Abstract
Background
Sarcopenic obesity arises from increased muscle catabolism triggered by inflammation and inactivity. Its significance lies in its role in contributing to morbidity and mortality in gastric cancer. This study aims to explore the potential correlation between sarcopenia, sarcopenic obesity, and gastric cancer, as well as their effect on survival.
Materials and methods
This retrospective study included 162 patients aged ≥ 18 years who were diagnosed with stomach cancer. Patient age, gender, diagnostic laboratory results, and cancer characteristics were documented. Sarcopenia was assessed using the skeletal muscle index (SMI) (cm2/m2), calculated by measuring muscle mass area from a cross-sectional image at the L3 vertebra level of computed tomography (CT).
Results
Among the 162 patients, 52.5% exhibited sarcopenia (with cut-off limits of 52.4 cm2/m2 for males and 38.5 cm2/m2 for females), and 4.9% showed sarcopenic obesity. Average skeletal muscle area (SMA) was 146.8 cm2; SMI was 50.6 cm2/m2 in men and 96.9 cm2 and 40.6 cm2/m2 in women, respectively. Sarcopenia significantly reduced mean survival (p = 0.033). There was no association between sarcopenic obesity and mortality (p > 0.05), but mortality was higher in sarcopenic obesity patients (p = 0.041). Patient weight acted as a protective factor against mortality, supporting the obesity paradox. Tumor characteristics, metabolic parameters, and concurrent comorbidities did not significantly impact sarcopenia or mortality.
Conclusion
Sarcopenia is more prevalent in the elderly population and is linked to increased mortality in gastric cancer patients. Paradoxically, higher body mass index (BMI) was associated with improved survival. Computed tomography offers a practical and reliable method for measuring muscle mass and distinguishing these distinctions.
Trial registration
This study was approved by Istanbul Training and Research Hospital Clinical Research Ethics Committee of the University of Health Sciences (29.05.2020/2383).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Sarcopenia is described as progressive and generalized loss of muscle mass, strength or function. Primary sarcopenia is a consequence of the aging process preceding frailty, while secondary sarcopenia is linked to factors like inactivity, poor nutrition, comorbidities such as organ failure, chronic inflammation, or cancer [1,2,3].
Various diagnostic methods are available for sarcopenia, including nuclear magnetic resonance imaging (MRI), computed tomography (CT), dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis for muscle mass, and tests for muscle function like gait speed, hand-grip strength, and low extremity strength [4,5,6,7]. Thus, it can easily be screened and diagnosed with a variety of tools and methods, most of which are simple and readily available.
Sarcopenic obesity is a recently coined term that describes the combination of sarcopenia and obesity, characterized by elevated body mass index (BMI), waist circumference (WC), total fat mass, body fat percentage, or visceral fat area [4]. It arises due to increased muscle catabolism triggered by inflammation and/or inactivity in patients with obesity [8]. Both sarcopenia and sarcopenic obesity are associated with higher overall mortality in cancer patients [9], rendering them valuable prognostic indicators in malignancy cases.
Gastric cancer, ranking fifth in terms of prevalence and third in cancer-related deaths [10], has been implicated in a reciprocal relationship with sarcopenia, possibly contributing to its development and progression. While the precise mechanisms remain unclear, muscle wasting in sarcopenia is believed to interact with systemic inflammation, altered immune function, and nutritional deficits, potentially facilitating cancer growth and metastasis [11]. Conversely, gastric cancer-related inflammation and metabolic changes may exacerbate sarcopenia [1, 12]. Regardless of the definition and measurement method employed, sarcopenia is linked to poor prognosis in gastric cancer [13,14,15].
In this context, this study aims to explore the relationship between gastric cancer and sarcopenia/sarcopenic obesity, investigating their potential as predictive tools for mortality in this patient group, as well as the additional benefits of CT for detecting these conditions.
Materials and methods
Patient population
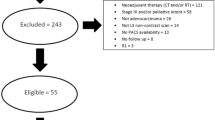
The study included adult patients diagnosed with gastric cancer at our hospital between 2015 and 2019, adhering to inclusion and exclusion criteria.
