Abstract
Introduction
Fibronectin is one of the most abundant proteins present in the inflamed joint. Here, we characterized the citrullination of fibronectin in the joints of rheumatoid arthritis (RA) patients and studied the prevalence, epitope specificity and human leukocyte antigen (HLA) association of autoantibodies against citrullinated fibronectin in RA.
Methods
Citrullinated residues in fibronectin isolated from RA patient synovial fluid were identified by mass spectrometry. The corresponding citrullinated and non-citrullinated peptides were synthesized and used to analyze the presence of autoantibodies to these peptides in RA sera and sera from other diseases and healthy controls by ELISA. The data were compared with risk factors like shared epitope HLA alleles and smoking, and with clinical features.
Results
Five citrullinated residues were identified in fibronectin from RA synovial fluid. RA sera reacted in a citrulline-dependent manner with two out of four citrullinated fibronectin peptides, one of which contains two adjacent citrulline residues, in contrast to non-RA sera, which were not reactive. The most frequently recognized peptide (FN-Cit1035,1036, LTVGLTXXGQPRQY, in which × represents citrulline) was primarily targeted by anti-CCP (cyclic citrullinated peptide) 2-positive RA patients. Anti-FN-Cit1035,1036 autoantibodies were detected in 50% of established anti-CCP2-positive RA patients and in 45% of such patients from a early arthritis clinic. These antibodies appeared to be predominantly of the immunoglobulin G (IgG) isotype and to be associated with HLA shared epitope alleles (odds ratio = 2.11).
Conclusions
Fibronectin in the inflamed synovia of RA patients can be citrullinated at least at five positions. Together with the flanking amino acids, three of these citrullinated residues comprise two epitopes recognized by RA autoantibodies. Anti-citrullinated fibronectin peptide antibodies are associated with HLA shared epitope alleles.
Similar content being viewed by others
Introduction
Citrullination or deimination is a post-translational modification, in which a peptidylarginine is converted into a peptidylcitrulline by the enzyme family of peptidylarginine deiminases (PAD). Citrullinated proteins occur at inflamed sites in healthy individuals as well as in patients [1, 2]. However, autoantibodies directed against citrullinated proteins (anti-citrullinated protein/peptide antibodies, ACPA) are very specific for rheumatoid arthritis (RA). More than 70% of RA patients display ACPA, measured via the anti-CCP2 (cyclic citrullinated peptide 2) test, in their sera [3, 4]. These antibodies are frequently present prior to disease onset and can predict the development of RA [5, 6].
It is still not fully understood how RA originates and develops, although there is experimental evidence for several steps in this process [7]. Both genetic and environmental factors have been demonstrated to contribute to the development of the disease and ACPA production. The association of several HLA-DRB1 alleles, which all share a highly conserved motif that is known as the shared epitope (SE), has already been reported many years ago [8, 9]. Other genes that have been identified as risk factors for RA include PTPN22, the TRAF1-C5 locus, PADI4, STAT4, IRF5 and CTLA-4 [10–15]. Smoking has been demonstrated to be an environmental risk factor for RA and also for ACPA production in RA patients carrying SE alleles [16, 17]. Other environmental risk factors that have been suggested to enhance the chance of developing RA include the exposure to mineral oil, diet restrictions and coffee intake [18–20]. However, these data still need confirmation.
Many citrullinated autoantigens (for example, fibrinogen, vimentin, α-enolase) and ACPA directed towards these citrullinated proteins have been identified in RA [21–26]. Currently, the CCP2 test, which is based on a synthetic citrullinated peptide not related to proteins occurring in the inflamed joints of RA patients, is the gold standard [27–29] for ACPA testing. ACPA have recently been included in the new American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria for the classification of RA, because they are present early in the disease and can predict disease development and outcome [5, 30]. The ACPA response in established RA patients is very heterogeneous and includes antibodies directed to many citrullinated proteins [31–33]. Because it has been suggested that ACPA play an important role in the development of the disease, it is important to learn more about the autoantigens that could be involved in the generation of ACPA [7]. Several citrullinated proteins occurring in the inflamed joints of RA patients have been identified previously. In part, their citrulline-containing epitopes have been mapped, particularly using synthetic peptides [24, 34] or material from cultured (non-synovial) cells (for example, HL-60 cells) [23]. It remains to be established whether these epitopes are relevant from a pathophysiological point of view. ACPA tests based upon citrullinated autoantigenic proteins may provide information on ACPA fine-specificities [35, 36] and may aid the differentiation between clinically distinct RA patient subgroups, although so far no correlations between ACPA fine-specificities and clinical phenotypes have been found [37–39].
One of the citrullinated proteins in inflamed synovial tissue identified previously is fibronectin (FN) [40, 41]. FN is a glycoprotein, which can be a component of the extracellular matrix (insoluble form) or present in body fluids (soluble form). FN is involved in a variety of processes, such as wound healing, haemostasis, thrombosis and embryogenesis [42]. Several findings have been published linking (citrullinated) FN with RA. For example, citrullinated FN was found to be present in synovial tissue and synovial fluid (SF) of RA patients [41, 43]. It has also been detected in pannus tissue and in immune complexes present in sera of RA patients [44, 45]. FN might play a role in articular cartilage destruction, because it has been observed that FN fragments can stimulate the production of multiple mediators of matrix destruction, such as various cytokines and metalloproteinases [46, 47]. However, the presence and characteristics of anti-citrullinated fibronectin antibodies in RA patients have not been studied yet.
In the current study, we have mapped citrullinated residues of FN isolated from the SF of RA patients and have used this information to investigate the B-cell response to citrullinated FN in RA.
Materials and methods
Patient material
Synovial fluid (SF) samples from RA patients were a kind gift from Prof. Dr. B. Bozic (Department of Rheumatology, University Medical Center, Ljubljana, Slovenia). After SF samples were obtained by joint punctures (arthrocentesis), using needles with a diameter from 1.6 to 2.2 mm, they were immediately centrifuged at 2,500 × g for 10 minutes at 4°C to separate insoluble and soluble components into pellet and supernatant fractions, respectively. Supernatant and pellet fractions were separately stored at -80°C within two hours after taking the samples. The pellets were resuspended in EGTA lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM KCl, 1 mM DTE, 0.1% NP40, 10 mM EGTA, 0.5 mM PMSF and protease inhibitor cocktail). Supernatant fractions were diluted in five volumes of EGTA lysis buffer. After sonification, SDS was added (final concentration 2%) and the fractions were heated and centrifuged at 12,000 × g. Supernatants were used for further analysis.
