Abstract
Background
Legg-Calvé-Perthes disease is a special self-limited disease in pediatric orthopedics with a high disability rate and a long-term course, and there is still no clear and effective therapeutic drug in clinic. This study aimed to investigate the potential efficacy of biochanin A, a kind of oxygen-methylated isoflavone compound, in treating Perthes disease based on network pharmacology, molecular docking and in vitro experiments.
Methods
IL-6 was used to stimulate human umbilical vein endothelial cells to construct endothelial cell dysfunction model. We demonstrated whether biochanin A could alleviate endothelial dysfunction through CCK8 assay, immunofluorescence. Targets of biochanin A from pharmMappeer, SWISS, and TargetNet databases were screened. Targets of endothelial dysfunction were obtained from Genecards and OMIM databases. Protein–protein interaction, Gene Ontology, and Kyoto Encyclopedia of Genes and Genomics analyses were used to analyze the potential target and the key pathway of the anti-endothelial dysfunction activity of biochanin A. To validate the potential target-drug interactions, molecular docking and molecular dynamics simulations were performed and the result was proved by western blot.
Results
It was found that biochanin A can promote the expression of ZO-1, reduce the expression of ICAM-1, which means improving endothelial dysfunction. A total of 585 targets of biochanin A from pharmMappeer, SWISS, and TargetNet databases were screened. A total of 10,832 targets of endothelial dysfunction were obtained from Genecards and OMIM databases. A total of 527 overlapping targets of endothelial dysfunction and biochanin A were obtained. AKT1, TNF-α, VCAM1, ICAM1, and NOS3 might be the key targets of the anti-endothelial dysfunction activity of biochanin A, and the key pathways might be PI3K-Akt and TNF signaling pathways. Molecular docking results indicated that the AKT1 and TNF-α had the highest affinity binding with biochanin A.
Conclusion
This study indicates that biochanin A can target AKT1 and TNF-α to alleviate endothelial dysfunction induced by IL-6 in Perthes disease, which provides a theoretical basis for the treatment of Perthes disease by using biochanin A.
Similar content being viewed by others
Introduction
Legg-Calve-Perthes disease (LCPD) is among the major teratological and disabling diseases of the hip in children. It typically occurs in children aged 4–8 years old (mean, 6.5 years old) [1]. Genetic factors combined with environmental ones characterize the systemic disease known as LCPD [2]. Endothelial dysfunction may lead to thrombosis, increase thrombomodulin levels, and ultimately lead to the occurrence and development of LCPD [3]. Affected children show reduced arterial diameter and function, and the smaller arteries become dysfunctional earlier than the larger ones, which suggests endothelial dysfunction may be a possible pathogenesis of LCPD [4]. In our previous study, circulating endothelial microparticles in LCPD were found to be the reason for endothelial dysfunction and were closely associated with plasma IL-6 concentration [5]. As of this writing, no drug can be effectively used in the clinical treatment of LCPD. Diminishing endothelial dysfunction may be the focus of drug therapy for LCPD.
Biochanin A is a natural oxygen-methylated isoflavone compound. It is a component of many plants, including Spatholobi caulis, soybean, chickpea, red clover, alfalfa and peanut [6]. Biochanin A shows anti-inflammatory [7], anti-tumor [8], anti-microbial [9], antioxidant [10], and neuroprotective activities [11]. This compound decreases lipopolysaccharide-induced inflammation through the inhibition of the expressions of NF-κB and MAPK pathways and the inflammatory factors tumor necrosis factor-α (TNF-α), IL-8, IL-1, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1). Thus, biochanin A diminishes endothelial dysfunction [12, 13]. Results of studies on animal models indicated that biochanin A induces the inhibition of NF-κB phosphorylation and the reduction of NOS-2, COX-2, inflammatory factor, and PGE2 secretion, thereby antagonizing interleukin-induced inflammation [14]. Biochanin A has potential for use as a therapeutic agent for LCPD.
Natural plant-derived biochanin A’s pharmacological effects are achieved via many targets and pathways. Studies have shown that diseases occur through complex network structures with many diverse phenotypes and targets, and drug research with only one target cannot meet the requirements of disease research [15, 16]. In 2007, Hopkins [17] proposed the idea of "network pharmacology," in which the multi-target action of drugs and the multi-target network of diseases are systematically integrated, and this has become the basis for predicting the drugs that can treat diseases. Thus, a theoretical basis for the study of potential mechanisms of action has been established [18]. Network pharmacology is built on systems biology theories and involves the utilization of high-throughput omics analysis, database search, and computing for exploring drug metabolic properties, toxicity, and function [19, 20]. For analyzing biochanin A’s potential targets and exploring its mechanism of action in LCPD treatment, molecular docking technology and molecular dynamics simulations are used [21].
