Abstract
The bioaccumulation of some heavy metals in coaster waters directly affects the ecosystem and human beings. Thus, the objectives of this study are to evaluate the interaction between fish and coastal water and assess the long-term implications of human consumption of some major organs obtained from Tilapia brevimanus and Euthynnus alletteratus. The study determined the concentrations of arsenic (As), cadmium (Cd), chromium (Cr), and lead (Pb) in twenty coaster water samples collected from two points and in the muscle, gills, and intestine of ten pieces of Tilapia brevimanus and ten pieces of Euthynnus alletteratus using a Perkin Elmer Model 306 Atomic Absorption Spectrometer. Furthermore, the accumulation of these metals and the assessment of the long-term implications of human consumption of the designated organs of the two species types were performed. The statistical significance level of the obtained results was determined using the T-test analysis tool, which revealed that the distribution of the metals varies in different organs of the fish species. The levels of pollutants were higher in Tilapia brevimanus organs than in Euthynnus alletteratus. The contents of As, Cd, Cr, and Pb in the gills were higher than those in the muscle. Also, the metal pollution index in the organs followed the order of intestine > gills > muscle for both Tilapia brevimanus and Euthynnus alletteratus, posing serious health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are regarded as critical contaminants in aquatic environments because of their great potential to enter and accumulate in food chains [1, 3, 4, 19]. Different metals accumulate to varying degrees in the bodies of fish. The variable affinity of metals in fish tissues and the different rates of absorption, deposition, and excretion cause these discrepancies [26]. Metal buildup in fish is influenced by pollution and can differ among fish species residing in the same body of water [6]. Several studies in the field and the laboratory has revealed a link between metal concentrations in fish and water [17]. It is important to keep in mind that the concentration of metal in the fish's body is only related to that in the water if the metal consumed by the fish is from the water [15].
Metals can be found in particulate or soluble forms in natural waterways. Labile and non-labile fractions of soluble species of fish are especially vulnerable to labile metal complexes. They include a variety of ionic forms with varying degrees of fish availability. Several studies have revealed that the levels of metals in the labile fraction, as well as the proportion of various metal ions, are highly influenced by environmental factors [16]. The results of numerous studies demonstrate that metals have synergistic or additive effects in most cases, but antagonistic effects can arise in specific mixes [22, 28]. Fish from different species in the same body of water may accumulate varying quantities of metals. Differences in metal buildup between animals may be attributed to lifestyles and diets.
Many studies have found that metals have varying affinities for different tissues [9, 10, 12, 26]. The liver, kidney, and gills store the majority of total body loads accumulated at varying metal concentrations in the water and at various exposure times. Metal concentrations were also found in the digestive tracts of fish from natural water sources [7]. The food uptake route is linked to high metal concentrations in the digestive tract of fish. The gonads, bones, and brain, for example, may have high metal levels, although the muscles, in comparison to the other tissues, normally have moderate metal concentrations but are frequently checked for metal content due to their usage for human food [8]. The buildup of critical metals like iron, zinc, copper, manganese, and cobalt, for example, is organ specific. Copper, for example, has a strong affinity for the liver even at low ambient quantities, whereas zinc concentrates in the gonads due to their primary metabolic functions in these organs. Metal contamination, on the other hand, causes metals to lodge in the same organs where they might cause hazardous effects [11]. Recent investigations on the bioaccumulation of hazardous metals such as As, Pb, Cd, and Cr for risk assessments in marine biota samples from the coastal Ondo area have not been published in spite of the ongoing expansion of small-scale businesses and oil exploration. It has become vital to assess the levels of metal concentration in marine species and the accompanying health risks of their consumption. The objectives of this study are to determine the concentration of metals in the coaster water and fish species (Tilapia brevimanus and Euthynnus alletteratus) from Ondo State and evaluate the probable health hazards associated with consumption of these fish products. The two species of coaster water fish from Ilaje were examined for the presence of four toxic metals (As, Pb, Cd, and Cr) using the Perkin Elmer Model 306 Atomic Absorption Spectrometer, and these species are all well-known and favored by consumers and fishermen around the community and beyond. The modeling of the data was performed using Ecolego 6.4. This was done based on the long-term pollution implications for the water and the organs of the fish. The simulation was predicated on the fact that water uptake and bioconcentration have kinetics that are approximately identical to first-order kinetics [24].