Sera from (established) rheumatoid arthritis (RA; n = 110), systemic lupus erythematosus (SLE; n = 31), and primary Sjőgren's syndrome (pSS; n = 31) patients were collected at the Department of Rheumatology of the University Medical Centre Nijmegen and the St. Maartenskliniek Nijmegen (The Netherlands). Sera from multiple sclerosis (MS; n = 31) patients were collected at the MS Centrum Nijmegen (The Netherlands). Type 1 diabetes (T1D; n = 32) sera were obtained from the Department of Immunohaematology and Blood Transfusion of the Leiden University Medical Centre (Leiden, The Netherlands). Early arthritis sera (EAC; n = 301) were collected at the Department of Rheumatology of the Leiden University Medical Center (Leiden, The Netherlands) [48]. Tuberculosis (TB; n = 29) sera were collected at Department of Internal Medicine, Tel Aviv Medical Center, Israel. Sera from healthy individuals (NS; n = 32) were collected at the Sanquin Blood Bank in Nijmegen. Sera were stored at -70°C until use.
ACPA levels in RA sera were measured using a commercial CCP2 ELISA kit (Euro-Diagnostica A.B., Malmo, Sweden).
These studies were approved by the local ethics committees; the need for patient consent was waived by the local ethics committees.
Sample preparation and tandem mass spectrometry analysis
Two SF samples from RA patients, the supernatant fraction of one and the pellet of the other, were depleted of albumin as described by Colantonio and coworkers [49], separated by SDS-PAGE and stained with colloidal Coomassie Brilliant Blue. Each lane of the gel, containing material from an individual SF sample, was sliced into 18 pieces and the polypeptides in these gel slices were digested after the addition of 20 μL trypsin solution (15 ng/μL trypsin in 25 mM NH4HCO3 and 5 mM n-octylpyranoglucoside). Peptides were extracted by adding 50% acetonitril, 0.5% trifluoroacetic acid, 5 mM n-octylpyranoglucoside followed by sonication. The protein digests resulting from each of the gel slices were separately analyzed by nano-LC-MS/MS (using a LTQ (linear trap quadrupole) Fourier Transform Ion Cyclotron Resonance mass spectrometer (LTQ FT, Thermo Scientific, Waltham, MA, USA)). Data were converted by BioWorks SEQUEST (Thermo Electron, Waltham, MA, USA) into a peak list, which allowed peptide identification with the Mascot Search database. Additionally, citrullination sites were checked manually. Mass deviations for precursor ions were set to 20 ppm and deviations for the mass of fragment ions were set at 0.8 Da. Fixed modifications, besides citrullination, such as oxidation and methylation, were taken along during the analysis.
Synthesis of citrullinated fibronectin peptides
Peptides (Table 1) were synthesized by a solid-phase procedure using Fmoc chemistry as described previously [50]. The peptides were at least 90% pure as deduced from their elution pattern on reversed phase HPLC.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISA) with fibronectin peptides were performed as described previously. Briefly, each well of a microtiter plate (Streptawell, Roche, Basel, Switzerland) was coated with 1 μg biotinylated peptide in 0.1 mL PBS/0.1% BSA overnight at 4°C. After washing three times with PBST0.1 (PBS, 0.1% Tween20), wells were incubated with serum samples 100-fold diluted at in PBST0.05 (PBS, 0.05% Tween20) containing 1% BSA for one hour at 37°C. After incubation, plates were washed three times with PBST0.1, followed by an incubation at 37°C with HRP-conjugated goat anti-human IgG, IgM, IgA, kappa, lambda (DAKO, Glostrup, Denmark) or with either HRP-conjugated rabbit anti-human IgG, rabbit anti-human IgM or rabbit anti-human IgA (DAKO, Glostrup, Denmark). Bound antibodies were detected by the conversion of 3,3',5,5'-tetramethylbenzidine (TMB) and, after terminating the reaction by the addition of sulfuric acid, the absorbance was measured at 450 nm. Cut-off values were determined as the mean value plus two times the standard deviation of normal human control sera.
ACPA fine specificity ELISA assays using peptides derived from citrullinated vimentin, fibrinogen and α-enolase were performed as described previously [39, 51].
Statistics
A two-tailed unpaired t-test with a CI of 95% was used to observe differences in reactivity between RA sera and non-RA sera with respect to the citrullinated peptides.
Univariate logistic regression analyses were performed for testing the association between several single risk factors (SE alleles as well as smoking) and anti-citrullinated fibronectin peptide antibodies in early arthritis patients. A Mann-Whitney U test was performed to address associations with the clinical phenotype (HAQ (health assessment questionnaire) score, VAS (visual analog scale) score, swollen joint count and Ritchie index) of the early arthritis patients.
Results
Citrullinated fibronectin in synovial fluid samples of RA patients
To study the immune response to citrullinated FN in RA, first the positions of the citrulline residues in FN isolated from the inflamed joints of RA patients were mapped. One supernatant fraction and one pellet fraction from SF samples obtained from two different RA patients (RA1 and RA2, respectively) were depleted of albumin by a differential precipitation procedure as described previously [49]. This resulted in two pellet fractions for each RA SF sample, which were separated by SDS-PAGE and stained with colloidal Coomassie Brilliant Blue (CBB) (Additional file 1: Figure S1). Subsequently, 18 equal slices, covering the largest polypeptides (slice number 1, molecular weight > 94,000) to the smallest polypeptides (slice number 18, molecular weight < 14,000) were excised from the stained gel for both samples (Figure 1A). The polypeptides present in the slices were digested with trypsin and analyzed by LC-MS/MS. The identity of polypeptides and the positions of citrullinated residues were determined by database searches using Mascot (Version 2.1.03, Matrix Science Inc, Boston, MA, USA). To confirm the presence of citrullinated residues and to distinguish from deamidation of glutamine or asparagine residues, the peptide fragmentation patterns were inspected manually. One of the citrullinated proteins found in the SF of both patients was FN.
Identification of (citrullinated) FN in RA synovial fluid. A. RA synovial fluid samples were depleted of albumin and separated by SDS-PAGE and stained with colloidal Coomassie Brilliant Blue. Subsequently, 18 equal slices were excised from the stained gel for both samples. The polypeptides present in the slices were digested with trypsin and analyzed by LC-MS/MS. The presence of citrullinated proteins in the gel was visualized by Western blotting using anti-modified citrulline (AMC) antibodies after modification of the proteins on the blot. The positions of molecular mass markers are indicated on the left (kDa). B. The positions of FN peptides detected in RA SF with LC-MS/MS for each of the 18 gel slices of both patient samples are schematically aligned with isoform 1 of fibronectin (FN1). The white bars represent peptides detected in RA1, the black bars peptides detected in RA2 and the grey bars peptides detected in both patients. The positions of the citrullinated residues detected are marked with asterisks. C. Schematic overview of the 15 different FN isoforms, resulting from alternative splicing events, documented in the UniProtKB database. The positions of the main alternatively spliced segments EDA, EDB and IIICS are indicated.