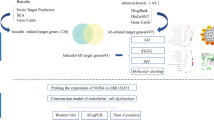
In conclusion, as shown in the Fig. 1, this study aims to explore the potential target proteins that biochanin A may bind to in LCPD through in vitro experiments and network pharmacology methods, thereby playing a role in improving endothelial dysfunction. In this study, we mainly verified that biochanin A can improve endothelial dysfunction through in vitro experiments. We carried out network pharmacology exploration based on this result, and verified that biochanin A may target protein kinase B (AKT1) and TNF-α through molecular docking technology, western blot. It provides a theoretical basis for the use of biochanin A as a clinical drug for the treatment of LCPD.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from the Cell Preservation Center of Wuhan University (CCTCC) and cultured in a 37 ℃, 5% CO2 incubator with RPIM-1640 complete medium (Gibco, USA) containing 10% fetal bovine serum (EVERY GREEN, China) and 1% penicillin streptomycin mixture (Gibco, USA). When the healing degree of cells in T25 or T75 culture bottles reaches 80%—90%, carry out routine digestion, and then conduct the next experiment on the 6-well plate or 96 well plate.
CCK8 assay
HUVECs were routinely digested and inoculated into 96 well plates with 3000 cells/well. After cell attachment, different concentrations of IL-6 (0, 1, 10, 100, 1000 pg/mL) (R&D System, USA) or biochanin A (0, 5, 10, 20, 40 μ M) (Desite, China) were added into the plates. After intervention, 10μL CCK8 reagent (HYcezmbio, China) was added to each well and the plate was incubated in the incubator in dark for 1 h. After incubation, the absorbance value (OD) was measured at the wavelength of 450 nm, and the cell activity was calculated.
Immunofluorescence
After routine digestion, HUVECs were inoculated into 96 well plates with 8000 cells/well. After cell attachment, intervention was divided into the following groups: IL-6 0 pg/mL, IL-6 100 pg/mL, IL-6 100 pg/mL + 5 μM biochanin A、IL-6 100 pg/mL + 10 μM biochanin A. After 24 h of intervention, discard the culture medium, wash it with PBS for three times. Add 4% paraformaldehyde to fix it for 20 min, wash it with PBS for three times after fixation. Add 0.1% Triton-X to penetrate the membrane for 5 min, then add 3% BSA to block for 1 h, wash it with 0.2% BSA twice at the end of blocking. Add primary antibody, and incubate it in a refrigerator at 4 ℃ for 12 h. After incubation, wash it twice with 0.2% BSA. Add secondary antibody, incubate it at room temperature for 2 h. Discard the secondary antibody and wash it twice with 0.2% BSA. Add DAPI to dye it for 5 min. After dyeing, wash twice with 0.2% BSA, and anti-fluorescence quenching agent was added. Observe and take photos under inverted fluorescence microscope. Mean fluorescence intensity was analyzed using ImageJ software.
Biochanin A targets
The drug target prediction principle is based on ligands’ and proteins’ structural characteristics [22], The following network databases were utilized: Swiss [23] (http://www.swisstargetprediction.ch/); pharmMappeer database [24] (http://lilab-ecust.cn/pharmmapper/); and TargetNet database [25] (http://targetnet.scbdd.com/). The term “Biochanin A” was entered as a search term in each database, and the term “Human” was entered as the study species. For potential drug target retrieval by utilizing the UniProt database (https://www.uniprot.org/uploadlists/), the UniProt ID was converted to Entrez ID through the UniProt database. Then, it was converted to Gene Symbol using DAVID. Biochanin A’s potential targets were identified when the repeated data were excluded.
Endothelial dysfunction targets
For the determination of the research target databases for common diseases, Genecards data [26] (https://www.genecards.org/) and OMIM database (https://www.omim.org/) were utilized. The related targets of endothelial dysfunction were searched by inputting the term “Endothelial Dysfunction” in these abovementioned databases. DAVID was utilized to convert all the ID into Gene symbol analysis and to remove the repeated targets. Thus, the predicted disease-related targets were obtained.