Description of the study area
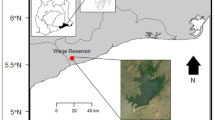
The investigation was conducted in the coastal waters of Ilaje, Ondo State, Southwest Nigeria, which is part of the transgression mud coast. The transgressive mud shore extends to the Benin River in the direction of the east [21]. It is bordered on the west by the shoreline of the Ijebu Waterside Local Government Area in Ogun State, on the north by the land mass of the Ondo State local governments of Okitipupa and Irele, and on the south by the Atlantic Ocean. The total length of the coast currently occupied by the Ilaje people of the river zone of Ondo State is estimated at 75 km. The largely coastal communities currently occupied by the Ilaje, a major river group of the Yoruba race, are known for a number of features of ecological zones, which include tropical lowland rainforests, sandy and sometimes clayey coastal cliffs, swamps and swamp forests, and freshwater mangroves [21]. The installation of crude oil prospecting enterprises by subsidiaries of the Nigerian National Petroleum Corporation (NNPC), Shell, and Chevron oil firms, as well as other joint ventures, characterizes the coastal region under examination. In addition, there is heavy commercial fishing activity, urban runoff from agricultural land, and direct dumping of municipal and industrial garbage into the coastal waters. The sample sites are shown on the map of the research region in Fig. 1.
Sample collections and analysis
The water samples were collected in January 2021, representing the peak of the dry season when the activities on the water are highest in Nigeria, from two points (SLP1 & SLP2) as shown in Fig. 1, along the shoreline at 100 m intervals below the surface film at a depth of 10 cm and 50 cm. All of the glassware and porcelain were soaked in 3 percent HNO3 for 24 h before the collection of our samples. They were rinsed with distilled water, put in the oven, and set aside to cool. A total of 20 water samples were taken with a 500 mL beaker, ten (10) from the first sampling point (SLP1) along the shoreline closed to the shore, and at 100 m intervals far into the coaster water (SLP2), the second set of ten (10) water samples were collected. Conc. HNO3 was added to the water samples to make them acidic.
The twenty (20) pieces of fish were divided into two sets: Tilapia brevimanus (10) and Euthynnus alletteratus (10). The average length for Tilapia brevimanus was 20 cm, and the average weight was 98 g, while for Euthynnus alletteratus, the average length was 30.8 cm, and the average weight was 610.5 g. These fish were caught at SLP1 and SLP2 as indicated in Fig. 1 from the water bodies with the assistance of local fishermen within two weeks. The samples were transported to the laboratory, where they were stunned by an accurate blow to the head with a blunt instrument. The force was proportionate to the size of the fish, and the blow was aimed just above the eyes to impact on the brain, causing the fish to be unconscious and then cut accordingly [23]. The inedible parts, such as scales, were removed, the samples were rinsed with distilled and deionized water to remove any sediment particles and adhering substances, and the edible parts (gills, intestines, and muscles) of each fish were extracted with a dissecting knife [30]. All procedures were performed in compliance with the institutionally approved guidelines [2]. The edible organs were washed, then separately oven-dried at 105 ± 2 °C in a Gallen Kamp moisture extraction oven until constant weight was obtained, homogenized using a grinder, and then stored in a desiccator in labeled plastic containers until digestion [9]. In a round-bottom flask, one gram of homogenized sample was weighed, and 10 mL of conc. HNO3 and 2 mL of HClO4 were added. 50 mL of distilled water was added and filtered after the digestion at 65 °C. To get to 100 mL, the filter paper was rinsed with more distilled water.
The digest was kept in pre-cleaned polypropylene vials before being examined using an Agilent (FS240AA) atomic absorption spectrometer. The standard was run first for the working curves, followed by the blank to overcome instrument drift, as is typical procedure in the laboratory. A Perkin Elmer model 306 atomic absorption spectrometer was used to analyze the specified heavy metal. 100 mL of the material was put into a 250-mL glass beaker after it had been vigorously agitated. The metal concentration was read in µg/L from the extracted standard calibration curve after the sample was inhaled into an oxidizing air/acetylene or nitrous oxide/acetylene flame. Each element's analyses were done in triplicate, and the average values were reported.