Figure 1B shows a schematic overview of all the FN peptide sequences obtained. This indicates that (fragments of) FN were present in material from many gel slices, indicative of a large variety of FN polypeptide lengths in SF samples. FN is a protein for which at least 15 different isoforms exist (Figure 1C); the canonical isoform (FN1) comprises a polypeptide of 2,386 amino acids, in which three repeats can be discerned [52]. FN-derived peptides that were found by these analyses covered most of the FN1 isoform, although for several regions no peptides were detected, which may at least in part be due to poor ionization efficiencies. For one of the alternatively spliced segments, extra domain A (EDA), no peptides were found in the data obtained, whereas several peptides demonstrating the absence of EDA were present. For IIICS, another alternatively spliced region, some peptide sequences were obtained with material from the high molecular weight fractions. Altogether, the mass spectrometry data covered 53% and 28% of the FN isoform 1 sequence for RA1 and RA2, respectively (Figure 1B and Additional file 1: Figure S2).
A search for deiminated arginines identified four citrullinated FN regions containing five citrullinated residues, located at amino acid positions 241, 1035, 1036, 1162 and 2356 (Figure 1B). In RA1, four citrullinated residues were identified (positions 241, 1035, 1036 and 1162), whereas in the second patient (RA2) four citrullinated residues were identified (positions 241, 1035, 1162 and 2356) (Figure 1A and Table 1).
Anti-citrullinated fibronectin peptide antibodies are specific for RA and are associated with the ACPA response
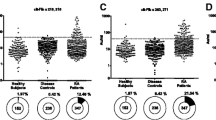
To investigate the antigenicity of citrullinated FN, peptides comprising the citrullination sites identified were synthesized, as well as their arginine-containing counterparts (Table 1). A single peptide was synthesized for the flanking citrullination sites at positions 1035 and 1036, similar to the peptide identified in RA SF. The recognition of these peptides by antibodies in established RA patient sera (n = 23) was analyzed by ELISA. Two of these peptides, which contained either a citrulline at position 241 or at position 1162 (FN-Cit241 and FN-Cit1162, respectively), were not recognized by RA sera. In contrast, the other two peptides (FN-Cit1035,1036 and FN-Cit2356) were reactive with RA sera, and for both this reactivity appeared to be citrulline-dependent (Figure 2A-D). To substantiate these data and to obtain an indication of the frequency by which these peptides are recognized, sera from a second, larger cohort of 80 established RA patients were analyzed. Also these sera were found to be frequently reactive with the FN-Cit1035,1036 and FN-Cit2356 peptides (Figure 2E, F). Forty-three percent of these sera appeared to recognize FN-Cit1035,1036, whereas eight percent was reactive with FN-Cit2356. To analyze the disease-specificity of antibodies to these citrullinated FN peptides, sera from 31 multiple sclerosis (MS), 32 type 1 diabetes (T1D), 31 primary Sjőgren's syndrome (pSS), 31 systemic lupus erythematosus (SLE) and 29 tuberculosis (TB) patients, and from 32 healthy individuals, in parallel with 75 established RA sera, were analyzed by ELISA. The results showed that less than two percent of the control sera was reactive with FN-Cit1035,1036, whereas one percent displayed reactivity with FN-Cit2356 (Figure 3).
Recognition of citrullinated fibronectin peptides by RA sera. The four fibronectin peptide sets (A, FN-Arg/Cit241; B, FN-Arg/Cit1035,1036; C, FN-Arg/Cit1162; D, FN-Arg/Cit2356) were analyzed by ELISA with 23 sera from established RA patients. Two peptide sets (E, FN-Arg/Cit1035,1036; F, FN-Arg/Cit2356) were analyzed with a larger cohort obtained from established RA sera (n = 80). OD450 = optical density at 450 nm.
Recognition of citrullinated fibronectin peptides by diseased and healthy control sera. The peptides FN-Arg/Cit1035,1036 and FN-Arg/Cit2356 were used to study the specificity of the anti-FN antibodies. A. Reactivity of RA sera and control sera to FN-Cit1035,1036. B. Reactivity of RA sera and control sera to FN-Cit2356. C. Reactivity of RA sera and control sera to FN-Arg1035,1036. D. Reactivity of RA sera and control sera to FN-Arg2356. RA (n = 75); pSS = primary Sjőgren's syndrome (n = 31); MS = multiple sclerosis (n = 31); SLE = systemic lupus erythematosus (n = 31); T1D = type 1 diabetes (n = 32); TB = tuberculosis (n = 29); NS = normal human sera (n = 32). Broken lines indicate cutoff values (mean + 2*SD of NS data); OD450 = optical density at 450 nm.
Recently, it was shown that RA patients can be divided into two subsets based upon the presence or absence of ACPA in their sera and the CCP2 test appeared to be very suitable to differentiate between these subsets [53]. The analysis of anti FN-Cit1035,1036 reactivity in 131 anti-CCP2-positive and 28 anti-CCP2-negative RA sera (Figure 4) showed that the anti-citrullinated fibronectin antibodies were hardly present in anti-CCP2-negative RA sera, corroborating the idea that these antibodies are part of the ACPA response in anti-CCP2-positive RA patients. The combined analyses of the established RA sera resulted in a prevalence of 50 percent for the autoantibodies to peptide FN-Cit1035,1036 (Table 2), LTVGLTXXGQPRQY (X represents citrulline), in CCP2-positive established RA sera (n = 82).
Anti-citrullinated fibronectin peptide antibodies in relation to anti-CCP2 positivity. The peptide set FN-Arg/Cit1035,1036 was used to study the presence of anti-FN antibodies in anti-CCP2 positive (n = 138) and anti-CCP2 negative (n = 28) RA sera. The broken line indicates the cut-off value (mean + 2*SD of data obtained with normal human control sera); OD450 = optical density at 450 nm.
Autoantibodies against citrullinated fibronectin peptide are present early in the disease
Autoantibodies against CCP2 peptides have been demonstrated to be detectable very early in the disease. Moreover, their presence in pre-disease sera predicts the development of RA [5, 6, 54]. The diversification of the ACPA response was found to occur mainly in the pre-disease stage. To investigate whether autoantibodies against citrullinated FN are also detectable in the early stages of RA development, a number of sera from early arthritis (EAC) patients (CCP2-negative, n = 23; CCP2-positive, n = 24) was analyzed. As observed before for the established RA sera, also the EAC sera reactive with the FN-Cit1035,1036 peptide represented a subgroup of the anti-CCP2-positive patients (Figure 5A). To substantiate these data and to obtain an indication of the frequency by which the FN-Cit1035,1036 peptide was recognized, additional CCP2-positive early arthritis sera (n = 278) were analyzed. Forty-five percent of these CCP2-positive EAC sera appeared to be reactive with the citrullinated fibronectin peptide containing citrulline residues at positions 1035 and 1036 (Figure 5B and Table 2). Only a small fraction of these early arthritis sera (4%) displayed some reactivity with the corresponding arginine-containing peptide (Figure 5B).