Protein–protein interaction (PPI) network
Through the above steps, the possible targets of biochanin A and LCPD were obtained, and their overlapping targets were analyzed through a Venn diagram. They are then imported using the PPI network database String (http://string.db.org/) to map of the PPI (protein protein interaction) network. The term “Biochanin A” was inputted in each database with “Human” as the corresponding study species. Statistical analysis of the interaction score in PPI network was performed by using R software (Version 3.6.3). The top 30 proteins with the highest number of interactions were visually displayed, and the top 100 proteins were used for subsequent analysis.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomics (KEGG) pathway analysis
GO and KEGG pathway analyses were used for describing genes and their interrelationships [27]. Gene symbol and Entrez ID data matrix were constructed for the top 100 co-targets of biochanin A and endothelial dysfunction by utilizing the R software (Version 3.6.3) and clusterProfiler package [28] (Version 3.18.1). The database was limited to org.hs.eg. db, and pvalueCutoff and qvalueCutoff = 0.05. Afterward, the GO and KEGG pathway enrichment analyses were performed, and the top 10 bar and top 20 bubble charts were created, respectively. Mapping of the pathway-target network was performed by using Cytoscape software (Version 3.8.0).
Molecular docking
Biochanin A’s structure SDF format was obtained from the PubChem database and converted into PDB format using Openbabel. This structure was opened by using AutoDockTools1.5.6 [29] and supplemented with hydrogen and charge as ligands. The PDB format of the 3D structures of key target proteins, such as AKT1, TNF-α, nitric oxide synthase 3 (NOS3), VCAM1, and ICAM1, which were screened based on the PPI network, was obtained from the RCSB protein database. The protein structure was separated from water molecules by using the PyMol software [30]. This structure was also opened by using the AutoDockTools1.5.6 software, and hydrogen and charge were added as the receptor. To determine the optional configuration, the AutoDockTools1.5.6 was also used for docking. Binding energy’s lowest conformation was analyzed, and the binding mode was obtained. For the visual operation, PyMol software was utilized.
Molecular dynamics simulation
Molecular dynamics simulations [31] were conducted using Gromacs 2020.6 software for a duration of 50 ns. Prior to the simulation, the system underwent energy optimization and energy minimization (canonical ensemble, NVT) utilizing the mdrun command and employing the fastest descent method. The starting step size was set to 0.01 nm with a maximum allowable force value of 1000 kJ/mol•nm. After the optimization of the system energy was completed, a 100 ps NVT simulation (isothermal isosteric) was performed on the system at a fixed volume and constant heating rate, causing the system temperature to slowly rise from 0 K to 310.15 K, further unifying the distribution of solvent molecules in the solvent box. Subsequently, a 100 ps NPT ensemble simulation (isobaric isobaric) was conducted using a Berendsen constant pressure device to perform pressure equilibrium on the solvent composite system, raising the system pressure to 1 bar. During Molecular Dynamics simulation, all hydrogen bonds were constrained using LINCS algorithm with an integration step of 2 fs. Electrostatic interactions were calculated using Particle-mesh Ewald (PME) method with a cutoff value of 1.2 nm. The nonbonded interaction cutoff was set at 10 A and updated every 10 steps. The simulated trajectories were periodically eliminated, followed by subsequent analysis including Root-mean-square deviation (RMSD), Root Mean Square Fluctuation (RMSF), radius of gyration (Rg) and hydrogen bond analyses. Finally, the initial and final conformations obtained from simulations were compared.
Western blot assay
After routine digestion, HUVECs were inoculated into 6-well plates at a rate of 3 × 105 cells/well. After cell attachment, the intervention groups were as follows: IL-6 0 pg/mL, IL-6 100 pg/mL, IL-6 100 pg/mL + 5 μM biochanin A, IL-6 100 pg/mL + 10 μM biochanin A. After 24 h of intervention, the medium was discarded, and 120μL cell lysate was added to each well. The cells were scraped off with the cell scraper and incubated on ice for 20 min. At the end of the incubation, the lysate was collected in a 1.5 mL EP tube and centrifuged at 12000 rpm/min for 20 min, and the supernatant was aspirated into another 1.5 mL EP tube. After extraction, the protein samples were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred to PVDF membrane (Biosharp, China) for blotting. The membrane was washed three times with 1 × TBST, blocked by adding fast blocking solution (Beyotime, China) for 15 min, and washed three times with 1 × TBST at the end of blocking. The corresponding primary antibodies, namely TNF-α (Signalway Antibody, China), AKT1 (Signalway Antibody, China) and p-AKT1 (Signalway Antibody, China), were added, and incubated at 4℃ for 12-14 h. At the end of the incubation, the membrane was washed with 1 × TBST for three times and the secondary antibody was added. The membrane was incubated on a shaker at room temperature for 1 h, and then washed with 1 × TBST for three times. The image of the membrane was displayed with Image Quant LAS 4000 system and analyzed with ImageJ software.