All of the water samples were securely maintained in iceboxes before being transferred to the laboratory, where they were kept in a refrigerator for five days before being tested. In a 250-mL conical flask, all the water samples were acidified to 100 mL and measured, then 5 mL of concentrated HNO3 was added and covered with a watch glass. The contents were heated for 50 min on a hot plate at 60 °C, then cooled to 40 °C. The flask and watch glasses were cleaned with distilled water and filtered into a clean conical flask, followed by a 5 mL distilled water rinse of the filter. The filtrate was transferred to a 100 mL volumetric flask, and the conical flask was washed with 5 mL distilled water. The sample was produced with distilled water up to the 100 mL mark, completely mixed, and examined using a (Perkin Elmer model 306 atomic absorption spectrometer.
Statistical analysis
The elemental concentration of the observed samples was statistically analyzed using the statistical package SPSS (version 16.0). The mean ± standard deviations of the metal concentration in fish species were determined. The statistical differences of mean metal levels among fish organs were also analyzed using multiple comparison tests in SPSS package program. Independent t-test, was also carried out.
Metal pollution index (MPI)
This was utilized to look at the overall heavy metal concentration in the coaster water as well as the different sorts of fish found in the sampling areas. The index was calculated by computing the geometrical mean of all metal concentrations in the coaster water and the organs of the fish types [14] using Eqs. 1.
where \(CF_i\) = concentration of the metal, n = total number of metals
Bio-concentration factor (BCF) estimation
In this study, BCF was computed as the ratio of pollutant concentration in fish organ types to pollutant content in ambient water at equilibrium conditions, as published by [31]. The following assumptions were resolved from this BCF estimation: (a) the surrounding conditions, feeding, excretion by fish, and chemical concentrations in the coaster water had reached equilibrium state; (b) the kinetic BCF, also known as the kinetic BCF, is equal to the equilibrium state; and (c), BCF is obtained by the ratio of the steady-state concentration in fish to that in water followed first-order kinetics [23, 27]
The following mathematical formulas demonstrate this assumption:
where, abiotic media represent water, k1 = first-order rate constant for uptake into fish (day−1) and k2 = first-order rate constant for excretion from fish (L/kg/d) and
where \(f_u\) = uptake rate constant, \(f_e\) = excretion rate constant, \(C_W\) = concentration in water,
\(C_F\) = concentration in fish types. If the BCF is greater than 1.0, then bioaccumulation for metal occurs by the fish samples [20].
An allometric relationship has been derived in the literature relating fish weight to the first-order rate constant for uptake into fish (L/kg/d) at the gill as [5],
where W is the weight of the fish in gram and \(k_{OW}\) represents the octanol–water partition coefficient obtained from [5] to be 3.50, which is the widely used molecular descriptor in quantitative structure–activity relationships for contaminants
Simulation using Ecolego 6.4
A simulation of the long-term pollution implications in the water and the organs of the fish kinds was performed using the results in the concentration of the metals found in water and the fish organs. The simulation was predicated on the observation that water uptake and bio-concentration have kinetics that are approximately identical to first-order kinetics [24]
This means that the following relationships can be used to approximate the change in concentration in the fish (CF) or water (CW) by [24]:
where \(P_{W,T}\), \(P_{F,T}\) = Predictive pollutant level in water and fish organs over time (t).
The intake of water was assumed to exchange through the gills and skin in this model. The gills are responsible for a variety of functions, including (i) respiratory gas exchange, (ii) water ingress and egress via osmosis, (iii) ionic and acid–base regulation, (iv) nitrogen excretion, as well as toxicants and heavy metals, and (v) passive uptake and elimination of natural and anthropogenic lipophilic compounds [5]. The gills are made up of four arches on either side of the pharynx, each with a double row of gill filaments. The secondary lamellae are a row of closely spaced leaflets found on the upper and lower sides of each filament. Its two parallel faces are spaced apart and pass through the pillar cells, which are necessary for pollutant uptake and removal in water.
