Abstract
Background
Maintaining osmotic equilibrium plays an important role in the survival of cold-water fishes. Heat stress has been proven to reduce the activity of Na+/K+-ATPase in the gill tissue, leading to destruction of the osmotic equilibrium. However, the mechanism of megatemperature affecting gill osmoregulation has not been fully elucidated.
Results
In this study, Siberian sturgeon (Acipenser baerii) was used to analyze histopathological change, plasma ion level, and transcriptome of gill tissue subjected to 20℃, 24℃and 28℃. The results showed that ROS level and damage were increased in gill tissue with the increasing of heat stress temperature. Plasma Cl− level at 28℃ was distinctly lower than that at 20℃ and 24℃, while no significant difference was found in Na+ and K+ ion levels among different groups. Transcriptome analysis displayed that osmoregulation-, DNA-repair- and apoptosis-related terms or pathways were enriched in GO and KEGG analysis. Moreover, 194 osmoregulation-related genes were identified. Amongst, the expression of genes limiting ion outflow, occluding (OCLN), and ion absorption, solute carrier family 4, member 2 (AE2) solute carrier family 9, member 3 (NHE3) chloride channel 2 (CLC-2) were increased, while Na+/K+-ATPase alpha (NKA-a) expression was decreased after heat stress.
Conclusions
This study reveals for the first time that the effect of heat stress on damage and osmotic regulation in gill tissue of cold-water fishes. Heat stress increases the permeability of fish’s gill tissue, and induces the gill tissue to keep ion balance through active ion absorption and passive ion outflow. Our study will contribute to research of global-warming-caused effects on cold-water fishes.
Similar content being viewed by others
Background
Since the 1850s, global warming has aggravated climate change [1], which causes the rise of global average temperature and probability of extreme high-temperature events during summertime [2,3,4]. In China, extreme high temperature occurs frequently in recent summers, resulting in continuous heat stress in breeding of cold-water fishes. Many studies have documented the significant effects of heat stress on cold-water fishes’ immunity, growth metabolism and behavior [4,5,6,7].
Freshwater makes fish hypoosmotic, which allows ions in the blood to passively diffuse into the surrounding freshwater. Internal osmotic pressure equilibrium is the foundation to maintain cells and physiological processes. To maintain the osmotic pressure, fish requires coordination of multiple organs, including gill, intestine, and kidney tissues [8,9,10]. The gill tissue is the critical organ for osmotic regulation system, which regulates ion exchange with the external environment via ion channel activity and tight connection to maintain osmotic equilibrium [11,12,13,14,15,16,17,18]. Importantly, gill tissue directly contacts with the external environment and, thereby, has more probability to subject to environmental stresses [19]. Previously, the effects of heat stress on morphological changes, damage, and oxidative stress were investigated in gill tissue [20,21,22,23,24,25]. Recently, studies have shown that heat stress could reduce the osmoregulation core enzyme (Na+/K+-ATPase) activity in gill tissue [25, 26]. However, how heat stress effects fish’s osmoregulation function is still unclear.
Sturgeon, widely distributed throughout the Northern Hemisphere [27], is an important economic cold-water fish for producing caviar [28]. In 2021, farming sturgeon reached to 104,280 ton in China, accounting for nearly 80% production over the world [29]. The proportion of Siberian sturgeon reached to 34% among the cultured sturgeon species in China. The southwest area of China is quit suitable for the breeding of Siberian sturgeon because of the relative low water temperature through whole year [30]. However, the resent crazy high-temperature weather swept the southwest China, which is taking huge challenge to sturgeon breeding.
Previous studies have showed that the optimal water temperature was around 20 °C for survival and growth of Siberian sturgeon [31, 32]. However, in the past 50 years, the average summer temperature in southwest China is around 24℃, and the highest temperature reaches to or overs 29 °C [33]. In the previous study of our group, the gill tissue of Siberian sturgeon was damaged via heat stress (at 24℃ and 28℃) [20]. However, the damaged mechanism is poorly understood. This study aims to investigate the effect mechanism of heat stress with focus on osmotic equilibrium in Siberian sturgeon, which will contribute to generate strategy against the emerging of heat stress issue in aquaculture.
Results
Heat stress induces damage of gill tissue
The histopathological change of the gill tissue subjected to heat stress was observed via H&E staining (Fig. 1). The results showed that the structure of gill filaments was complete and clearly visible at 20℃, while the epithelial cells of gill filaments occurred degeneration and hyperostosis after heat stress at 24℃ and 28℃, particularly at 28℃ where necrosis was appeared (Fig. 1A).
