17–19]. Thus, selecting an optimal NAVA level for a patient based on variability analysis is not suitable.
NAVA levels influence the overall matching (Range90) between patient inspiratory demand and delivered Vt. Overall, NAVA50 gave the best matching (lowest Range90) for the entire cohort. However, cohort results can be misleading for individual patients. More specifically, Patient 10 had a minimum Range90 value at NAVA150, 4 patients had minimum value at NAVA100 (Patients 1-3, 11) and 7 patients (Patients 4-9, 12) at NAVA50. These results show the clear inter-patient variability, and the perils of only considering cohort-based measures.
The results show the clinical potential of using Vt/ʃEadi and the Range90 metric to titrate patient-specific NAVA level over a heterogeneous patient cohort to obtain the best possible Vt/ʃEadi matching. Practically, such an approach could be used in real time if it was implemented in the ventilator to choose the best NAVA level for a given patient at a given time. This approach could also prove useful for adapting NAVA level over time, during a patient’s stay, and especially during weaning from mechanical ventilation, where only limited data are currently available [10].
It was observed that several patients had very similar values of Range90 in two different NAVA levels. Three patients with only ±10% difference were Patient 3 (NAVA100 and NAVA150), Patient 6 (NAVA50 and NAVA150) and Patient 10 (NAVA50 and NAVA150). These results indicate that the effect of the different NAVA levels were less significant in the matching of ventilator supply and patient demand. This finding also indicates that NAVA level titration through interpolation of Range90 would not be effective as the supply and demand matching does not correspond linearly to NAVA level. Similarly, titrating NAVA towards higher or lower levels in some cases (Patients 6 and 10) may be beneficial in terms of ventilator supply and demand matching.
Figures 2 and 3 show Vt/ʃ Eadi cumulative distribution and Vt-ʃ Eadi plots for Patient 2, 7 and 10 with Range90 values at each NAVA level. These 3 patients had minimum Range90 values at different NAVA levels. More specifically, the Range90 metric suggested that Patient 2 should be ventilated at the original NAVA100 level, Patient 7 could have the original NAVA level of 0.80 reduced by 50% for better matching, and Patient 10 would be better matched at the higher NAVA150 level.
Correlation coefficients facilitate examination of the relationship between Vt with ʃEadi, independent of the effects of NAVA level on the magnitude of Eadi signals. The correlation coefficient between Vt and ʃEadi may potentially be another metric to aid in titrating NAVA level. The correlation coefficients for different NAVA levels in this study were similar, indicating that NAVA was able to consistently match supply with demand at different levels. However, individual patients showed otherwise. For example, Patient 2 (NAVA50), Patient 4 (NAVA50), Patient 7 (NAVA150) and Patient 11 (NAVA50), showed significantly lower R values compared to other NAVA levels, indicating significant supply and demand mismatch at a specific patient level. Equally, the small changes in the value of R between NAVA levels may not be clinically significant, indicating that correlation coefficient was not as sensitive to changes in NAVA level as the Range90 metric. The Range90 metric consistently identified Vt/ʃEadi mismatch between NAVA levels compared to Pearson’s correlation, yielding a potentially more sensitive metric.
Limitations
Several potential limitations of this study must be pointed out. First, NAVA100 was defined to match the value of peak airway pressure during PS as set by clinicians. However, there was no standardisation of PS settings, and the appropriate level of assistance remains debated.
This study was conducted during NIV ventilation. During NIV, leaks can occur at the patient-mask interface and can influence delivered Vt. However, for this study, the mask was tightly attached to the patient by an experienced therapist in order to minimize the chance of leaks. Additionally, the therapist remained at the bedside during the whole recording to adapt the mask if necessary. These precautions made major leaks at the patient mask interface very unlikely.
Only 3 levels of NAVA were explored for each patient, separated by ±50% from the original NAVA100 level. At ±50%, the absolute changes of NAVA level can be very small or large depending on initial NAVA level. One consequence of such widely spaced NAVA levels is that, potentially none of the 3 tested NAVA levels in the trials were optimal. Thus, a more refined set of NAVA levels might well show a better result with this metric at a different NAVA level.