Results and discussion
Analysis of metal distribution
The mean ± standard deviation of concentrations of Pb, Cd, As, and Cr in the sampled points is presented in Table 1. In SLP1 samples, the mean value of heavy metals follows the sequence: Cd > Cr > As > Pb, with values (in µg/L) of 94.15 ± 0.95, 19.41 ± 1.57, 15.54 and 15.54 ± 1.11, respectively. The contents of Cd in SLP1 are higher than the permissive concentrations (5 µg/L) of the continental crust recommended by NESREA (2007). Yu et al. [30] attributed an increase in cadmium content to the corrosion of galvanized iron, emissions from burning fossil fuels, corrosion agents, and color pigments. In contrast, the average values of metals in SLP2 display the order as Cr > Cd > As > Pb, with average values (in µg/L) of 0.95 ± 0.01, 0.89 ± 0.01, 0.78 ± 0.01, and 0.72 ± 0.01, respectively. The concentrations of all metals studied were higher at point SLP1 than at point SLP2. However, average values of Pb and As are within the permissive level.
Analysis of metal concentrations in fish tissue
The average contents of Pb, Cd, As and Cr in the muscle, gills and intestines of Tilapia brevimanus and Euthynnus alletteratus collected from the study area are presented in Table 2. The order of the concentration of heavy metals in the fish species examined in the research region is: intestine > Glls > Muscle. Most of the world's population eats fish muscles as food. The species of Tilapia brevimanus and Euthynnus alletteratus are important sources of protein and are eaten all throughout Nigeria. However, considerable variations were observed in the metals concentrations among various species. Amidst the species, Tilapia brevimanus has the highest concentrations of almost all the four metals observed. This may be attributed to its large size (higher biomass), it has been observed than larger fish tend to accumulate higher amount of heavy metals.
Lead (Pb) is a pervasive contaminant that might enter the coast river by the discharge of industrial effluents from several businesses, including printing, dyeing, oil refineries, textiles and other sources. The highest lead concentrations were found in the intestine of Tilapia brevimanus (219.21 ± 13.18 µg/L), while the lowest lead concentrations (0.52 ± 0.01 µg/L) were found in the muscle of Euthynnus alletteratus. The t-test result showed there was a statistically significant difference in Pb concentration between the fish species organs (Muscle and Gills) while Intestine showed no significant difference. Since, the lead content in the muscle of freshwater fish corresponded to 0.52 ± 0.01 µg/L (Euthynnus alletteratus) and 0.82 ± 0.08 (Tilapia brevimanus), a careful attention should be paid to edible freshwater fish species in the study area. The accumulation pattern of Pb in fish tissues was in the descending order of gill > muscle > intestine in all the species examined.
Cadmium (Cd) is a dangerous pollutant and a highly poisonous element that is present in a variety of sources and is transmitted through the water and air. The muscle of Tilapia brevimanus had the lowest quantity of Cd (101.71 ± 1.45 µg/L), whereas the intestine of Euthynnus alletteratus had the greatest content of Cd (177.43 ± 1.27 µg/L). Furthermore, for the two fish species, the intestine had a greater Cd concentration than the muscle and gill, this is because metallothionein proteins exist, which have the ability to bind particular heavy metals like Cd and Cu for detoxification [25]. The lowest level of Cd in the muscle of Tilapia brevimanus was greater than the threshold value 0.5 g/g for fish and predators. Except for the gills, t-test result also revelead a statistical significant difference in the organ of the two fish sepcies. It was clearly seen from the experimental investigation that Cd levels in the tissue of the species from Ilaje coast river were greater than the standards mentioned above, also, long-term accumulation of Cd in fish may be harmful to human health. Different industrial and household channels that are induced in the Ilaje coast river are to responsible for the load of Cd present.
Arsenic is a poisonous substance frequently found in aquatic environments. Low doses of exposure to inorganic As may result in numerous consequences on the liver, skin, cardiovascular, and hematological systems, among other organs. The lowest concentration of As is found in the muscle of Tilapia brevimanus while the intestine of this same species had the greatest concentration. As found in these species tissue were in organic forms, which are less dangerous than inorganic [18]. However, the arsenic concentration was beyond the permissive level, with no statistical significant difference in the organ of the two fish sepcies except for the muscle, having (p < 0.05).
The concentration (µg/L) of chromium (Cr) in the chosen fish tissue ranged from 65.12 ± 3.36 to 284.71 ± 1.82. The lowest concentration of Cr was found in the muscle of Tilapia brevimanus. The WHO and FEPA both recommended a daily acceptable chromium content of 0.15 g/g, however, the European Union Commission recommended 1 g/g. In this investigation, the Cr concentration was greater than the recommended values from regulatory bodies. T-test result also showed a statistical significant different the mean concentration of Cr present in the fish species (muscle and Intestine). The Ilaje coast river's fish tissues and river water are been shown to contain significant concentrations of Cr, which puts the fish at severe risk.