Heat stress elevates ROS level of gill tissue
ROS level of gill tissue was measured via immunofluorescence after heat stress. As shown in Fig. 2, heat stress both at 24℃ and 28℃ induced distinct ROS accumulation in gill filaments. Amongst, the ROS level at 28℃ was significantly high than that at 24℃.
Heat stress effects plasma Na+, K+ and Cl− ion contents
Plasma Na+, K+ and Cl− ion contents were measured after heat stress in Siberian sturgeon (Fig. 3). The results displayed that plasma Na+ and K+ ion contents was not significantly changed after heat stress at 24℃ and 28℃ than at 20℃. By contrast, plasma Cl− ion content was obviously decreased after heat stress at 28℃ (p < 0.05) but no significant change at 24℃ than the control group.
Library construct, de novo assembly and annotation
Nine RNA-seq libraries were constructed from the gill tissue after different temperatures exposures in Siberian sturgeon. All datasets from the Illumina sequencing platform can be found in the National Center for Biotechnology Information (NCBI) Short Read Archive (SRA) database under accession number (PRJNA765171). After sequencing and filtering with raw data, 30 to 44 million clean reads were acquired in each library, whose Q30 values ranged from 94.29% to 96.04% in the libraries. Clean reads from all groups were mapping with the corresponding unigene, the mapping rate is 69.09%-74.76% (Table S1). Total of 172,158 unigenes were acquired from de novo assembly. All the unigenes were annotated by five databases, including NR, GO, eggNOG, KEGG and Swiss (Table 1).
Identification of differentially expressed genes (DEGs), GO and KEGG enrichment analysis
Total 8,570 DEGs (FDR < 0.005, log2 (foldchange) ≥ 2)were identified in 24℃ vs 20℃group, containing 4,593 up-regulated genes, and 4,275 down-regulated genes (Fig. S2 A-B). In 28℃ vs 20℃ group, 11,835 DEGs were identified, containing 5,950 up-regulated genes and 5,885down-regulated genes (Fig. S2 C-D).
In 24℃ vs 20℃ group, top 30 GO terms were shown in Fig. 4A, mainly associating with DNA-repair and apoptosis, e.g., “mismatch repair complex”, “nucleotide-excision repair, DNA incision, mismatched DNA binding” and “regulation of reactive oxygen species biosynthetic process”. In 28℃ vs 20℃ group, top 30 GO terms relate to osmoregulation, DNA-repair and apoptosis, including “active ion transmembrane transporter activity”, “ATPase coupled active ion transmembrane transporter activity”, “nucleotide-excision repair, DNA incision,0 mismatched DNA binding” and “regulation of reactive oxygen species biosynthetic process” (Fig. 4B).
In KEGG enrichment analysis, top 30 KEGG pathways were presented in 24℃ vs 20℃ group, which involved in pathways of DNA repair (e.g., “Fanconi anemia pathway” “Mismatch repair” “Base excision repair” and “Nucleotide excision repair”) and apoptosis (e.g., “TNF signaling pathway” “p53 signaling pathway” and “apoptosis”) (Fig. 5A). In 28℃ vs 20℃ group, top 30 KEGG pathways included DNA repair related pathways, e.g., “Fanconi anemia pathway”, “Mismatch repair”, “Base excision repair”, “Homologous recombination”, “Nucleotide excision repair” and “Non − homologous end-joining”, apoptosis-related pathways, e.g., “Protein processing in endoplasmic reticulum”, “Necroptosis” and “Apoptosis” and osmoregulation-related pathways, e.g., “Collecting duct acid secretion”, “Proximal tubule bicarbonate reclamation”, “Protein digestion and absorption”, “Vasopressin-regulated water reabsorption” and “Aldosterone-regulated sodium reabsorption” (Fig. 5B).