Inclusion criteria comprised age ≥ 18, any gender, presence of a medical file in our oncology clinic, availability of height-weight data, and an abdominal CT scan covering the entire abdomen and the third lumbar spine level, conducted prior to treatment.
Exclusion criteria encompassed patients lacking BMI data, those with only post-operative CT scans, and patients whose CT scans did not include the third lumbar spine level.
A digital database was established for laboratory values, tumor histopathological features, treatment protocols, complications, and dates of diagnosis and death. A computer program (PROBEL) facilitated data processing. Out of 1136 patients diagnosed with malignant neoplasm of the stomach (C16) in our system, 162 met the study's criteria. Non-sarcopenic patients were selected as the control group.
Patients were categorized based on concomitant comorbidities and treatment-related complications. Lauren's classification was used for adenocarcinomas [16], and the WHO classification was employed for cancer types [17]. Tumor locations were classified as esophagogastric junction, cardia, corpus, antrum, and pylorus. Treatment modalities included chemotherapy, radiotherapy, and surgery. Subtotal and partial gastrectomy were grouped together, along with the wedge resection category comprising patients undergoing mass excision.
Imaging analysis
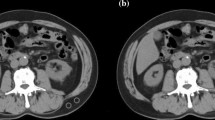
CT examinations were performed using a 64-detector scanner (Aquilion, Canon Medical Systems, Japan) and a 128-detector scanner (Philips, Marifet, The Netherlands), with analysis carried out using the 3D Slicer (Version 4.10.2) open-source software program. Segmentation analysis was conducted on a single slice where the transverse processes of the L3 vertebra corpus were visiblein the axial plane [18, 19]. The muscles were specified as psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal oblique, and rectus abdominis. Measurements were made by tissue-specific threshold technique (between -30 and 130), and a masking method was used on selected images accordingly. Muscle tissues were segmented on CT images, and the results obtained by quantification were extracted for each patient. The cross-sectional muscle area was normalized to the patient's height to calculate the skeletal muscle index (SMI) (Fig. 1).
The presence of sarcopenia was determined by dividing SMA value (calculated on CT) by the square of the patient's height (m2) and was expressed as SMI, with cutoff values set at 52.4 cm2/m2 for men and 38.5 cm2/m2 for women based on previous large-scale studies [20].
Patients were categorized by BMI (weight/(height)2) as underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29 kg/m2), and having obesity (≥ 30 kg/m2). Sarcopenic obesity was defined as the coexistence of sarcopenia and obesity in patients with a BMI ≥ 25 kg/m2 [10].
Statistical analysis
SPSS 22.0 program was used in the analysis. We used mean, standard deviation, median lowest, highest, frequency, and ratio values in the descriptive statistics of the data. Independent t-test or Mann–Whitney U tests were used in the analysis of quantitative independent data, and the chi-square test was used for the analysis of qualitative independent data. Multivariate Cox regression (hazard regression) was used to calculate the effect of skeletal muscle index on survival. Univariate regression analysis was used to calculate the survival effect of all variables. A p-value of < 0.05 was considered statistically significant for all analyses.
Results
Demographic and clinical descriptive features
The study included 162 patients; 66.7% (n = 108) were male. The mean age was 63.3 ± 10.3 years, and 52.5% (n = 85) were ≥ 65 years old. BMI distribution was as 2.5% (n = 4) low, 40.1% (n = 65) normal, 32% (n = 52) overweight, and 25.3% (n = 41) with obesity. When overweight and obesity (BMI ≥ 25 kg/m2) were evaluated together, obesity prevalence was 34.5% (n = 56) (Table 1).
Cancer types were intestinal (n = 46, 28.5%), diffuse (n = 37, 22.8%), indeterminate (n = 65, 40.1%), and non-adenocarcinoma (n = 14, 8.6%); tumors types were papillary (n = 3, 1.9%), tubular (n = 27, 16,7%), mucinous (n = 14, 8.6%), signet ring and weak cohesive (n = 14, 21%) and the mixed (n = 84, 51,9%). The tumors were stage 1 in 11.1% (n = 18), stage 2 in 27.2% (n = 44), stage 3 in 30.9% (n = 50), and stage 4 in 30.9% (n = 50) of the patients.