Potential health risk assessment and bio-concentration factor
The MPI values for the analyzed heavy metals in muscle, gills and instines of the two fish species are displayed in Fig. 2. The distribution of the tested heavy metals for the two fish species was in the ascending order of muscle < gills < intestine. In the intestines of the two fishes, the BCF were high in ascending sequence of Pb > Cr > As > Cd, followed by the gills and muscles in the same pattern, as shown in Fig. 3. It was discovered that all of the chosen fish's organs had been exposed to the heavy metal concentration through the water. In the present study, the BCF of the heavy metals in the species-specific different fish tissues, i.e., gill, liver, and muscle showed that there was an appreciable chance of bioaccumulation of the different heavy metals in the fish body organ tissues.
Modeling results
The results of the simulations of the concentration rate of Pb, As, Cd and Cr in the muscles, gills, and intestines of the Tilapia brevimanus and Euthynnus alletteratus over six years are presented in the Fig. 4. The concentration rate of Cd increased sharply over the six year periods in all the organs (Muscles, Gills and Intestines) of the Tilapia brevimanus and Euthynnus alletteratus.
Metal stimulation in muscles of Tilapia brevimanus and Euthynnus alletteratus
The simulation shows that (Fig. 4 bT), for Tilapia brevimanus, considering the muscles, in terms of the Cd, it ranged from 0.1017 mg/kg (zero year) to 0.2230 mg/kg (six years) showing 54.39% growth rate with a mean value of 0.1656 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 bE), it ranged from 0.1051 mg/kg (zero year) to 0.2291 mg/kg (six years) showing 54.12% growth rate with a mean value of 0.1702 mg/kg for over six years.
In terms of the Cr (Fig. 4 bT), the muscles of Tilapia brevimanus simulation indicated that it concentrations moved from 0.0651 mg/kg (zero year) to 0.0795 mg/kg (six years) showing 17.52% growth rate with a mean value of 0.0727 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 bE) it moved from 0.0616 mg/kg (zero year) to 0.0749 mg/kg (six years) showing 17.80% growth rate with a mean value of 0.0689 mg/kg for over six years.
As simulation for Tilapia brevimanus considering the muscles (Fig. 4 bT) indicated that it extended from 0.0697 mg/kg (zero year) to 0.0812 mg/kg (six years) showing 14.12% growth rate with a mean value of 0.0761 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 bE), it shifted from 0.0652 mg/kg (zero year) to 0.076 mg/kg (six years) showing 14.41% growth rate with a mean value of 0.0712 mg/kg for over six years.
In the case of Pb, (Fig. 4 bT) revealed that in the muscles, of Tilapia brevimanus, the values slightly oscillated from 0.00082 mg/kg (zero year) to 0.00085 mg/kg (six years) showing 3.53% growth rate with a mean value of 0.00084 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 bE), it slightly moved from 0.00052 mg/kg (zero year) to 0.00054 mg/kg (six years) showing 3.70% growth rate with a mean value of 0.00053 mg/kg for over six years.
Metal stimulation in gills of Tilapia brevimanus and Euthynnus alletteratus
In the case of the gills, for Tilapia brevimanus (Fig. 4 aT), the Cd concentration ranged from 0.1171 mg/kg (zero year) to 0.2506 mg/kg (six years) showing 53.27% growth rate with a mean value of 0.1866 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 aE), it ranged from 0.1172 mg/kg (zero year) to 0.2509 mg/kg (six years) showing 53.26% growth rate with a mean value of 0.1867 mg/kg for over six years.
For Cr concentration in the gills of Tilapia brevimanus (Fig. 4 aT), the values oscillated from 0.1245 mg/kg (zero year) to 0.1466 mg/kg (six years) showing 15.78% growth rate with a mean value of 0.1364 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 aE), it ranged from 0.1252 mg/kg (zero year) to 0.1473 mg/kg (six years) showing 15.06% growth rate with a mean value of 0.1372 mg/kg for over six years.