Anti-citrullinated fibronectin peptide antibodies in early arthritis patient sera. A. Sera from anti-CCP2-negative (n = 23) and anti-CCP2-positive (n = 24) early arthritis patients were analyzed in the FN-Cit1035,1036 ELISA. B. Sera from anti-CCP2-positive early arthritis patients (n = 278) were analyzed in the FN-Cit1035,1036 ELISA. The broken line indicates the cut-off value (mean + 2*SD of data obtained with normal human control sera); OD450 = optical density at 450 nm.
It is known that after disease onset ACPA isotype switching may occur [55]. To investigate isotype switching of antibodies to citrullinated FN, 23 anti-FN-Cit1035,1036-positive sera for which both samples taken at baseline and approximately seven years after disease onset were available, were selected and analyzed in ELISA with isotype-specific (IgG, IgM and IgA) secondary antibody conjugates. At baseline (t = 0), 87% of the reactive sera contained immunoglobulins of the IgG isotype (Figure 6A), whereas 13% contained IgM (Figure 6C) and 4% contained IgA (Figure 6E) type reactivities. After a median follow-up time of seven years (t = 7) the frequency of IgG type antibodies to FN-Cit1035,1036 was slightly decreased to 74% (Figure 6B). In these patients the frequency of IgM type anti-FN-Cit1035,1036 antibodies decreased to 4% (Figure 6D) In contrast, at this stage the frequency of IgA type antibodies to this citrullinated FN peptide increased to 13% (Figure 6F). All IgM- and IgA-positive patients were also positive for IgG, whereas the simultaneous presence of IgM and IgA reactivities in the same patients was observed in one patient. Except for two patients, in which the IgG type anti-FN-Cit1035,1036 antibodies disappeared, the presence of these IgG antibodies did not markedly change in time (Figure 6G).
Anti-FN-Cit 1035,1036 antibodies are predominantly of the IgG isotype. Sera from a subset of anti-FN-Cit1035,1036-positive early arthritis patients (n = 23) taken at baseline (t = 0) and after a median follow-up of seven years later (t = 7) were analyzed for different anti-FN-Cit1035,1036 antibody isotypes in ELISA. A. IgG isotype reactivity at t = 0. B. IgG isotype reactivity at t = 7. C. IgM isotype reactivity at t = 0. D. IgM isotype reactivity at t = 7. E. IgA isotype reactivity at t = 0. F. IgA isotype reactivity at t = 7. G. Anti-FN-Cit1035,1036 IgG reactivity at t = 0 and t = 7. The broken lines indicate the cut-off values determined by normal human control sera.
Anti-citrullinated fibronectin peptide antibodies are associated with HLA SE alleles
The HLA-DRB1*01, HLA-DRB1*04, HLA-DRB1*10 and HLA-DRB1*14 alleles comprise the group of HLA SE alleles, which are associated with RA [56]. The reactivity to FN-Cit1035,1036 was compared with the HLA-DRB1 alleles of the 278 early arthritis patients. The results showed that the presence of HLA SE alleles is associated with the production of anti-citrullinated fibronectin antibodies, because patients carrying HLA SE alleles are more than two times more likely to have autoantibodies against citrullinated fibronectin (OR = 2.11; Table 3 and Additional file 1: Table S1). When addressing individual HLA-DRB1 alleles, only HLA-DRB1*04 (OR = 1.5), and HLA-DRB1*10 (OR = 1.57) showed a weak to moderate association with the presence of anti-FN-Cit1035,1036 antibodies. Although a more pronounced association was observed with HLA-DRB1*08 (OR = 2.45) and HLA-DRB1*16 (OR = 2.43), these data should be interpreted with care because the carriage of these alleles is rare and the data are based on only a low number of patients. Interestingly, a negative association was observed between the presence of anti-citrullinated fibronectin antibodies and two additional HLA-DRB1 alleles, HLA-DRB1*09 and HLA-DRB1*11 (OR = 0.19 and OR = 0.41, respectively) (Additional file 1: Table S1).
In addition, the potential relationship of smoking with the production of anti-citrullinated fibronectin antibodies was assessed and a weak association was found (OR = 1.42). We did not observe significant associations between the presence of anti-citrullinated fibronectin antibodies and clinical parameters, such as VAS score, HAQ score, Ritchie index or swollen joint count at baseline (data not shown).
Discussion
The analysis of citrullinated proteins in the synovial fluids of two rheumatoid arthritis patients revealed fibronectin as one of the multiply citrullinated proteins in both patients. Two of the four synthetic peptides that were derived from the citrullinated regions of FN appeared to be reactive with ACPA in the sera of RA patients. The most frequently targeted FN peptide, FN-Cit1035,1036, which contains two adjacent citrullines, was recognized by 50% of established and 45% of early ACPA-positive RA patients. Like ACPA in general, anti-FN-Cit1035,1036 antibodies appeared to be associated with HLA-DRB1 shared epitope alleles.
FN is a complex protein, characterized by the presence of three types of repeats in its polypeptide sequence, for which many isoforms resulting from alternative splicing events have been described (Figure 1C). The UniProtKB database provides details of 15 isoforms, with isoform 1 (FN1 in Figure 1C) as the canonical sequence. The overall sequence coverage of the mass spectrometry datasets relative to FN1 comprises 53% and 28% for both synovial fluid samples, respectively. It is likely that multiple FN isoforms are expressed in the inflamed synovia of RA patients and our data do not allow drawing conclusions on their relative abundance. Three regions of FN are especially prone to alternative splicing; at the protein level these are termed extra domain A (EDA), extra domain B (EDB) and type III connecting segment (IIICS). Although peptides were found that match part of the IIICS sequence, no peptides were detected for EDA and EDB. Moreover, a few peptides provide evidence for the absence of EDA and EDB in the isoform(s) they originate from, because their sequences covered the regions immediately N- and C-terminal from EDA or from EDB. The EDA domain, also designated EIIIA, has been implicated in inflammation, because it was shown to be involved in Toll-like receptor (TLR) 4 activation [57]. Although our data do not support the presence of EDA-containing FN isoforms in the synovial fluid of RA patients, it has been demonstrated previously that EDA-containing FN is produced in the RA synovium and is expressed abundantly in RA SF [58, 59]. Moreover, recently Lefebvre and colleagues showed that EDA-containing FN stimulated leukotriene synthesis and neutrophil recruitment via TLR activation in a mouse model [60]. Although we could not detect any peptides in the EDA region, our data do not exclude the presence of EDA-containing FN isoforms in RA synovial fluid samples, because we have only analyzed a limited number of patients or because of technical limitations, such as ionization efficiencies.