KEGG enrichment analysis of DEGs. KEGG enrichment analysis of DEGs identified in 24℃ vs 20℃ (A) and 28℃ vs 20℃ (B) groups, respectively. Osmoregulation-related terms are marked with yellow color; DNA-repair-related terms are marked with green color; apoptosis-related terms are marked with orange color
GO and KEGG enrichment analysis of osmoregulatory DEGs
To investigate the effect of heat stress on osmoregulatory function, osmoregulatory DEGs were identified based on the genes enriched in the osmoregulatory terms of the GO enrichment analysis. In 24℃ vs 20℃ group, a total of 117 DEGs were composed of 92 up-regulated genes and 25 down-regulated genes. In 28℃ vs 20℃ group, 194 DEGs were identified, containing 172 up-regulated genes and 22 down-regulated genes. GO enrichment analysis showed that these DEGs were enriched into three major functional categories: biological processes (BP), cellular components (CC), and molecular functions (MF). In both 24℃ vs 20℃ and 28℃ vs 20℃ groups, many DEGs related to osmoregulation were enriched in “regulation of metal ion transport”, “inorganic ion homeostasis”, “cellular ion homeostasis”, “inorganic ion transmembrane transport”, and “regulation of ion transport” (Fig. 6).
In 24℃ vs 20℃ and 28℃ vs 20℃groups, top 10 KEGG pathways were shown in Fig. S3, of which mainly associate with osmoregulation function, such as “Tight junction”, “Collecting duct acid secretion”, “Vasopressin − regulated water reabsorption”, “Aldosterone − regulated sodium reabsorption” and “Endocrine and other factor-regulated calcium reabsorption”.
Expression of osmoregulatory DEGs
The expression of osmoregulatory DEGs at 24℃ vs 20℃ and 28℃ vs 20℃ groups were visualized by heat map (Fig. 7). Amongst, expression of seven ion absorption (AE2, CLC-Kb, ATP1a, ATP2a, CLC-2, NHE3 and ATP4a), three resistance ion loss (OCLN, JAM and CLDN) and one water molecules' transport (AQP3) genes were changed after heat stress at 24℃ or 28℃. The seven ion absorption genes showed different expression changes, of which the expression level of ATP2a was significantly downregulated but upregulated in other genes, after heat stress at 24℃ or 28℃. The expression levels of three resistance ion loss genes were significantly increased at both 24℃ and 28℃, but the expression level of water molecules' transport gene was only increased at 28℃.
The expression levels of 5 osmoregulatory DEGs were detected by qPCR in gill tissue after heat stress. As shown in Fig. 8B, the expression levels of OCLN, NHE3, CLC-2 and AE2 genes were upregulated, while the expression level of NKA-a gene was significantly downregulated at 24℃ or 28℃. The mRNA expression of these genes was consistent with the RNA-Seq results, indicating the transcriptome data is reliable.
Validation of RNA-seq data. A and B: Comparison of the expression of 5 selected DEGs by RNA-seq and qRT-PCR. The gene expression of RNA-seq was presented based on the RPKM-values. The qPCR results were calculated by normalizing to the reference genes (β-actin and EF-1α), Mean ± SD. One-way ANOVA plus Bonferroni post-tests; different letters indicate statistically significant differences (p < 0.05)
Discussion
Global warming has increased average summer temperatures and the frequency of extreme weather, posing the thermal threat to cold-water fishes [3]. Gill tissue directly contacts with the water environment and maintains the osmotic equilibrium in fishes [16, 18]. However, less information is available concerning the effects of heat stress on osmoregulation in gill tissue of cold-water fishes. To investigate the effect and mechanism of gill osmoregulation after heat stress in cold-water fishes, this study analyzed the changes of gill structure and ROS level, plasma ion concentration, and gill transcriptome in Siberian sturgeon.
The gill tissue is particularly sensitive to environmental temperature changes, making it be a perfect organ to study the effect of heat stress on cold-water fishes [20, 34]. In this study, we observed degeneration and hyperplasia of the gill tissue after heat stress at both 24℃ and 28℃, particularly at 28℃ where necrosis occurred. These results indicate that damage of gill tissue is emphasized with the increasing of heat stress temperature from 24℃ to 28℃, because the emergence of necrotic means possible dysfunction of the gill tissue [35]. Previous study has demonstrated that damage of gill tissue closely related to oxidative stress response [36]. Consistently, ROS level of gill tissue in Siberian sturgeon was upregulated after heat stress at both 24℃ and 28℃.
Na+, K+, and Cl− are the major ions in the blood, and the change of their content has a great influence on the internal homeostasis of fish [10, 37]. The concentration of Na+ and K+ in the blood of Siberian sturgeon did not alter after the heat stress, while the content of Cl− reduced dramatically at 28 °C compared to the control group (Fig. 3). However, no significant changes of Na+, K+, and Cl− concentration were observed in blood ions of European seabass (Dicentrarchus labrax) and Atlantic salmon (Salmo salar) exposed to rising temperature [4, 26, 38], which is different from our results. We speculate that it may be related to kidney damage in fish. Kidney is the organ of Cl− reabsorption in fish [37]. Moreover, renal tissue damage can affect the expression of chloride channel genes [39].