The relationship of patient characteristics with sarcopenia
The mean SMA of < 65 years was 146 cm2 and SMI 50.4 cm2/m2, and the SMA of ≥ 65 years was 125.6 cm2 and SMI 46.6 cm2/m2. SMA and SMI values were higher for those < 65 ages (p = 0.001 and p = 0.006, respectively) and in men when compared to women (p < 0.001). The mean SMA was 146.8 cm2 SMI 50.6 cm2/m2for men, and 96.9 cm2 SMI 40.6 cm2/m2 for women. Sarcopenia was found in 52,5% (n = 85) of the patients; 61,1% (n = 66) were men. Sarcopenia was significantly higher (p = 0.003) > 65 ages and in men (p = 0.002) (Table 2).
The mean age was 61 ± 11.2 years in non-sarcopenic and 65.4 ± 9 years, in sarcopenic patients. Increase in sarcopenia with age was statistically significant (p = 0.003). The mean BMI was 28.8 kg/m2 in patients without sarcopenia and 24.2 kg/m2 in patients with sarcopenia. There was a negative correlation between BMI and sarcopenia (p < 0.001).
Sarcopenia was found in 19.5% and 48.1% of the patients in the obesity and overweight groups, respectively and it was significantly higher in non-obesity patients (75.4%) (p < 0.001).No correlation was found between the existence of sarcopenia and laboratory parameters or co-morbid diseases in gastric cancer patients.
Sarcopenic patients were more likely to have cardia-located malignancies(p = 0.004). The complication rate after treatment was 64,2% (n = 104). There was no statistical significance for complication occurrence and undergoing surgery, chemotherapy, and radiotherapy in sarcopenic and non-sarcopenic patients.
Patients and the treatment modalities (chemotherapy, partial gastrectomy and total gastrectomy)
In this cohort, 86.4% (n = 140) of the patients had chemotherapy and 13.6% (n = 22) did not. Sarcopenia was found in 52.9% (n = 74) of the patients who had chemotherapy and 50% (n = 11) of the patients who did not and the difference was not statistically significant (p = 0,803) (Table 3).
51.3% (n = 60) of patients who had an operation had sarcopenia and 48.7% (n = 57) did not (totally 72.2%, n = 117). 50% (n = 81) of the patients had total gastrectomy.Operation increased survival rates but having an operation or type of operation did not have a significant relationship with sarcopenia (p = 0,956).
The relationship of patient characteristics with mortality
The mortality was higher in the existence of sarcopenia (p = 0.012). Patients were alive in 40% of the sarcopenic and 59.7% of the non-sarcopenic patients. As BMI increased, mortality rates decreased (p = 0.019). Low albumin value was a statistically significant predictor of mortality in non-sarcopenic patients (p = 0.009) (Table 4).Complications increased mortality (p < 0.001); the surgical treatment group had lower mortality (p < 0.001) when compared to chemotherapy and radiotherapy (p = 0.692, p = 0.842) groups.
The mortality was significantly higher in the esophagogastric location (p = 0.014). There was a positive correlation between mortality and Lauren classification (p = 0.004) but not with WHO classification (p = 0.362).The mortality increased significantly as tumor stage advanced, in both non-sarcopenic and sarcopenic groups (Table 5). According to the Lauren classification, the mortality rate in indeterminate type gastric cancer was higher in the sarcopenic patients.
Mortality increased by 1.03 with age (95% C.I. 1.009–1.062) (p = 0,008). The mortality risk was 5,621 times higher in Stage III (95% C.I. 1,317–23,985) (p = 0,020) and 33,426 times higher in Stage IV (95% C.I. 7,495–149.073) (p < 0,001) compared to Stage I. Mortality was 0,9 times higher in the population with lower BMI (95% CI 0.858–0.984) (p = 0.016).