The interpretation of the data is further complicated by the fact that FN-derived peptides were found in material from many gel slices, which indicates a high heterogeneity of FN polypeptide lengths. It is likely that this is at least in part due to the presence of proteolytic enzymes in the synovial fluid from inflamed joints, which cleave the FN polypeptides in different fragments. It is known that during inflammation proteases are active in synovial fluid and can contribute to RA pathogenesis [61, 62]. Indeed, the fragmentation of FN in inflammatory SF [63], as well as in cartilage of RA and osteoarthritis patients [64] has been reported previously.
Our analyses identified five citrullinated residues in FN from RA patient SF. Except for two adjacent citrullines, these residues are found in distant regions of the FN polypeptide chain. Our data do not provide information on the extent to which these residues are citrullinated in RA SF and it is likely that differences between patients exist. This is substantiated by the observation that in material from RA2 for the region containing the two adjacent citrullines only one of these two residues (1035) was found to be citrullinated, whereas both were citrullinated in RA1. Two other studies have previously reported the presence of citrullinated FN in SF and synovial tissue of RA patients [41, 43]. However, these studies did not reveal the positions of the citrullinated residues. Our data do not exclude the possibility that FN is also citrullinated at other positions in RA SF, because material from only two patients was analyzed in detail and the sequence coverage was not more than 53%. Moreover, if citrullination enhances FN's susceptibility to proteolytic cleavage, citrullinated peptides may have escaped detection as a result of cleavages by endogenous proteases that cleave the polypeptide close to the citrullinated residue.
A synthetic peptide approach to investigate the recognition of citrullinated epitopes of FN by RA autoantibodies revealed that the major autoepitope is located in the region containing the two adjacent citrullines (amino acids 1035 and 1036). Only one of the other three citrullinated peptides was recognized by some RA sera. The fact that RA sera were only reactive with two of these peptides is consistent with the results of other studies showing that the amino acids flanking the citrulline residue contribute to the formation of auto-epitopes [1]. Several studies [24, 34, 65] with synthetic citrullinated peptides (derived from vimentin, fibrinogen and α-enolase) showed that not all peptides containing citrullinated residues are recognized by patient sera, indicating that not only the citrulline is important, but also the amino acids surrounding the citrullinated residue. However, it should be noted that the use of synthetic peptides in general does not allow the identification of reactivities with conformational and/or discontinuous epitopes. Therefore, our data do not exclude the possibility that the citrullinated residues identified are part of conformational epitopes and that autoantibodies to these epitopes may be present in RA patient sera. Although autoantibodies to FN have been detected in RA as well as SLE patients before [66, 67], our data are the first to describe antibodies that target FN in a citrulline-dependent manner. The autoantibodies that can be detected with the FN-Cit1035,1036 peptide represent a subset of ACPA, which is substantiated by the lack of correlation between the levels of reactivity with CCP2 and with FN-Cit1035,1036 (Additional file 1: Figure S3). In total, 50% of the (anti-CCP2-positive) established RA patients showed reactivity to FN-Cit1035,1036, compared to two percent of the controls (non-RA and healthy individuals; Table 2).
The possibility existed that the anti-FN-Cit1035,1036 subset of ACPA overlapped with another subset that has been identified previously, as a result of epitope similarities. ACPA fine-specificity data were available for most of the EAC patient sera. The reactivity of these sera with FN-Cit1035,1036 was compared with their reactivity with two citrullinated peptides derived from vimentin (vimentin-1-16: STCitSVSSSSYCitCitMFGG; vimentin-59-74: VYATCitSSAVCitLCitSSVP), two peptides derived from fibrinogen (α-fibrinogen-27-43: FLAEGGGVCitGPRVVERH; β-fibrinogen-36-52: NEEGFFSACitGHRPLDKK) and one peptide derived from α-enolase (α-enolase-5-20: KIHACitEIFDSCitGNPTV) [39]. These analyses showed that most of the EAC sera recognized multiple citrullinated epitopes (Figure 7A). A large overlap of anti-FN-Cit1035,1036 with reactivities to any of the other peptides was observed, as might be expected from previous data [26, 68]. However, some sera appeared to react exclusively with FN-Cit1035,1036 and not with the other citrullinated epitopes (Figure 7B). These data are underscoring the large heterogeneity of the ACPA response in RA and indicate that the anti-FN-Cit1035,1036 antibodies are one of the most abundant ACPA subclasses that can be detected with synthetic peptides derived from citrullinated synovial proteins. Taken together, these data indicate that this citrullinated FN peptide may be used to investigate the fine-specificity of ACPA in more detail and may be complementary to other citrullinated molecules in ACPA profiling.
Anti-FN-Cit 1035,1036 in relation to other ACPA. The reactivity of EAC sera (n = 228) with FN-Cit1035,1036 was compared with the presence of antibodies to other citrullinated peptides, which are derived from vimentin (1 to 16: STCitSVSSSSYCitCitMFGG and 59 to 74: VYATCitSSAVCitLCitSSVP), fibrinogen (α-fibrinogen 27 to 43: FLAEGGGVCitGPRVVERH; β-fibrinogen 36 to 52: NEEGFFSACitGHRPLDKK) and α-enolase (5 to 20: KIHACitEIFDSCitGNPTV) [39]. A. Fraction of patients recognizing 0 to 6 citrullinated peptides. B. Heat map showing the presence of antibodies to the citrullinated peptides obtained by an unsupervised cluster analysis. Red and green mark positive and negative sera, respectively. Missing values are depicted in grey.
The anti-FN-Cit1035,1036 antibodies present in early arthritis patients appeared to be predominantly of the IgG isotype. Only a small percentage of sera was found to contain IgM or IgA type anti-FN-Cit1035,1036 antibodies. After a median follow-up of seven years, the IgG type reactivities were hardly changed, whereas the prevalence of IgM reactivity against FN-Cit1035,1036 was decreased, and that of IgA was somewhat increased. Similar observations have been reported for samples from undifferentiated arthritis patients (who developed RA) taken either at baseline or after one year follow-up [36]. It remains an open question whether the anti-citrullinated FN antibodies play a pathophysiological role. FN-containing immune complexes are likely to be formed in the inflamed joints of RA patients and this may occur already early during disease development. Further studies will be required to elucidate whether citrullinated FN is involved in the inflammatory process.
The presence of anti-citrullinated FN antibodies in the early arthritis patients was associated with HLA SE alleles (OR = 2.11). Of the individual SE alleles, only HLA-DRB1*04 and HLA-DRB1*10 showed a weak association with the presence of anti-citrullinated FN peptide antibodies (Additional file 1: Table S1). Previously, Snir and co-workers demonstrated that antibodies against multiple citrullinated antigens (for example, vimentin, fibrinogen, α-enolase and the C1-epitope of type II collagen) were associated with SE alleles, particularly with HLA-DRB1*04 [26, 69]. Our data show a negative association of HLA-DRB1*09 and HLA-DRB1*11 with the presence of anti-citrullinated FN peptide antibodies (OR = 0.19 and OR = 0.41, respectively). This may be in agreement with the previously reported association of the HLA-DRB1*0901 haplotype with reduced levels of anti-CCP antibodies [70]. Paradoxically, HLA-DRB1*09 has been reported previously to be associated with RA in Asian as well as Caucasian individuals [71, 72]. In addition, it should be noted that, because ACPA-positive RA patients comprise a strong prevalence for SE-allelles, other HLA-DRB1 alleles are less frequently present than SE-alleles and, therefore, might seem to be protective. As a consequence, the negative association observed with HLA-DRB1*09 and HLA-DRB1*11 might also be the result of skewing [73].