Temperature is an important environmental factor affecting the physiological function of fish [40,41,42]. Osmotic regulation is one of the important physiological functions to maintain fish survival [12, 37]. The GO analysis of DEGs showed that heat stress at 24℃ and 28℃ activated gene expression enriched into “regulating ion transport” (Fig. 6). KEGG pathway enrichment analysis showed that “Tight junction” was enriched (Fig. S3). Tight junctions are essential for establishing a selectively permeable barrier to diffuse ions through the paracellular space between neighboring cells [43]. Previous studies demonstrated that activating Tight junction pathway could reduce ion loss in gill tissue [17, 44]. Thus, our results indicate that heat stress may affect the osmotic function of gill tissue.
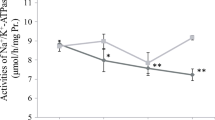
To maintain ion equilibrium in fresh water, gill tissue performs osmotic regulation by lowering passive ion outflow and actively absorbing ions from water [12, 14]. Five genes related to fish osmosis were selected to qPCR analysis from the DEGs. OCLN, one of integral membrane proteins, constitutes the chordates zonula occludes, and it plays an important role in osmotic regulation of fish [43]. In our results, OCLN expression was increased at 24℃ and 28℃ (Fig. 8B). When fish migrate from seawater to freshwater, gill tissue resists ion loss by increasing OCLN expression [17, 45, 46]. At the same time, the expression levels of the ion absorption genes (NHE3, CLC2, AE2) tended to increase with temperature rising (Fig. 8B). It has been demonstrated that the gill tissue improves active ion absorption capacity and resists ion loss by increasing the expression levels of NHE3, CLC-2, and AE2, when fishes migrate from high-salt environment to freshwater [15, 47,48,49]. Numerous previous studies have shown that NKA-α expression decreases when water temperature increases [22, 50, 51], this was substantiated by the change results of heat stress at 28℃ and 24℃ when compared to 20℃ in our study (Fig. 8B).
In summary, our study reveals that heat stress can regulate osmotic function of gill tissue in cold-water fishes. As shown in Fig. 9, we speculate that heat stress results in gill damage and decreases activity of Na+-K+-ATPase, which may further increase the permeability of fish’s gill tissue and boost fish ion loss in fresh water. Besides, to reduce ion loss, the gill tissue keeps ion balance through active ion absorption and passive ion outflow. When gill tissue is slightly damaged, it can withstand ion loss by upregulating CLDN gene expression and increasing the tightness of the tissue's connections. Otherwise, when gill tissue is severely damaged and permeability increases, gill tissue will activate NHE3, AE2, CLC-2 to absorb ions from water and, thereby, maintain the in vivo osmotic equilibrium.
Graphic represents the effects of heat stress on the osmotic function of gill tissue in cold-water fishes. Orange box indicates heat stress-induced lower osmotic pressure in gill tissue, blue box means the osmotic regulation of gill tissue against the heat stress. The dots on left and right sides present the gene expression change in 24℃ vs 20℃ and 24℃ vs 20℃ groups, respectively. Red, blue and orange dots suggest significant upregulation (p < 0.05), downregulation (p < 0.05), and change without significant difference (p > 0.05), respectively
Materials and Methods
Fish breeding
A total of 300 healthy Siberian sturgeon (length of 25.0 ± 2.3 cm, weight of 86.0 ± 4.0 g) were purchased from Sichuan Runzhao Fisheries Co., Ltd., Sichuan, China. All fishes were bred in 40 × 80 × 50 cm3 indoor tanks with an average temperature of 20℃. The sturgeons were fed with 1% body weight of commercial feed (Haida Group Co., Ltd, Guangdong, China) three times per day (at 8:00 am, 14:00 pm, and 20:00 pm, respectively) and bred at least one week of acclimatization before temperature administration. The handling of animals was performed in accordance with the protocols approved by the Animal Protection Committee of Sichuan Agricultural University.
Heat stress administration
After one week of acclimatization, 270 fishes were randomly divided into three groups (n = 90): the control group (at 20℃) and two heat stress groups (at 24℃ and 28℃, respectively). The fishes of two heat stress groups underwent an increasing temperature of 1 ± 0.5℃/day within 8 days to 24℃ and 28℃, respectively, then were maintained at the heat stress temperature for 12 days (Fig. S1).