In terms of the As for Tilapia brevimanus (Fig. 4 aT), considering the gills, the simulation indicated that it ranged from 0.0745 mg/kg (zero year) to 0.0865 mg/kg (six years) showing 13.85% growth rate with a mean value of 0.0810 mg/kg for over six years while for Euthynnus alletteratus (Fig. 4 aE), it raised from 0.0731 mg/kg (zero year) to 0.0849 mg/kg (six years) showing 13.92% growth rate with a mean value of 0.079 mg/kg for over six years.
In the case of Pb concentration in the gill, (Fig. 4 aT) revealed that in Tilapia brevimanus, the values slightly oscillated from 0.00041 mg/kg (zero year) to 0.00043 mg/kg (six years) showing 4.65% growth rate with a mean value of 0.00042 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 aE) it slightly moved from 0.00061 mg/kg (zero year) to 0.00063 mg/kg (six years) showing 3.17% growth rate with a mean value of 0.00062 mg/kg for over six years.
Metal stimulation in intestines of Tilapia brevimanus and Euthynnus alletteratus
In the case of the intestines, the concentration rate of Cd (Fig. 4 cT) increased sharply from 0.1565 mg/kg (zero year) to 0.3213 mg/kg (six years) showing 51.28% growth rate with a mean value of 0.2380 mg/kg for over six years for Tilapia brevimanus whilst for Euthynnus alletteratus (Fig. 4 cE) it increased from 0.1774 mg/kg (zero year) to 0.3588 mg/kg (six years) showing 50.54% growth rate with a mean value of 0.2671 mg/kg for over six years.
In terms of Cr concentration (Fig. 4 cT) in the Tilapia brevimanus, the simulation indicated that it concentrations raised from 0.2802 mg/kg (zero year) to 0.3240 mg/kg (six years) showing 13.51% growth rate with a mean value of 0.3029 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 cE), it increased from 0.2847 mg/kg (zero year) to 0.3291 mg/kg (six years) showing 13.49% growth rate with a mean value of 0.3077 mg/kg for over six years.
In terms of the concentration of As in Tilapia brevimanus (Fig. 4 cT), it increased from 0.0897 mg/kg (zero year) to 0.1033 mg/kg (six years) showing 13.18% growth rate with a mean value of 0.0968 mg/kg for over six years in the intestines whilst for Euthynnus alletteratus (Fig. 4 cE), it shifted from 0.0882 mg/kg (zero year) to 0.1016 mg/kg (six years) showing 13.23% growth rate with a mean value of 0.0951 mg/kg for over six years in the intestines.
In terms of the concentration rate of Pb (Fig. 4 cT), in the intestines of Tilapia brevimanus, the values slightly oscillated from 0.2192 mg/kg (zero year) to 0.22388 mg/kg (six years) showing 2.08% growth rate with a mean value of 0.2215 mg/kg for over six years whilst for Euthynnus alletteratus (Fig. 4 cE) it barely increased from 0.2153 mg/kg (zero year) to 0.2199 mg/kg (six years) displaying 2.09% growth rate with a mean value of 0.2175 mg/kg for over six years.
From this work, the results obtained showed that the concentration of heavy metals at the first sampling point (SLP1) of the coastal waters of Ilaje is higher than the concentration obtained in the SLP2. This shows that the high concentration of heavy metals in the first sampling point (which is close to the coast), could be due to human activities on the coast. The first point (SLP1) of water represents a potential health risk for human and aquatic life (because the concentration of heavy metals is high compared to the limits set by the NESREA, (2007), in particular the surrounding communities whose main source of livelihood and food comes from these waters. The deduction that can be drawn from the above results is that tilapia fish (Tilapia brevimanus) have a higher concentration of heavy metals than small tunas (Euthynnus alletteratus) and that the organs of these fish have different bioaccumulation rates as this was also observed by Sehar et al. (2014). Thus, their concentrations in individual organs will be different. This is due to the concentration of the water in which the fish are found [29]. In Tilapia brevimanus, Pb was shown to have the highest concentration in the intestines and the lowest in the muscles, Cd was the highest in the intestines and also the lowest in the muscles. As turned out to be very high in the intestine and the concentration of Cr was highest in the gills. According to this analysis, the intestines have the highest rate of bioaccumulation and muscle the least and similar observation has been reported by [12, 13].