We did not detect a significant association between clinical phenotype and the presence of anti-citrullinated fibronectin in ACPA-positive RA patients. Recently, Scherer and colleagues also observed no effect on radiographic joint damage in patients that were positive for several citrullinated epitopes [37]. Nevertheless, a weak correlation between the presence of autoantibodies against citrullinated FN and smoking, a risk factor for ACPA generation, particularly in individuals that carry the SE alleles [16, 17], was observed.
Conclusion
Five citrullinated residues were identified in fibronectin isolated from the inflamed joints of RA patients. An epitope containing two adjacent citrullines at positions corresponding to residues 1035 and 1036 appeared to be most frequently recognized by RA sera. Our data not only show that antibodies against citrullinated FN are present in RA patients, but also demonstrate that the anti-FN antibodies represent a subgroup of anti-CCP2 antibodies and that they can already be detected very early in the disease. Moreover, anti-FN-Cit1035,1036 antibodies are associated primarily with HLA SE alleles.
Abbreviations
- ACPA:
-
anti-citrullinated protein antibodies
- AMC:
-
anti-modified citrulline
- BSA:
-
bovine serum albumin
- CBB:
-
Coomassie Brilliant Blue
- CCP:
-
cyclic citrullinated peptide
- CI:
-
coincidence interval
- EAC:
-
early arthritis clinic
- EDA:
-
extra domain A
- EDB:
-
extra domain B
- FN:
-
fibronectin
- HAQ:
-
health assessment questionnaire
- HLA:
-
human leukocyte antigen
- IIICS:
-
type III connecting segment
- LC-MS/MS:
-
liquid chromatography - tandem mass spectrometry
- LTQ:
-
linear trap quadrupole
- MS:
-
multiple sclerosis
- NS:
-
normal sera
- OR:
-
odds ratio
- PAD:
-
peptidylarginine deiminase
- PBS:
-
phosphate-buffered saline
- pSS:
-
primary Sjogren's syndrome
- RA:
-
rheumatoid arthritis
- RF:
-
rheumatoid factor
- SE:
-
shared epitope
- SF:
-
synovial fluid
- SLE:
-
systemic lupus erythematosus
- T1D:
-
type 1 diabetes
- TB:
-
tuberculosis
- TLR:
-
Toll-like receptor
- TMB:
-
3,3',5,5'-tetramethylbenzidine
- VAS:
-
visual analog scale
References
Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI: Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006, 38: 1662-1677. 10.1016/j.biocel.2006.03.008.
Vossenaar ER, Smeets TJ, Kraan MC, Raats JM, van Venrooij WJ, Tak PP: The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 2004, 50: 3485-3494. 10.1002/art.20584.
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ: Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998, 101: 273-281. 10.1172/JCI1316.
van Venrooij WJ, van Beers JJ, Pruijn GJ: Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci. 2008, 1143: 268-285. 10.1196/annals.1443.013.
Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ: Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48: 2741-2749. 10.1002/art.11223.
van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, Toes RE, Huizinga TW: Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004, 50: 709-715. 10.1002/art.20044.
van Venrooij WJ, Pruijn GJ: An important step towards completing the rheumatoid arthritis cycle. Arthritis Res Ther. 2008, 10: 117-10.1186/ar2504.
Khani-Hanjani A, Lacaille D, Horne C, Chalmers A, Hoar DI, Balshaw R, Keown PA: Expression of QK/QR/RRRAA or DERAA motifs at the third hypervariable region of HLA-DRB1 and disease severity in rheumatoid arthritis. J Rheumatol. 2002, 29: 1358-1365.
van Gaalen FA, van Aken J, Huizinga TW, Schreuder GM, Breedveld FC, Zanelli E, van Venrooij WJ, Verweij CL, Toes RE, de Vries RR: Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004, 50: 2113-2121. 10.1002/art.20316.
Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, Liew A, Khalili H, Chandrasekaran A, Davies LR, Li W, Tan AK, Bonnard C, Ong RT, Thalamuthu A, Pettersson S, Liu C, Tian C, Chen WV, Carulli JP, Beckman EM, Altshuler D, Alfredsson L, Criswell LA, Amos CI, Seldin MF, Kastner DL, Klareskog L, Gregersen PK: TRAF1-C5 as a risk locus for rheumatoid arthritis-a genomewide study. N Engl J Med. 2007, 357: 1199-1209. 10.1056/NEJMoa073491.
Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono M, Ohtsuki M, Furukawa H, Yoshino S, Yukioka M, Tohma S, Matsubara T, Wakitani S, Teshima R, Nishioka Y, Sekine A, Iida A, Takahashi A, Tsunoda T, Nakamura Y, Yamamoto K: Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003, 34: 395-402. 10.1038/ng1206.
Lee HS, Remmers EF, Le JM, Kastner DL, Bae SC, Gregersen PK: Association of STAT4 with rheumatoid arthritis in the Korean population. Mol Med. 2007, 13: 455-460.
Sigurdsson S, Padyukov L, Kurreeman FA, Liljedahl U, Wiman AC, Alfredsson L, Toes R, Ronnelid J, Klareskog L, Huizinga TW, Alm G, Syvanen AC, Ronnblom L: Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 2007, 56: 2202-2210. 10.1002/art.22704.
Yanagawa T, Gomi K, Nakao EI, Inada S: CTLA-4 gene polymorphism in Japanese patients with rheumatoid arthritis. J Rheumatol. 2000, 27: 2740-2742.
Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Chang SY, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla F, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CI, Sninsky JJ, Gregersen PK: A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004, 75: 330-337. 10.1086/422827.
Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, Kloppenburg M, de Vries RR, le Cessie S, Breedveld FC, Toes RE, Huizinga TW: Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006, 65: 366-371. 10.1136/ard.2005.041079.
Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L: A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54: 38-46. 10.1002/art.21575.
Sverdrup B, Kallberg H, Bengtsson C, Lundberg I, Padyukov L, Alfredsson L, Klareskog L: Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res Ther. 2005, 7: R1296-R1303. 10.1186/ar1824.
Rosell M, Wesley AM, Rydin K, Klareskog L, Alfredsson L: Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology. 2009, 20: 896-901. 10.1097/EDE.0b013e3181b5f0ce.