Sampling
Thirty fishes were randomly selected from each group and anesthetized by 200 mg/L MS-222 (yuxi biological technology co., Lianyungang, China). Blood was collected in tube with heparin sodium for detection of plasma ion contents, and gill tissues were sampled for histopathological observation, ROS immunofluorescence, transcriptome analysis, and qPCR analysis.
Histopathological observation
The acquired gill tissues were fixed with 10% formaldehyde solution for at least 24 h, then dehydrated in graded ethanol solutions, cleared in xylene, embedded in paraffin wax and sectioned into 3–5 μm sections. Sections were prepared, then mounted on slides for hematoxylin and eosin (H&E). After staining, the sections were observed and imaged by a Motic BA400Digital microscope (Motic China Group, Co., Ltd, Xiamen, China).
ROS immunofluorescence
The refrigerant gill tissues were embedded by OCT (Sakura, USA), then sliced by freezing microtome (Thermo, CRYOSTAR NX50, US). The slice thickness was 8–10 μm. The frozen slides were restored to room temperature, then incubated with spontaneous fluorescence quenching reagent (Servicebio, China). ROS staining solution (Servicebio, Wuhan, China) was used to stain slides at 37℃ for 30 min in dark place, following washing three times with PBS (pH 7.4) and then incubating with DAPI solution (Servicebio, Wuhan, China) at room temperature for 10 min in dark place. Finally, the sections were observed by Fluorescent Microscopy (Pannoramic MIDI, 3DHISTECH, HUN).
Determination of plasma Na+, K+ and Cl− contents
The blood was centrifuged at 4000 × g for 10 min, the plasma was collected and frozen at -80℃ until for use. Plasma Na+, Cl− and K+ contents were measured by detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All the procedures were performed following strict adherence to the manufacturer’s instructions.
RNA extraction, library construction, and Illumina sequencing
Total RNA was extracted from the gill tissue by TRIzol® Reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed by DNase I (Takara, Dalian, China). Then, RNA quality was determined by 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA, USA)) and quantified by ND-2000 (NanoDrop Technologies, Shanghai, China). The high-quality RNA sample (OD260/280 = 1.8 ~ 2.2, OD260/230 ≥ 2.0) was used to construct sequencing libraries. RNA-seq transcriptome libraries were prepared following TruSeqTM RNA sample preparation Kit from Illumina (San Diego, CA, USA). Libraries were selected for cDNA target fragments of 200–300 bp on 2% Low Range Ultra Agarose followed by PCR amplified using Phusion DNA polymerase (NEB) for 15 PCR cycles. After quantified by TBS380, paired-end libraries were sequenced by Illumina NovaSeq 6000 platform (Shanghai BIOZERON Co., Ltd).
De novo assembly and annotation
The raw paired-end reads were firstly trimmed, and quality was controlled by Trim Galore and Fast QC software. Then, clean data from all samples was used to de novo assembly with Trinity (http://trinityrnaseq.sourceforge.net/). All the assembled transcripts were annotated against NR, GO, eggNOG, KEGG and Swiss databases. NR database was used to identify the proteins that have the highest sequence similarity with the given transcripts. BLAST2GO (http://www.blast2go.com/b2ghome) program was used to get GO annotations of unique assembled transcripts for describing biological processes, molecular functions, and cellular components.
DEGs identification, GO and KEGG enrichment analysis
After assembly, the gene expression abundance was calculated as FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values that were used to analyze the expression change of genes in the gill tissues of Siberian sturgeon after heat stress. The DEGs of heat stress groups than the control group were identified via identified by DEseq R packages within adjusted p < 0.005, |log2 (foldchange)|≥ 2. The acquired DEGs then underwent GO and KEGG enrichment analysis [52,53,54].
Verification by quantitative real-time PCR (qRT-PCR)
5 DEGs were selected to verify the RNA-Seq results. These genes were measured by qRT-PCR. The Primer 6.0 software was used to design primers (Table 2). Total RNA was isolated from the gill with an animal tissue total RNA extraction kit (Fuji, Chengdu, China). cDNA was synthesized from 2 μg of RNA using a RT Easy™ II kit (Fuji). qPCR was performed using a SYBR green real-time PCR kit (Takara, Kyoto, Japan) and a Thermo Cycler (BioRad, Hercules, CA, USA).