Conclusion
This study revealed that the first sample point SLP1 (close to the shoreline) of water poses potential health risk to human and aquatic life (especially Cd concentration is high in comparison to limits set by NESREA, most especially the surrounding communities whose main source of livelihood and food comes from these waters. The insinuation from this study is that Tilapia brevimanus has more heavy metal concentration compared to Euthynnus alletteratus and the organs of these fishes have different bioaccumulation rates. The metal pollution index is in the order: intestine > gills > muscle for both Tilapia brevimanus and Euthynnus alletteratus. This search achieves that, the concentration of heavy metals in areas close to the coast is higher than the concentration in the high seas. In addition, Tilapia brevimanus and Euthynnus alletteratus can serve as good biomarkers to measure the concentrations of heavy metals. Fish intestines are recommended to be avoided as long-term exposure of humans to heavy metals could lead to immune system dysfunction among many other possible health risks.
Availability of data and materials
All experimental data for this study are available by contacting the corresponding author.
References
Adebayo AS, Olufemi AP, Ogundele LT, Okunnuwa OQ, Toyeje AB, Olowookere CJ (2022) Ecological and human health risk assessments of metals in soil and tailing from Ife-Ijesha gold mining are, Southwest Nigeria. Environ Earth Sci 81(18):462
Adeyemo OK, Alarape SA, Adetunji VE, Saka AB, Adebowale OO, Ubiogoro OO, Agbede SA (2021) Safety hazards along animal food supply chain in Nigeria. Food Secur Safety Afr Perspect 869–883.
Ashayeri NY, Keshavarzi B (2019) Geochemical characteristics, partitioning, quantitative source apportionment, and ecological and health risk of heavy metals in sediments and water: a case study in Shadegan Wetland, Iran. Mar Pollut Bull 149:110495
Balakrishnan T, Sundaramanickam A, Shekhar S, Balasubramanian T (2015) Distribution and seasonal variation of heavy metals in sediments of Muthupet lagoon, southeast coast of India. J Ecol Eng 16(3):49–60
Brooke DN, Crookes MJ, Merckel DA (2012) Methods for predicting the rate constant for uptake of organic chemicals from water by fish. Environ Toxicol Chem 31(11):2465–2471
Burada A, Odor D, Teodorof L, Nastase C, Nastase A, Navodaru I, Georgescu L (2014) Mercury levels in fish tissues with and without commercial value from Danube delta Biosphere reserve. J Environ Prot Ecol 15(3):842–850
Chen C-W, Kao C-M, Chen C-F, Dong C-D (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66(8):1431–1440
Çoǧun HY, Kargın F (2004) Effects of pH on the mortality and accumulation of copper in tissues of Oreochromis niloticus. Chemosphere 55(2):277–282
Fu L, Lu X, Niu K, Tan J, Chen J (2019) Bioaccumulation and human health implications of essential and toxic metals in freshwater products of Northeast China. Sci Total Environ 673:768–776
Issa S, Elkokaney OI, Khedr R, Genena D, Abdel Rahman SM (2023) Lead, cadmium, zinc, and copper concentrations in different Arabian Gulf fish species. Egypt Soc Clin Toxicol J 11(1):146–160
Jezierska B, Witeska M (2006) The metal uptake and accumulation in fish living in polluted waters. In: Soil and water pollution monitoring, protection and remediation. Springer, pp 107–114
Kaçar E (2022) Relationship of concentrations of some heavy metals with fish size in muscle tissue of Carassius gibelio (Bloch, 1782) from the Tigris River (Turkey). Erzincan Univ J Sci Technol 15(2):475–481
Kalipci E, Cüce H, Ustaoğlu F, Dereli MA, Türkmen M (2023) Toxicological health risk analysis of hazardous trace elements accumulation in the edible fish species of the Black Sea in Türkiye using multivariate statistical and spatial assessment. Environ Toxicol Pharmacol 97:104028
Karaouzas I, Kapetanaki N, Mentzafou A, Kanellopoulos TD, Skoulikidis N (2021) Heavy metal contamination status in Greek surface waters: a review with application and evaluation of pollution indices. Chemosphere 263:128192
Labianca C, De Gisi S, Notarnicola M (2018) Assessing the correlation between contamination sources and environmental quality of marine sediments using multivariate analysis. EEMJ. https://doi.org/10.30638/eemj.2018.237