Finally, it is important to note that while the Range90 metric showed better matching for specific NAVA levels, the advantage of using this specific NAVA level is not yet clinically proven. Prior work has shown better matching of Vt to ʃ Eadi demand results in less asynchrony in comparing NAVA ventilation and PS [20]. However, the use of Range90 to titrate NAVA levels for better physiological outcome remains to be prospectively tested and the results here show only the sensitivity to different level settings and inter-patient variability thus demonstrating the potential clinical interest.
Conclusions
Based on matching and correlation analysis, it was found that each patient reacted differently to different NAVA levels. This finding indicates significant inter-patient variability and patient-specific response. Using the proposed concept of supply and demand ratios (Range90), more optimal NAVA levels can be found and titrated for each patient based on the simple Range90 metric. This approach can later be used in real time to adapt NAVA levels if included in software.
Abbreviations
- CVR:
-
Robust coefficient of variation
- Eadi:
-
Diaphragm electrical activity
- ʃEadi:
-
Patient inspiratory demand (Eadi Area)
- FiO2:
-
Fraction of inspired oxygen
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- NAVA:
-
Neurally adjusted ventilatory assist
- NIV:
-
Non invasive ventilation
- PEEP:
-
Positive end expiratory pressure
- Pin:
-
Peak inspiratory pressure
- PS:
-
Pressure support
- Ti:
-
Pressurisation time or inspiratory time
- Vt:
-
Tidal volume
- BMI:
-
Body mass index
- COPD:
-
Chronic obstruct pulmonary disease
- SAPSII:
-
Simplified acute physiology score.
References
Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, Epstein SK, Hill NS, Nava S, Soares M-A, et al.: Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004, 350: 2452–2460. 10.1056/NEJMoa032736
Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A: Early noninvasive ventilation averts extubation failure in patients at risk. Am J Respir Crit Care Med 2006, 173: 164–170. 10.1164/rccm.200505-718OC
Vignaux L, Vargas F, Roeseler J, Tassaux D, Thille A, Kossowsky M, Brochard L, Jolliet P: Patient–ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med 2009, 35: 840–846. 10.1007/s00134-009-1416-5
Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre P-F, Jolliet P, Tassaux D: Neurally adjusted ventilatory assist improves patient–ventilator interaction. Intensive Care Med 2011, 37: 263–271. 10.1007/s00134-010-2052-9
Schmidt M, Dres M, Raux M, Deslandes-Boutmy E, Kindler F, Mayaux J, Similowski T, Demoule A: Neurally adjusted ventilatory assist improves patient–ventilator interaction during postextubation prophylactic noninvasive ventilation*. Crit Care Med 2012, 40: 1738–1744. 1710.1097/CCM.1730b1013e3182451f3182477 10.1097/CCM.0b013e3182451f77
Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, Sinderby C: Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist *. Crit Care Med 2010, 38: 518–526. 510.1097/CCM.1090b1013e3181cb1090d1097b 10.1097/CCM.0b013e3181cb0d7b
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte F, Navalesi P: Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 2008, 34: 2010–2018. 10.1007/s00134-008-1208-3
Schmidt M, Demoule A, Cracco C, Gharbi A, Fiamma M-N, Straus C, Duguet A, Gottfried SB, Similowski T: Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology 2010, 112: 670–681. 610.1097/ALN.1090b1013e3181cea1375 10.1097/ALN.0b013e3181cea375
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L: Neural control of mechanical ventilation in respiratory failure. Nat Med 1999, 5: 1433–1436. 10.1038/71012
Rozé H, Lafrikh A, Perrier V, Germain A, Dewitte A, Gomez F, Janvier G, Ouattara A: Daily titration of neurally adjusted ventilatory assist using the diaphragm electrical activity. Intensive Care Med 2011, 37: 1087–1094. 10.1007/s00134-011-2209-1
Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, Sinderby C: Titration and implementation of neurally adjusted ventilatory assist in critically Ill patients. Chest 2009, 135: 695–703. 10.1378/chest.08-1747
Barwing J, Linden N, Ambold M, Quintel M, Moerer O: Neurally adjusted ventilatory assist vs. pressure support ventilation in critically ill patients: an observational study. Acta Anaesthesiologica Scandinavica 2011, 55: 1261–1271. 10.1111/j.1399-6576.2011.02522.x
Lecomte F, Brander L, Jalde F, Beck J, Qui H, Elie C, Slutsky AS, Brunet F, Sinderby C: Physiological response to increasing levels of neurally adjusted ventilatory assist (NAVA). Respiratory Physiology & Neurobiology 2009, 166: 117–124. 10.1016/j.resp.2009.02.015
Ververidis D, van Gils M, Passath C, Takala J, Brander L: Identification of Adequate Neurally Adjusted Ventilatory Assist (NAVA) during systematic increases in the NAVA level. Biomedical Engineering, IEEE Transactions on 2011, 58: 2598–2606.