To distinguish between specific and nonspecific reaction products, a melting curve was obtained at the end of each run (Table 2). The 2−△△Ct method was used to calculate relative changes in mRNA transcript expression from the qPCR results (ΔCT = CT target gene – Ct reference gene, ΔΔCT = ΔCT experimental—ΔCT control).
Data analysis
All data were expressed as mean ± standard deviation. Differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni’s Multiple Comparison Test with the significance as p < 0.05.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the Sequence Read Archive of National Center for Biotechnology Information database with accession number PRJNA765171 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA765171). The datasets analyzed during this study are included in this published article and its supplementary information files. Please contact Shiyong Yang (yangshiyong@sicau.edu.cn) if someone wants to request the data from this study.
Abbreviations
- qRT-PCR:
-
Quantitative real-time PCR
- GO:
-
Gene Ontology
- BP:
-
Biological process
- CC:
-
Cellular component
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Meye RKPaLA. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2014.
Jentsch A, JKaCB. A new generation of climate-change experiments: events, not trends. Front Ecol Environ. 2007;5:365–74.
Lazoglou G, Anagnostopoulou C, Tolika K, Kolyva-Machera F. A review of statistical methods to analyze extreme precipitation and temperature events in the Mediterranean region. Theoret Appl Climatol. 2018;136(1–2):99–117.
Islam MJ, Slater MJ, Kunzmann A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: the case of European seabass, Dicentrarchus labrax. Sci Total Environ. 2020;749:141458.
Xu Y, Wang Z, Zhang Y, Liang J, He G, Liu X, Zheng Z, Deng Y, Zhao L. Transcriptome analysis reveals acclimation responses of pearl oysters to marine heatwaves. Sci Total Environ. 2022;810:151189.
Daniel Mameri PB. Maria Teresa Ferreira, José Maria Santos: Heatwave effects on the swimming behaviour of a Mediterranean freshwater fish, the Iberian barbel Luciobarbus bocagei. Sci Total Environ. 2020;730:139–52.
Liu Y, Cheng J, Xia Y, Li X, Liu Y, Liu PF. Response mechanism of gut microbiome and metabolism of European seabass (Dicentrarchus labrax) to temperature stress. Sci Total Environ. 2022;813:151786.
Kersten S, Arjona FJ. Ion transport in the zebrafish kidney from a human disease angle: possibilities, considerations, and future perspectives. Am J Physiol Renal Physiol. 2017;312(1):F172–89.
Whittamore JM. Osmoregulation and epithelial water transport: lessons from the intestine of marine teleost fish. J Comp Physiol B. 2012;182:1–39.
Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R28-47.
Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85(1):97–177.
Evans DH. Freshwater fish gill ion transport: August Krogh to morpholinos and microprobes. Acta Physiol (Oxf). 2011;202(3):349–59.
Chasiotis H, Kolosov D, Bui P, Kelly SP. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: a review. Respir Physiol Neurobiol. 2012;184(3):269–81.
Dymowska AK, Hwang PP, Goss GG. Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol. 2012;184(3):282–92.
Ellis LV, Bollinger RJ, Weber HM, Madsen SS, Tipsmark CK. Differential Expression and Localization of Branchial AQP1 and AQP3 in Japanese Medaka (Oryzias latipes). Cells. 2019;8(5):422.
Guo B, Tang Z, Wu C, Xu K, Qi P. Transcriptomic analysis reveal an efficient osmoregulatory system in Siberian sturgeon Acipenser baeri in response to salinity stress. Sci Rep. 2018;8(1):14353.
Kolosov D, Kelly SP. C-type natriuretic peptide regulates the molecular components of the rainbow trout gill epithelium tight junction complex. Peptides. 2020;124:170211.
Tipsmark CK, Sorensen KJ, Madsen SS. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J Exp Biol. 2010;213(3):368–79.
Xu D, Sun L, Liu S, Zhang L, Yang H. Histological, ultrastructural and heat shock protein 70 (HSP70) responses to heat stress in the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015;45(2):321–6.
Yang S, Yang X, Li Y, Li D, Gong Q, Huang X, Wu J, Huang A, Kong F, Han X, et al. The multilevel responses of Acipenser baerii and its hybrids (A. baerii ♀ × A. schrenckii ♂) to chronic heat stress. Aquaculture. 2021;541:736773.