Limbu SM, Zhou L, Sun S-X, Zhang M-L, Du Z-Y (2018) Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ Int 115:205–219
Liu J, Guo J, Cai Y, Ren J, Lu G, Li Y, Ji Y (2022) Multimedia distribution and ecological risk of bisphenol analogues in the urban rivers and their bioaccumulation in wild fish with different dietary habits. Process Saf Environ Prot 164:309–318
Muñoz O, Devesa V, Suñer MA, Vélez D, Montoro R, Urieta I, Macho ML, Jalón M (2000) Total and inorganic arsenic in fresh and processed fish products. J Agric Food Chem 48(9):4369–4376
Njinga RL, Olufemi AP, Adebayo AS (2022) Major chemical carcinogens and health exposure risks in some therapeutic herbal plants in Nigeria. PLoS ONE 17(11):e0276365
Nowrouzi M, Pourkhabbaz A (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve, Iran. Chem Speciat Bioavailab 26(2):99–105
Nubi AO, Popoola SO, Dada OA, Oyatola OO, Unyimadu JP, Adekunbi OF, Salami AM (2022) Spatial distributions and risk assessment of heavy metals and PAH in the southwestern Nigeria coastal water and estuaries, Gulf of Guinea. J Afr Earth Sc 188:104472
Odongo M, Mutagaya SA, Wanasolo W, Oketch D (2022) Investigation of levels of some selected heavy metals in raw bovine milk from Oyam District, Uganda and estimation of potential health risks. Am J Appl Indus Chem 6(1):1–6
Orata F, Birgen F (2016) Fish tissue bio-concentration and interspecies uptake of heavy metals from waste water lagoons. J Pollut Eff Cont. https://doi.org/10.4172/2375-4397.1000157
Park J, Lee S, Lee E, Noh H, Seo Y, Lim H, Shin H, Lee I, Jung H, Na T (2019) Probabilistic ecological risk assessment of heavy metals using the sensitivity of resident organisms in four Korean rivers. Ecotoxicol Environ Saf 183:109483
Ploetz DM, Fitts BE, Rice TM (2007) Differential accumulation of heavy metals in muscle and liver of a marine fish, (king mackerel, Scomberomorus cavalla Cuvier) from the Northern Gulf of Mexico, USA. Bull Environ Contam Toxicol 78:134–137
Police S, Maity S, Chaudhary DK, Dusane CK, Sahu SK, Kumar AV (2021) Estimation of trace and toxic metals in marine biota and associated health risk assessment in Thane Creek, Mumbai, India. Environ Chem Ecotoxicol 3:234–240
Rahayu DR, Anggoro S, Soeprobowati TR (2020) Metal concentrations and bio-concentration factor (BCF) in surface water and economical fish species from wadaslintang multipurpose Dam, Wonosobo, Indonesia, IOP Conference Series: Earth and Environmental Science. IOP Publishing, pp 012024. https://doi.org/10.1088/1755-1315/593/1/012024
Taghizadeh SF, Karimi G, Tzatzarakis M, Tsakiris I, Ahmadpourmir H, Azizi M, Afshari A, Ghorani V, Yarmohammadi F, Tsatsakis A (2022) Probabilistic risk assessment of exposure to multiple metals and pesticides through consumption of fruit juice samples collected from Iranian market. Food Chem Toxicol 170:113493
Varol M, Şen B (2012) Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 92:1–10
Yu B, Wang X, Dong KF, Xiao G, Ma D (2020) Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar Pollut Bull 159:111505
Zhelyazkov G, Yankovska-Stefanova T, Mineva E, Stratev D, Vashin I, Dospatliev L, Valkova E, Popova T (2018) Risk assessment of some heavy metals in mussels (Mytilus galloprovincialis) and veined rapa whelks (Rapana venosa) for human health. Mar Pollut Bull 128:197–201
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
RLN: conceptualization, data acquisition, writing—original draft, revising and editing the manuscript. ASA: site location and selection, software, data acquisition and analysis, writing—original draft, manuscript revision. APO: data acquisition, writing—original draft, statistical analyses, software, manuscript revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The author declared that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Njinga, R.L., Adebayo, A.S. & Olufemi, A.P. Bioaccumulation of heavy metals in water and organs of Tilapia brevimanus and Euthynnus alletteratus from a coaster water in southwestern Nigeria. Environ Sci Eur 35, 85 (2023). https://doi.org/10.1186/s12302-023-00794-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00794-5