Statistical analysis
All data analysis was carried out in SPSS 26.0, and the mapping software used GraphPad 7.0, AI and other software for comprehensive processing. One-way ANOVA was used to analyze the differences between groups. And the post-test used in this study is Tukey test. When the p value was less than 0.05, it was considered that the difference was statistically significant.
Results
Biochanin A alleviates IL-6-induced endothelial dysfunction
After the intervention of HUVECs with different concentrations of IL-6 and biochanin A, the optimal intervention concentration was determined by CCK8 assay. The results showed that 0-100 pg/mL IL-6 and 0-10 μM biochanin A did not affect the cell viability. When 100 pg/mL IL-6 was combined with 0-10 μM biochanin A, there was no toxic effect on cells (Fig. 2 A-C). Based on this, we further performed immunofluorescence and found that the expression of ICAM-1 increased and ZO-1 decreased after IL-6 intervention, indicating endothelial dysfunction at this time. However, when biochanin A was added, ICAM-1 expression decreased and ZO-1 expression recovered (Fig. 2D, E). These results indicated that biochanin A alleviated IL-6-induced endothelial dysfunction.
Biochanin A alleviates endothelial dysfunction induced by IL-6. A CCK8 result of different concentration of biochanin A showed that 0–10 μM biochanin A did not have toxic effect on HUVECs. (n = 3) B CCK8 result of different concentration of IL-6 showed that 0–100 pg/mL IL-6 did not have toxic effect on HUVECs. (n = 3) C CCK8 result of 100 pg/mL IL-6 combined with 0–10 μM biochanin A showed that the intervention did not have toxic effect on HUVECs. (n = 3) D The result of immunofluorescence showed that 100 pg/mL IL-6 caused the decreased expression of ZO-1 and biochanin A restored the expression of ZO-1. (n = 3) E The result of immunofluorescence showed that 100 pg/mL IL-6 caused the increased expression of ICAM-1 and biochanin A decreased the expression of ICAM-1. (n = 3) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. #p < 0.05, 20 μM biochanin A compared with 0 μM biochanin A
Intersection targets of biochanin A and endothelial dysfunction
The pharmMappeer, SWISS, and TargetNet databases were searched for potential target genes of biochanin A. Duplicate data were excluded, and 585 target genes of biochanin A were obtained. A total of 10,832 target genes of endothelial dysfunction were obtained by consulting disease-related databases, such as Genecards and OMIM, by using the term "Endothelial Dysfunction" after duplicate data removal. The Venn diagram was constructed, and it revealed that 527 targets intersected with one another (Fig. 3A).
Biochanin A and prediction of key therapeutic targets for endothelial dysfunction. A Venn diagram of the target gene of biochanin A and endothelial dysfunction; B PPI network of the co-targets of biochanin A and endothelial dysfunction; C The top 30 proteins in PPI protein interaction with other proteins
PPI network of targets
For drawing the PPI network, details of the protein interaction obtained from the Venn diagram were imported into the PPI network database String (Fig. 3B). R software was used for the further evaluation of the PPI analysis results. Figure 3C shows the top 30 proteins that interacted the most with other proteins in the PPI network. AKT1 was the protein with the highest number of interactions, thereby suggesting that it plays a critical role in biochanin A’s prevention and treatment of endothelial dysfunction. The top 100 proteins in the PPI network that showed the highest number of interactions with other proteins were included in the subsequent analysis.
GO enrichment analysis
GO enrichment analysis process includes the cellular component (CC), molecular function (MF), and biological process (BP). A total of 527 potential targets were involved in the following: “G protein − coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger”, “adenylate cyclase − modulating G protein − coupled receptor signaling pathway”, “adenylate cyclase − inhibiting G protein − coupled receptor signaling pathway”, “cellular calcium homeostasis” and “second − messenger − mediated signaling”. The MFs had relationships with “G protein − coupled amine receptor activity”, “G protein − coupled peptide receptor activity”, “peptide receptor activity”, “G protein − coupled serotonin receptor activity”, and “neurotransmitter receptor activity”. According to CC analysis, the protein’s proportion in the membrane microregion was higher. The 10 BPs are listed in Supplementary Table I. This finding indicated that biochanin A regulated many different MFs and participated in varied BPs to produce effects (Fig. 4).