The mean survival time was 29.8 ± 3.1 and 39.9 ± 3.4 months in sarcopenic and non-sarcopenic patients, respectively. Sarcopenia significantly reduced the mean survival time (p = 0.033) (Fig. 2). There was no significant correlation between age, gender, obesity, WHO classification, and survival time with other locations except esophagogastric location (p > 0.005). The indeterminate subtype (mean 26.4 ± 3.5 months, C.I.:19,594–33.342) had a significantly lower survival time (p = 0.004). As the tumor stage advanced, the survival timesignificantly decreased (p < 0.001). The mean survival time in the esophagogastric location was significantly low (15.3 ± 3.6 months, p = 0.030).The correlation between sarcopenic obesity and mortality was insignificant (p > 0.05).
Also, cox regression analysis was made to evaluate the survival time. In univariate analysis; age (p = 0.011), BMI (p = 0.014), SMA (p = 0.021), SMI (p = 0.023), stage (p = 0.000), complication (p = 0.001), operation (p = 0.000) and RT (p = 0.049) had statistically significant effet on survival time. In multivariate analysis; age (p = 0.001), BMI (p = 0.001), SMA (p = 0.005), stage (p = 0.000) and operation (p = 0.000) had significant-independent effect on survival time (Table 6).
Association of sarcopenic obesity with research endpoints
In the examination performed by grouping the patients with overweight (BMI 25–29 kg/m2) and obesity (BMI ≥ 30 kg/m2) as "sarcopenic obesity", "sarcopenia + & obesity-", "sarcopenia- & obesity + " and "sarcopenia- & obesity-" the sarcopenic obesity group had a correlation with age and gender. Sarcopenic obesity was more prevalent in ≥ 65 years of age and common in men (p = 0.019 and p = 0.015, respectively).
There was apositive correlation between sarcopenic obesity patients and the presence of mortality and post-treatment complications. The post-treatment complication (p = 0.019) and mortality (p = 0.041) rates were higher in the sarcopenic obesity group. Those with obesity and sarcopenia had increased mortality compared to those who had obesitywithout sarcopenia (p = 0.041).
Non-cardia tumor localization was higher in the sarcopenic obesity group but not statistically significant. There was no statistically significant relationship between sarcopenic obesity and the tumor stage, tumor location or the presence of co-morbid disease.
Discussion
The prevalence of sarcopenia in gastric cancer varies between 12.5% and 69.8% [10]. In our study, 52.5% of the patients were sarcopenic. The prevalence of sarcopenic obesity was 25.3% when BMI was ≥ 30 kg/m2 and 34.5% when BMI was ≥ 25 kg/m2. In a study examining the relationship between geriatric gastrointestinal cancers and sarcopenia, and sarcopenic obesity, the prevalence of sarcopenia was 30%, similar to our findings [21]. In a meta-analysis, sarcopenia had a prevalence of 10% in individuals aged ≥ 60 and increased to 20% when bioelectrical impedance analysis was used for diagnosis [22]. The differences in prevalence between studies may originate from using different methodologies such as DEXA, CT, and anthropometric and physical performance measures. There is no consensus yet on the exact numerical values for the assessment of sarcopenia.
Sarcopenia increases with age in the healthy population and is more common in patients over 65, as in the presented study [23]. Sarcopenic obesity was also higher in gastric cancer patients aged ≥ 65 years compared to sarcopenic patients without obesity or other non-sarcopenic patient groups, similar to previous studies [24]. Batsis et al. [25] reported the prevalence of sarcopenic obesity as 42.9% in men and 18.1% in women in a population ≥ 60 years old, supporting the presence of sarcopenia in gastric cancer patients increasing with age.
As in ours, sarcopenia is more common [18, 26, 27] and associated with fatigue in men [28]. Hypogonadism developing with cancer in men is assumed to have more negative effects than in women [20]. In advanced cancers, hypogonadism is associated with low muscle mass, fatigue, decreased physical activity, and survival [29, 30]. Testosterone replacement to prevent the loss of muscle mass due to cancer is still controversial [31]. Kim et al. [32] showed that obesity increased early in gastric cancer and well-to-moderately differentiated adenocarcinoma in men. Obesity was associated with dysplasia that develops independently of H. pylori infection in women. We found that 76% of the men with obesity were in the non-sarcopenic group. In the cohort, 51% of men and 68.5% of women had obesity. There was no correlation between tumor stage and obesity for genders.