Li G, Lv X, Zhou J, Shen C, Xia D, Xie H, Luo Y. Are the surface areas of the gills and body involved with changing metabolic scaling with temperature? J Exp Biol. 2018;221(Pt 8):jeb174474.
Masroor W, Farcy E, Gros R, Lorin-Nebel C. Effect of combined stress (salinity and temperature) in European sea bass Dicentrarchus labrax osmoregulatory processes. Comp Biochem Physiol A Mol Integr Physiol. 2018;215:45–54.
Shirangi SA, Kalbassi MR, Khodabandeh S, Jafarian H, Lorin-Nebel C, Farcy E, Lignot JH. Salinity effects on osmoregulation and gill morphology in juvenile Persian sturgeon (Acipenser persicus). Fish Physiol Biochem. 2016;42(6):1741–54.
Sun Z, Lou F, Zhang Y, Song N. Gill Transcriptome Sequencing and De Novo Annotation of Acanthogobius ommaturus in Response to Salinity Stress. Genes (Basel). 2020;11(6):631.
Bernard B, Leguen I, Mandiki SNM, Cornet V, Redivo B, Kestemont P. Impact of temperature shift on gill physiology during smoltification of Atlantic salmon smolts (Salmo salar L.). Comp Biochem Physiol A Mol Integr Physiol. 2020;244:110685.
Vargas-Chacoff L, Arjona FJ, Ruiz-Jarabo I, Garcia-Lopez A, Flik G, Mancera JM. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J Therm Biol. 2020;88:102526.
Bemis WE, Findeis EK. The sturgeons’ plight. Nature. 1994;370:602.
Wang L, Wu J, Wang CA, Li J, Zhao Z, Luo L, Du X, Xu Q. Dietary arginine requirement of juvenile hybrid sturgeon (Acipenser schrenckii♀ × Acipenser baerii♂). Aquac Res. 2017;48(10):5193–201.
Liu X. China Fishery Statistics Yearbook 2021. China: China Agriculture Science and Technique Press; 2021.
Shen L, Shi Y, Zou YC, Zhou XH, Wei QW. Sturgeon Aquaculture in China: status, challenge and proposals based on nation-wide surveys of 2010–2012. J Appl Ichthyol. 2014;30(6):1547–51.
Mai LK xyL, Da-jiang sun. Effects of water temperature on growth of acipenser schisterii, sturgeon microsoma and juvenile Siberian sturgeon. Chin J Fish 2014, 27(15):22.
Pourkazemi M, Shakurian M, Hasani MHS, Pourali HR, Pourasaadi M, Yousefi A. Effects of daily temperature fluctuations on growth and hematology of juvenile Acipenser baerii. J Appl Ichthyol. 2011;27(2):591–4. https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0426.2011.01667.x.
Li j-mbq-lmx. Analysis of Characteristics of temperaturevariations in Southwest China in recent 50 years. Resources and Environment in the Yangtze River Basin 2006, 15(3):346-351.
Liu Y. Histological change and heat shock protein 70 expression in different tissues of Japanese fl ounder Paralichthys olivaceus in response to elevated temperature. Chin J Oceanol Limnol. 2015;33(1):11–9.
Ballesteros ML, Rivetti NG, Morillo DO, Bertrand L, Ame MV, Bistoni MA. Multi-biomarker responses in fish (Jenynsia multidentata) to assess the impact of pollution in rivers with mixtures of environmental contaminants. Sci Total Environ. 2017;595:711–22.
Souza JP, Baretta JF, Santos F, Paino IMM, Zucolotto V. Toxicological effects of graphene oxide on adult zebrafish (Danio rerio). Aquat Toxicol. 2017;186:11–8.
Edwards SL, Marshall WS. Principles and Patterns of Osmoregulation and Euryhalinity in Fishes. In: Euryhaline Fishes. 2012. p. 1–44.
Vargas-Chacoff L, Regish AM, Weinstock A, McCormick SD. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J Fish Biol. 2018;93(3):550–9.
Kuo IY, Ehrlich BE. Ion channels in renal disease. Chem Rev. 2012;112(12):6353–72.
Sun JL, Zhao LL, Cui C, Du ZJ, He Z, Wang Y, Li XW, Yang S. Influence of long-term temperature stress on respiration frequency, Na(+)/K(+)-ATPase activity, and lipid metabolism in common carp (Cyprinus carpio). J Therm Biol. 2019;83:165–71.
Bowden TJ. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008;25(4):373–83.