Mikuls TR, Cerhan JR, Criswell LA, Merlino L, Mudano AS, Burma M, Folsom AR, Saag KG: Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2002, 46: 83-91. 10.1002/1529-0131(200201)46:1<83::AID-ART10042>3.0.CO;2-D.
Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G: The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001, 166: 4177-4184.
Vossenaar ER, Després N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ, Menard HA: Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004, 6: R142-R150. 10.1186/ar1149.
Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ: Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis ResTher. 2005, 7: R1421-R1429. 10.1186/ar1845.
Sebbag M, Moinard N, Auger I, Clavel C, Arnaud J, Nogueira L, Roudier J, Serre G: Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur J Immunol. 2006, 36: 2250-2263. 10.1002/eji.200535790.
Van Steendam K, Tilleman K, De Ceuleneer M, De Keyser F, Elewaut D, Deforce D: Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis ResTher. 2010, 12: R132-10.1186/ar3070.
Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, Lundberg K, Engström A, Venables PJ, Toes RE, Holmdahl R, Klareskog L, Malmström V: Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010, 62: 44-52. 10.1002/art.25036.
Bizzaro N, Tonutti E, Tozzoli R, Villalta D: Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin Chem. 2007, 53: 1527-1533. 10.1373/clinchem.2007.087569.
Coenen D, Verschueren P, Westhovens R, Bossuyt X: Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem. 2007, 53: 498-504. 10.1373/clinchem.2006.078063.
Mutlu N, Bicakcigil M, Tasan DA, Kaya A, Yavuz S, Ozden AI: Comparative performance analysis of 4 different anti-citrullinated protein assays in the diagnosis of rheumatoid arthritis. J Rheumatol. 2009, 36: 491-500. 10.3899/jrheum.080656.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, et al: 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62: 2569-2581. 10.1002/art.27584.
Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, Saxne T, Malmstrom V, Venables PJ: Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008, 58: 2287-2295. 10.1002/art.23618.
Matsuo K, Xiang Y, Nakamura H, Masuko K, Yudoh K, Noyori K, Nishioka K, Saito T, Kato T: Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis ResTher. 2006, 8: R175-10.1186/ar2085.
Goeb V, Jouen F, Gilbert D, Le Loët X, Tron F, Vittecoq O: Diagnostic and prognostic usefulness of antibodies to citrullinated peptides. Joint Bone Spine. 2009, 76: 343-349. 10.1016/j.jbspin.2008.12.006.
Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, Huizinga TW, de Vries RR, Toes RE, Ioan-Facsinay A: Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62: 117-125. 10.1002/art.25059.
Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, Elias B, Ménard HA, Newkirk M, Fritzler MJ, Toes RE, Huizinga TW, El-Gabalawy HS: Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008, 58: 3000-3008. 10.1002/art.23763.
van der Woude D, Syversen SW, van der Voort EI, Verpoort KN, Goll GL, van der Linden MP, van der Helm-van Mil AH, van der Heijde DM, Huizinga TW, Kvien TK, Toes RE: The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2010, 69: 1110-1116. 10.1136/ard.2009.116384.
Scherer HU, van der Woude D, Willemze A, Trouw LA, Knevel R, Syversen SW, van der Linden MP, Lie B, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH, Kvien TK, Toes RE: Distinct ACPA fine specificities, formed under the influence of HLA shared epitope alleles, have no effect on radiographic joint damage in rheumatoid arthritis. Ann Rheum Dis. 2011, 70: 1461-1464. 10.1136/ard.2010.146506.
Fisher BA, Plant D, Brode M, van Vollenhoven RF, Mathsson L, Symmons D, Lundberg K, Ronnelid J, Venables PJ: Antibodies to citrullinated alpha-enolase peptide 1 and clinical and radiological outcomes in rheumatoid arthritis. Ann Rheum Dis. 2011, 70: 1095-1098. 10.1136/ard.2010.138909.
Willemze A, Bohringer S, Knevel R, Levarht EN, Stoeken-Rijsbergen G, Houwing-Duistermaat JJ, van der Helm-van Mil AH, Huizinga TW, Toes RE, Trouw LA: The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Ann Rheum Dis. 2012, 71: 268-274. 10.1136/annrheumdis-2011-200421.
van Beers JJBC, Zendman AJW, van Venrooij WJ, Pruijn GJM: Citrullination in the arthritic synovium; the citrullinome, the antibodies against citrullinated proteins and their connection with RA pathogenesis. From Etiopathogenesis to the Prediction of Autoimmune Diseases: Relevance of Autoantibodies Report on the 8th Dresden Symposium on Autoantibodies September 12-15, 2007;Dresden. Edited by: Conrad K, Chan EKL, Fritzler MJ, Sack U, Shoenfeld Y, Wiik AS. 2007, Pabst Science Publishers: Lengerich, Germany, 5: 378-388. Autoantigens, Autoantibodies, Autoimmunity
Chang X, Yamada R, Suzuki A, Kochi Y, Sawada T, Yamamoto K: Citrullination of fibronectin in rheumatoid arthritis synovial tissue. Rheumatology (Oxford). 2005, 44: 1374-1382. 10.1093/rheumatology/kei023.
Potts JR, Campbell ID: Fibronectin structure and assembly. Curr Opin Cell Biol. 1994, 6: 648-655. 10.1016/0955-0674(94)90090-6.
Tabushi Y, Nakanishi T, Takeuchi T, Nakajima M, Ueda K, Kotani T, Makino S, Shimizu A, Hanafusa T, Takubo T: Detection of citrullinated proteins in synovial fluids derived from patients with rheumatoid arthritis by proteomics-based analysis. Ann Clin Biochem. 2008, 45: 413-417. 10.1258/acb.2007.007205.
Herbert KE, Coppock JS, Griffiths AM, Williams A, Robinson MW, Scott DL: Fibronectin and immune complexes in rheumatic diseases. Ann Rheum Dis. 1987, 46: 734-740. 10.1136/ard.46.10.734.
Scott DL, Delamere JP, Walton KW: The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981, 62: 362-368.
Xie D, Homandberg GA: Fibronectin fragments bind to and penetrate cartilage tissue resulting in proteinase expression and cartilage damage. Biochim Biophys Acta. 1993, 1182: 189-196.
Homandberg GA, Meyers R, Williams JM: Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J Rheumatol. 1993, 20: 1378-1382.
van Aken J, van Bilsen JH, Allaart CF, Huizinga TW, Breedveld FC: The Leiden Early Arthritis Clinic. Clin Exp Rheumatol. 2003, 21: S100-S105.
Colantonio DA, Dunkinson C, Bovenkamp DE, Van Eyk JE: Effective removal of albumin from serum. Proteomics. 2005, 5: 3831-3835. 10.1002/pmic.200401235.
Hiemstra HS, Duinkerken G, Benckhuijsen WE, Amons R, de Vries RR, Roep BO, Drijfhout JW: The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc Natl Acad Sci USA. 1997, 94: 10313-10318. 10.1073/pnas.94.19.10313.