In a study on solid tumors involving the respiratory system and gastrointestinal tract, 15% of 250 patients with obesity had sarcopenia, but 85% did not [18]. Sarcopenic obesity was more common in men, patients aged ≥ 65 years, and those with colorectal cancer, independent of TNM stage and weight loss history. Sarcopenic obesity was an independent predictor of survival. The development of sarcopenia with aging is a multifactorial change. The body fat rate increases up to age 70 and then enters the declining phase [33]. Vertebral compression causes shortening in height which affects BMI [34]. Decreased physical activity, loss of mitochondrial volume, and fall in oxidative capacity decrease the resting metabolic rate [35, 36]. By age, DHEA sulfate, testosterone, and estrogen values reduce. The hormones maintain muscle mass [37, 38] and activation of the inflammatory pathway, playing a critical role in the development of sarcopenia [39, 40].
Zhuang et al. [41] found a significant decrease in survival in TNM stage 2 and stage 3 gastric cancer, and sarcopenia was an unfavorable prognostic factor. The increase in stage and mortality was correlated in our study. Mortality was high in both sarcopenic and non-sarcopenic patients. Albumin was associated with decreased survival in gastric cancer patients [42]. We found an association between low albumin value and mortality in patients without sarcopenia. The low albumin level of the patients who died in sarcopenic patient group was statistically nonsignificant.
The relationship between sarcopenia and obesity with cancer has recently gained importance; obesity in cancer patients is increasing parallel to the high obesity prevalence [43]. In our study, sarcopenia was lower in those with higher BMI; BMI was 0.9 times protective against mortality. The mortality rate was significantly higher in the sarcopenia + obesity group. Obesity was protective against mortality. Lee et al. [44] reported similar results to our findings; they observed a decline in disease-related survival and postoperative survival as BMI decreased. Martin et al. [45] showed that BMI predicted survival, and the heaviest patients had the longest survival time. Weight loss of more than 8% was associated with reduced survival.
Obesity is an advantage for survival since catabolism increases in cancer patients [46]. It was reported that weight loss of 15% in the first month following surgery was the only independent significant risk factor [47]. The postoperative weight loss in non-adipose body mass was critical in discontinuing adjuvant chemotherapy [48]. Early termination of treatment in patients with low BMI negatively affects the survival time. The patients who had obesity, sarcopenia, and sarcopenic obesity had gradually increased postoperative complication risks [19]. Low BMI was involved with more severe complications and poor prognosis in advanced stages. High BMI in gastric cancer patients who underwent gastrectomy showed a superior outcome compared with patients with normal BMI [49]; this concept is called the obesity paradox. The obesity paradox states that patients with obesity have advantages over normal-weight patients, contrary to the expectation that excess BMI is associated with an increased risk of death. Moreover, being overweight and having obesity does not increase the risk in some conditions; obesity may even be protective against mortality [49, 50]. Our research proved the obesity paradox; weight was a protective factor for mortality. BMI was 0.9 times protective against mortality (p = 0,016).
We found that sarcopenia was associated with overall survival in gastric cancer patients; sarcopenia significantly increased mortality. Since 52.5% of all patients are sarcopenic, sarcopenia should be considered a critical parameter in gastric cancer patients. The presence of obesity in the sarcopenic group was protective against mortality. Survival was less than ten months in sarcopenic patients. In a meta-analysis, sarcopenia was associated with augmented overall mortality compatible with our study [9].
Patients with sarcopenic obesity have a higher tendency to cardiovascular disease (CVD) [51]. There was a higher rate of CVD in patients with metabolic syndrome and coexistence of sarcopenia [52]. We found the lowest survival time was 27.6 ± 3.4 months in patients with DM. It was 29.3 ± 2.8 months in the presence of cardiovascular disease and 32.7 ± 3.4 months in the presence of hyperlipidemia, but it was not statistically significant.