Passath C, Takala J, Tuchscherer D, Jakob SM, Sinderby C, Brander L: Physiologic response to changing positive end-expiratory pressure during neurally adjusted ventilatory assist in sedated, critically Ill adults. Chest 2010, 138: 578–587. 10.1378/chest.10-0286
Moorhead K, Piquilloud L, Lambermont B, Roeseler J, Chiew Y, Chase JG, Revelly J-P, Bialais E, Tassaux D, Laterre P-F, et al.: NAVA enhances tidal volume and diaphragmatic electro-myographic activity matching: a Range90 analysis of supply and demand. J Clin Monit Comput 2013, 27: 61–70. 10.1007/s10877-012-9398-1
Patroniti N, Bellani G, Saccavino E, Zanella A, Grasselli G, Isgrò S, Milan M, Foti G, Pesenti A: Respiratory pattern during neurally adjusted ventilatory assist in acute respiratory failure patients. Intensive Care Med 2012, 38: 230–239. 10.1007/s00134-011-2433-8
Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS: Inspiratory Muscle Unloading by Neurally Adjusted Ventilatory Assist During Maximal Inspiratory Efforts in Healthy Subjects*. Chest 2007, 131: 711–717. 10.1378/chest.06-1909
Bertrand P-M, Futier E, Coisel Y, Matecki S, Jaber S, Constantin J-M: Neurally adjusted ventilator assist versus pressure support ventilation for noninvasive ventilation during acute respiratory failure: a cross-over physiological study. Chest 2013, 143: 30–36. 10.1378/chest.12-0424
Piquilloud L, Tassaux D, Bialais E, Lambermont B, Sottiaux T, Roeseler J, Laterre P-F, Jolliet P, Revelly J-P: Neurally adjusted ventilatory assist (NAVA) improves patient–ventilator interaction during non-invasive ventilation delivered by face mask. Intensive Care Med 2012, 38: 1624–1631. 10.1007/s00134-012-2626-9
Acknowledgements
This work was supported in part by the FNRS 474 (Belgium), the FRST (New Zealand), the University of Liege, the 475, Belgian French Community (ARC—Academie Wallonie Europe). The authors wish to thank Lise Piquilloud, Philippe Jolliet and Jean-Pierre Revelly for their contribution in data collection and input to the analysis of results. The authors also wish to thank GIGA Cardiovascular Science in University of Liege for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declared that they have no competing interest.
Authors’ contributions
YSC, TD, CP, and JGC defined the metric. All authors had input to analysis of results. BL, JR and EB implemented trials clinically with input from all others. All authors had input in writing and revising the manuscript.
Electronic supplementary material
12938_2013_678_MOESM1_ESM.doc
Additional file 1: Effects of Various Neurally Adjusted Ventilatory Assist (NAVA) levels on the matching between electric diaphragmatic activity and tidal volume. (DOC 582 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chiew, Y.S., Chase, J.G., Lambermont, B. et al. Effects of Neurally Adjusted Ventilatory Assist (NAVA) levels in non-invasive ventilated patients: titrating NAVA levels with electric diaphragmatic activity and tidal volume matching. BioMed Eng OnLine 12, 61 (2013). https://doi.org/10.1186/1475-925X-12-61
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-925X-12-61