Martin BT, Dudley PN, Kashef NS, Stafford DM, Reeder WJ, Tonina D, Del Rio AM, Scott Foott J, Danner EM. The biophysical basis of thermal tolerance in fish eggs. Proc Biol Sci. 1937;2020(287):20201550.
Tsukita S, MFaMI. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2009;2(4):285–93.
Chen CC, Marshall WS, Robertson GN, Cozzi RRF, Kelly SP. Mummichog gill and operculum exhibit functionally consistent claudin-10 paralog profiles and Claudin-10c hypersaline response. Biol Open. 2021;10(7):058868.
Tipsmark CK, Baltzegar DA, Ozden O, Grubb BJ, Borski RJ. Salinity regulates claudin mRNA and protein expression in the teleost gill. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R1004-1014.
Chasiotis H, Effendi JC, Kelly SP. Occludin expression in goldfish held in ion-poor water. J Comp Physiol B. 2009;179(2):145–54.
Breves JP, Keith PLK, Hunt BL, Pavlosky KK. clc-2c is regulated by salinity, prolactin and extracellular osmolality in tilapia gill. J Mol Endocrinol. 2017;59(4):391–402.
Blondeau-Bidet E, Hiroi J, Lorin-Nebel C. Ion uptake pathways in European sea bass Dicentrarchus labrax. Gene. 2019;692:126–37.
Li J, Xue L, Cao M, Zhang Y, Wang Y, Xu S, Zheng B, Lou Z. Gill transcriptomes reveal expression changes of genes related with immune and ion transport under salinity stress in silvery pomfret (Pampus argenteus). Fish Physiol Biochem. 2020;46(4):1255–77.
Monroe I, Wentworth S, Thede K, Aravindabose V, Garvin J, Packer RK. Activity changes in gill ion transporter enzymes in response to salinity and temperature in fathead minnows (Pimephales promelas). Comp Biochem Physiol A Mol Integr Physiol. 2019;228:29–34.
Chadwick JG, McCormick SD. Upper thermal limits of growth in brook trout and their relationship to stress physiology. J Exp Biol. 2017;220(Pt 21):3976–87.
Kanehisa MaG S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51.
Acknowledgements
Not applicable.
Funding
The present study has been made possible by research grants from Provincial 14th Five Years’ Animal Breeding of Sichuan Province (2021YFYZ0015); the Science and Technology Achievement Transfer and Transformation Demonstration Project of Sichuan Province (2021ZHCG0065); the Applied Basic Research from Technological Office of Sichuan Province (in 2022); and the Two Sides Supporting Plan in Sichuan Agricultural University.
Author information
Authors and Affiliations
Contributions
Shiyong Yang and Datian Li designed the experiment and wrote the manuscript. Xiaoli Huang designed the experiment. Datian Li, Chaoyang Zhang, Langkun Feng, ChaoZhan Yan, ZiHan Xu, and Yu Jie Zhang carried out the experiment, organized the data, and carried out statistical analyses. Datian Li, Hongli Liu and Dandan Xi carried out data visualization. Taiming Yan, Quan Gong, Jun Du, Zhi He, ShiYong Yang, and Jiayun Wu provided the experimental samples. XiaoGang Du, YunKun Li ShiYong Yang and DaTian Li discussed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols involved in fishes in this study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Sichuan Agricultural University and ARRIVE guidelines (https://arriveguidelines.org). All procedures and investigations were reviewed and approved by the Animal Research and Ethics Committees of Sichuan Agricultural University and performed in accordance with the guidelines of the committee (Approval No.20190031).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig.S1. Temperature control Day1 to 7: temperature acclimation; Day7 to 14: Reaching targeted temperature. Day15 to 27: Summer water temperature exposure. Fig. S2. DEGs analysis. (A, B) DEGs analyzed in 24℃-vs-20℃. (C, D) DEGs in 28℃-vs-20℃. Each dot represents one gene. Red dots represent up-regulated genes and blue dots represent down-regulated genes. Gray dots represent genes with no differential expression. Fig. S3. KEGG pathway of osmoregulation DEGs. (A) 24℃-vs-20℃. (B) 28℃-vs-20℃. Table S1. The sequence quality and mapping results in the nine samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Li, D., Feng, L. et al. Transcriptome analysis reveals the high temperature induced damage is a significant factor affecting the osmotic function of gill tissue in Siberian sturgeon (Acipenser baerii). BMC Genomics 24, 2 (2023). https://doi.org/10.1186/s12864-022-08969-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08969-9