It was shown that SMI was an independent predictor for poor prognosis in metastatic gastric cancer patients who underwent chemotherapy [53]. Mirkin et al. [54] showed that preoperative chemotherapy significantly increased the incidence of sarcopeniaand perioperative complications in patients who had chemotherapy before gastrectomy in gastric cancer. Sarcopenia and sarcopenic obesity had a relationship with the early suspension of neoadjuvant chemotherapy in resected gastric cancer [10].
Sarcopenia and sarcopenic obesity are associated with early termination of neoadjuvant chemotherapy [10]. The management of sarcopenia is critical; it increases complications and mortality. Huang et al. [55] emphasized the importance of sarcopenia classification as a determinative and independent factor among postoperative complications. They found that the worst post-gastrectomy results occurred in patients with advanced sarcopenia. Sarcopenia should be evaluated preoperatively, as it increases the complication risks [15]. Sarcopenic obesity was an independent preoperative risk factor for postoperative site infection in patients who underwent laparoscopic total gastrectomy [56]. It was shown that postoperative complications were associated with age ≥ 65 and sarcopenia but not with other parameters (BMI, cardiopulmonary comorbidity, DM, tumor characteristics, etc.) [43]. In the overweight and obesity group, the risk of postoperative complications was six times higher. We detected 64.2% of the patients had complication rates without any correlation with sarcopenia and mortality; we presumed that the tumor burden of advanced disease caused it.
Computed tomography can easily show sarcopenia and sarcopenic obesity with high accuracy for diagnosis and staging (the reported error margin is 1.4%) [57]. Our results highlighted the importance of assessing body composition integrity and interpreting it with CT imaging, which commonly provides valuable prognostic information. CT is a quick and practical method for assessing mortality in cancer patients.
Exercise and nutrition programs may stop the development of sarcopenia by increasing protein synthesis [58]. Dietary proteins prevent an inevitable decrease in muscle mass, even in old age [59]. Nutrition programs can be initiated preoperatively. Leucine amino acid, omega-3 polyunsaturated fatty acid supplementation, and exercise programs for protecting skeletal muscles and function provide synergy [43]. Protein, creatine, and β-hydroxy β-methyl butyric acid supplementation are effective for increasing muscle mass [60].
Benjamin et al. [61]found a significant decrease in the total psoas area and skeletal muscle attenuation and a lower overall survival rate in patients who underwent neoadjuvant chemotherapy compared to the operated patients. They suggested that available muscle mass is a ready-to-consume resource of the sick body and can be critical in evaluating treatment options and choosing the appropriate treatment for the body's reserve, leading to personalized therapy. Fat and muscle mass constitute a potential reserve against the stress of cancer, so it will be wise to determine the body's resources and make a survival plan accordingly. Sarcopenia and sarcopenic obesity are the risk factors for gastric cancer, and we should provide tight BMI and SMI control in those patients [62].
Conclusions
Sarcopenia is more common in men and those ≥ 65, and is associated with higher mortality in gastric cancer. Conversely, a higher BMI has a protective role against mortality in these patients. Therefore, maintaining muscle mass and appropriate body composition is a significant step towards reducing mortality in cancer patients. Computed tomography is helpful in assessing body composition, diagnosing sarcopenia, and predicting prognosis from the onset of the disease in clinical relevance.
Limitations
We defined sarcopenia by measuring muscle mass. Since the study was retrospective, tests measuring muscle strength, such as 'walking speed' and 'grip strength' could not be performed. A prospective study can yield more detailed results using dynamic and passive techniques.
This study has a low number of patients and an unequal distributionof gender. The patient cohort was small due to the inadequate appropriate CT images of the patients, lack of height and weight information, and follow-up of patients in other centers. A higher number of patients with an equal number of patients from both genders would have been ideal for further research.
Availability of data and materials
The datasets used and/or analyzed during the current study can be accessed from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- CT:
-
Computerized tomography
- SMA:
-
Skeletal muscle area
- SMI:
-
Skeletal muscle index
References
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Borba VZC, Costa TL, Moreira CA, Boguszewski CL. MECHANISMS OF ENDOCRINE DISEASE: Sarcopenia in endocrine and non-endocrine disorders. Eur J Endocrinol. 2019;180(5):R185–99.