Verpoort KN, Cheung K, Ioan-Facsinay A, van der Helm-van Mil AH, Vries-Bouwstra JK, Allaart CF, Drijfhout JW, de Vries RR, Breedveld FC, Huizinga TW, Pruijn GJ, Toes RE: Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007, 56: 3949-3952. 10.1002/art.23127.
White ES, Baralle FE, Muro AF: New insights into form and function of fibronectin splice variants. J Pathol. 2008, 216: 1-14. 10.1002/path.2388.
van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW: Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis ResTher. 2005, 7: R949-R958. 10.1186/ar1767.
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ: The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43: 155-163. 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3.
Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, Ioan-Facsinay A, Drijfhout JW, van Tol MJ, Breedveld FC, Huizinga TW, Toes RE: Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006, 54: 3799-3808. 10.1002/art.22279.
Holoshitz J: The rheumatoid arthritis HLA-DRB1 shared epitope. Curr Opin Rheumatol. 2010, 22: 293-298. 10.1097/BOR.0b013e328336ba63.
Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF: The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001, 276: 10229-10233. 10.1074/jbc.M100099200.
Chevalier X, Claudepierre P, Groult N, Zardi L, Hornebeck W: Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 1996, 23: 1022-1030.
Hino K, Shiozawa S, Kuroki Y, Ishikawa H, Shiozawa K, Sekiguchi K, Hirano H, Sakashita E, Miyashita K, Chihara K: EDA-containing fibronectin is synthesized from rheumatoid synovial fibroblast-like cells. Arthritis Rheum. 1995, 38: 678-683. 10.1002/art.1780380516.
Lefebvre JS, Levesque T, Picard S, Pare G, Gravel A, Flamand L, Borgeat P: Extra domain A of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 2011, 63: 1527-1533. 10.1002/art.30308.
Mantle D, Falkous G, Walker D: Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers. Clin Chim Acta. 1999, 284: 45-58. 10.1016/S0009-8981(99)00055-8.
Ronday HK, van der Laan WH, Tak PP, de Roos JA, Bank RA, TeKoppele JM, Froelich CJ, Hack CE, Hogendoorn PC, Breedveld FC, Verheijen JH: Human granzyme B mediates cartilage proteoglycan degradation and is expressed at the invasive front of the synovium in rheumatoid arthritis. Rheumatology (Oxford). 2001, 40: 55-61. 10.1093/rheumatology/40.1.55.
Barilla ML, Carsons SE: Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000, 29: 252-265. 10.1016/S0049-0172(00)80012-8.
Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD: Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006, 54: 2912-2922. 10.1002/art.22045.
Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, Mikuls TR, Venables PJ: Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58: 3009-3019. 10.1002/art.23936.
Atta MS, Lim KL, Ala'deen DA, Powell RJ, Todd I: Investigation of the prevalence and clinical associations of antibodies to human fibronectin in systemic lupus erythematosus. Ann Rheum Dis. 1995, 54: 117-124. 10.1136/ard.54.2.117.
Kim CW, Cho EH, Lee YJ, Kim YH, Hah YS, Kim DR: Disease-specific proteins from rheumatoid arthritis patients. J Korean Med Sci. 2006, 21: 478-484. 10.3346/jkms.2006.21.3.478.
van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, Drijfhout JW, Huizinga TW, Toes RE, Pruijn GJ: Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010, 69: 1554-1561. 10.1136/ard.2009.124537.
Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, Engstrom A, Venables PJ, Lundeberg J, Holmdahl R, Klareskog L, Malmstrom V: Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009, 68: 736-743. 10.1136/ard.2008.091355.
Okada Y, Suzuki A, Yamada R, Kochi Y, Shimane K, Myouzen K, Kubo M, Nakamura Y, Yamamoto K: HLA-DRB1*0901 lowers anti-cyclic citrullinated peptide antibody levels in Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2010, 69: 1569-1570. 10.1136/ard.2009.118018.
Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Khalili H, Wolfe F, Lum RF, Massarotti E, Weisman M, Bombardier C, Karlson EW, Criswell LA, Vlietinck R, Gregersen PK: Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007, 56: 1745-1753. 10.1002/art.22703.
Milicic A, Lee D, Brown MA, Darke C, Wordsworth BP: HLA-DR/DQ haplotype in rheumatoid arthritis: novel allelic associations in UK Caucasians. J Rheumatol. 2002, 29: 1821-1826.
van der Woude D, Lie BA, Lundstrom E, Balsa A, Feitsma AL, Houwing-Duistermaat JJ, Verduijn W, Nordang GB, Alfredsson L, Klareskog L, Pascual-Salcedo D, Gonzalez-Gay MA, Lopez-Nevot MA, Valero F, Roep BO, Huizinga TW, Kvien TK, Martin J, Padyukov L, de Vries RR, Toes RE: Protection against anti-citrullinated protein antibody-positive rheumatoid arthritis is predominantly associated with HLA-DRB1*1301: a meta-analysis of HLA-DRB1 associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in four European populations. Arthritis Rheum. 2010, 62: 1236-1245.
Acknowledgements
The authors would like to thank Prof. Dr. Borut Bozic (Department of Rheumatology, University Medical Centre, Ljubljana, Slovenia) for providing the synovial fluid samples and Prof. Dr. Walther van Venrooij for critically reading the manuscript. This work was supported in part by the Dutch Arthritis Association.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JvB carried out the proteomic studies and part of the immunoassays, and participated in design of the study, interpretation of the data and drafting of the manuscript. AW participated in the analysis and interpretation of the data, performed the statistical analysis and contributed to the preparation of the manuscript. JS-V performed part of the immunoassays. JD participated in the design of the study, generated the synthetic peptides and participated in the preparation of the manuscript. RT participated in the design of the study, the interpretation of the data and the preparation of the manuscript. GP conceived of the study, participated in its design and coordination, contributed to the interpretation of the data and helped to draft the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
13075_2011_3494_MOESM1_ESM.PDF
Additional file 1: Supplementary Table 1 and Supplementary Figures 1 to 3. Supplementary Table 1: Association of anti-citrullinated fibronectin antibodies and HLA-DRB1 alleles in anti-CCP2-positive early arthritis patients. Supplementary Figure 1: Overview of the handling of synovial fluid samples. Supplementary Figure 2: Fibronectin-derived peptides identified in the synovial fluid of RA patients. Supplementary Figure 3: Correlation between anti-CCP2 and anti-FN-Cit1035,1036 reactivities. (PDF 69 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
van Beers, J.J., Willemze, A., Stammen-Vogelzangs, J. et al. Anti-citrullinated fibronectin antibodies in rheumatoid arthritis are associated with human leukocyte antigen-DRB1shared epitope alleles . Arthritis Res Ther 14, R35 (2012). https://doi.org/10.1186/ar3744
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar3744