Nezameddin R, Itani L, Kreidieh D, El Masri D, Tannir H, El Ghoch M. Understanding sarcopenic obesity in terms of definition and health consequences: a clinical review. Curr Diabetes Rev. 2020;16(9):957–61.
Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11(4):294–305.
Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–88.
Xie W-q, Xiao G-l, Fan Y-b, He M, Lv S, Li Y-s. Sarcopenic obesity: research advances in pathogenesis and diagnostic criteria. Aging Clinical Experimental Res. 2021;33:247–52.
Marano L, Carbone L, Poto GE, Gambelli M, Nguefack Noudem LL, Grassi G, et al. Handgrip strength predicts length of hospital stay in an abdominal surgical setting: the role of frailty beyond age. Aging Clin Exp Res. 2022;34(4):811–7.
Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18(5):388–95.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Palmela C, Velho S, Agostinho L, Branco F, Santos M, Santos MPC, et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. Journal of gastric cancer. 2017;17(1):74–87.
Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–8.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64.
Kamarajah SK, Bundred J, Tan BH. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22:10–22.
Kawamura T, Makuuchi R, Tokunaga M, Tanizawa Y, Bando E, Yasui H, et al. Long-term outcomes of gastric cancer patients with preoperative sarcopenia. Ann Surg Oncol. 2018;25:1625–32.
Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 2018;268(1):58–69.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathologica Microbiologica Scandinavica. 1965;64(1):31–49.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol: WJG. 2014;20(19):5679.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Zhang Y, Wang J, Wang X, Tian H, Gao T, Tang L, et al. Computed tomography–quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr Oncol. 2018;25(5):411–22.
Burney BO, Hayes TG, Smiechowska J, Cardwell G, Papusha V, Bhargava P, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol. 2012;97(5):E700–9.
BIÇAKLI DH, Çehreli R, Özveren A, Meseri R, Uslu R, Uyar M, et al. Evaluation of sarcopenia, sarcopenic obesity, and phase angle in geriatric gastrointestinal cancer patients: before and after chemotherapy. Turk J Med Sci. 2019;49(2):583–8.
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:1–10.
Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–31.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37.
Batsis J, Mackenzie T, Barre L, Lopez-Jimenez F, Bartels S. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the national health and nutrition examination survey III. Eur J Clin Nutr. 2014;68(9):1001–7.
Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non− small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(4):1133S–S1137.
Wallengren O, Iresjö B-M, Lundholm K, Bosaeus I. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015;23:79–86.
Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, Bacon SL, et al. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010;1:177–85.
Burney BO, Garcia JM. Hypogonadism in male cancer patients. J Cachexia Sarcopenia Muscle. 2012;3:149–55.
Dev R, Hui D, Del Fabbro E, Delgado-Guay MO, Sobti N, Dalal S, et al. Association between hypogonadism, symptom burden, and survival in male patients with advanced cancer. Cancer. 2014;120(10):1586–93.
Del Fabbro E, Garcia J, Dev R, Hui D, Williams J, Engineer D, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer. 2013;21:2599–607.
Kim HJ, Kim N, Kim HY, Lee HS, Yoon H, Shin CM, et al. Relationship between body mass index and the risk of early gastric cancer and dysplasia regardless of Helicobacter pylori infection. Gastric Cancer. 2015;18:762–73.
Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr. 2012;95(3):594–602.
Xu W, Perera S, Medich D, Fiorito G, Wagner J, Berger LK, et al. Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone. 2011;48(2):307–11.
Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(1):203–10.
Wilson M-MG, Morley JE. Invited review: aging and energy balance. J Appl Physiol. 2003;95(4):1728–36.
L McIntire K, R Hoffman A. The endocrine system and sarcopenia: potential therapeutic benefits. Curr Aging Sci. 2011;4(3):298–305.
Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. International journal of endocrinology. 2012;2012.
Jo E, Lee S-R, Park B-S, Kim J-S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. 2012;24:412–22.
Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24(6):623.
Zhuang C-L, Huang D-D, Pang W-Y, Zhou C-J, Wang S-L, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine. 2016;95(13):e3164.
Ouyang X, Dang Y, Zhang F, Huang Q. Low Serum Albumin Correlates with Poor Survival in Gastric Cancer Patients. Clin Lab. 2018;64(3):239–45.
Lou N, Chi C-H, Chen X-D, Zhou C-J, Wang S-L, Zhuang C-L, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: a prospective study. European Journal of Surgical Oncology (EJSO). 2017;43(1):188–95.
Lee JH, Park B, Joo J, Kook M-C, Kim Y-I, Lee JY, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer. 2018;21:913–24.
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47.
Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10(4):433–42.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Aoyama T, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015;22:2560–6.
Chen H-N, Chen X-Z, Zhang W-H, Yang K, Chen X-L, Zhang B, et al. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10-year, single-institution cohort study. Medicine. 2015;94(42):e1769.
Caan BJ, Kroenke CH. Next steps in understanding the obesity paradox in cancer. AACR. 2017;26(1):12.
Chin SO, Rhee SY, Chon S, Hwang Y-C, Jeong I-K, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE. 2013;8(3):e60119.
Chen X, Chen W, Huang Y, Xu J, Zeng Y, Shi M, et al. A quantified risk-scoring system including the visceral fat area for peritoneal metastasis of gastric cancer. Cancer Manag Res. 2019;11:2903.
Hayashi N, Ando Y, Gyawali B, Shimokata T, Maeda O, Fukaya M, et al. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep. 2016;35(3):1727–31.
Mirkin KA, Luke FE, Gangi A, Pimiento JM, Jeong D, Hollenbeak CS, et al. Sarcopenia related to neoadjuvant chemotherapy and perioperative outcomes in resected gastric cancer: a multi-institutional analysis. J Gastrointest Oncol. 2017;8(3):589.
Huang D-D, Zhou C-J, Wang S-L, Mao S-T, Zhou X-Y, Lou N, et al. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161(3):680–93.
Nishigori T, Tsunoda S, Okabe H, Tanaka E, Hisamori S, Hosogi H, et al. Impact of sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann Surg Oncol. 2016;23:524–31.
Mitsiopoulos N, Baumgartner R, Heymsfield S, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22.
Mason C, Xiao L, Imayama I, Duggan CR, Foster-Schubert KE, Kong A, et al. Influence of diet, exercise, and serum vitamin d on sarcopenia in postmenopausal women. Med Sci Sports Exerc. 2013;45(4):607–14.
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8.
Pacifico J, Geerlings MA, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp Gerontol. 2020;131:110801.
Benjamin AJ, Buschmann MM, Zhang SQ, Wroblewski K, Kindler HL, Roggin KK, et al. The impact of changes in radiographic sarcopenia on overall survival in older adults undergoing different treatment pathways for pancreatic cancer. J Geriatr Oncol. 2018;9(4):367–72.
Kim YM, Kim J-H, Baik SJ, Chun J, Youn YH, Park H. Sarcopenia and sarcopenic obesity as novel risk factors for gastric carcinogenesis: a health checkup cohort study. Front Oncol. 2019;9:1249.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MEHŞ and FA designed this study. MEHŞ and EŞ drafted the manuscript. MEHŞ, EŞ and AHY collected clinical data. MEHŞ, FA and AHY performed data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data collection and the methods used in this study were carried out inaccordance with all relevant guidelines and regulation. The data collectionprogram for this study was approved by the This study was approved by University of Health Sciences, İstanbul Training and Research Hospital Clinical Research Ethics Committee (29.05.2020/2383) with a waiver for individualinformed consent for this retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Şahin, M.E.H., Akbaş, F., Yardimci, A.H. et al. The effect of sarcopenia and sarcopenic obesity on survival in gastric cancer. BMC Cancer 23, 911 (2023). https://doi.org/10.1186/s12885-023-11423-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11423-y