KEGG pathway analysis
An enrichment analysis of KEGG pathway was conducted for the intersection targets, and the top 20 significant pathways (P < 0.05) were obtained for visual display. Among these, the neuroactive ligand-receptor interaction was the signal pathway with the highest score and that involved the highest number of targets (Fig. 5A). The 20 pathways are listed in Supplementary Table II. Biochanin A possibly exerts pharmacological effects through cell biological phenotypes, as follows: cell apoptosis, cell migration, cell survival, vascular smooth muscle function, vascular tension, prostaglandin production and inflammation. Studies showed maps of signal pathways related to endothelial dysfunction and the key targets contained in each pathway. Biochanin A may pass through AKT1, TNF, EGFR, MAPK1, PIK3CA, MAPK8, MTOR, MAPK14, IL2, NOS3, AR, ICAM1, RELA, JAK2, MAP2K1, VCAM1, IGF1R, SRC, PTGS2, MMP9, F2R, AGTR1, and MMP2. Endothelial function was treated through the regulation of EGFR tyrosine kinase inhibitor resistance and the following: AGE-RAGE signaling pathway, cAMP signaling pathway, TNF signaling pathway, VEGF signaling pathway, GMP-PKG signaling pathway, PI3K-Akt signaling pathway, HIF-1 signaling pathway, MAPK signaling pathway, mTOR signaling pathway, and other pathway obstacles (Fig. 5B). The Supplementary Figures 1 and 2 shows the pathway-target network diagrams of PI3K-Akt and TNF signaling respectively [32,33,34].
The top 100 key targets in the PPI network were selected for KEGG gene pathway enrichment analysis. A: Bubble map of the KEGG gene pathway enrichment analysis. B: Map of signal pathways that are associated with endothelial dysfunction and the key targets contained in each pathway. Green, red, and yellow circles represent drugs, key targets, and related pathways, respectively.
Molecular docking
Many studies of molecular docking have shown that stable complexes are formed when the free binding energy is negative. The lower the binding energy required for the ligand to bind to the receptor, the more likely docking is to occur. We tested the following potential target proteins (Fig. 6A): AKT1(PDB: 1unq), TNF-α (PDB: 2az5), ICAM1 (PDB: 1iam), VCAM1 (PDB: 1vsc) and NOS3(PDB: 4d1p). They are key targets in the network of interactions, and the docking binding energy results are -5.19 kcal/mol, -4.61 kcal/mol, -3.87 kcal/mol, -3.54 kcal/mol and -4.731 kcal/mol, respectively. Biochanin A binds to Glu-91 of AKT1 protein and ASN-46 and ILe-136 of TNF-α protein, respectively, with minimal and negative binding energy. This finding indicated that the ligand had a good binding activity with the target protein and resulted in stable binding. Other targets could also bind biochanin A to form stable complexes. Figure 6A presents the docking results, which indicated that biochanin A can closely bind to the predicted target. This is probably the main reason for the reduction of endothelial function. Subsequently, we performed western blot experiment. The result showed that biochanin A could increase the expression level of p-AKT1 and decrease the expression level of TNF-α in HUVECs intervened by IL-6, which was a further validation of the result of molecular docking (Fig. 6B-D).
Molecular docking. A The result of molecular docking. B The result of Western blot showed that biochanin A reduced the expression of TNF-α and increased the phosphorylation level of AKT1. Full-length blots are presented in Supplementary Figure 3. (n = 3) C, D Statistical analysis of the phosphorylation level of AKT1 (C) and the expression level of TNF-α (D). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Molecular dynamics simulation
Analyze the simulated trajectory and evaluate the RMSD, RMSF, and Rg. RMSD serves as the standard for evaluating system stability. Based on the analysis of the RMSD diagram and the movement trajectory of small molecule protein complexes, it could be seen that although the RMSD curve of small molecules fluctuated during the simulation process, there was never a phenomenon of detachment from the protein binding cavity, such as AKT1 protein and TNF- α. After 10 ns and 4 ns, the RMSD curve gradually stabilized, indicating the formation of stable complexes between proteins and small molecules during this simulated period (Fig. 7A, D). The RMSF value of the protein is relatively low, indicating that the protein is relatively stable during the simulation process, and this sampling is relatively reliable (Fig. 7B, E). During the entire simulation process, there was no significant disturbance of the protein side chain relative to the main chain, and it remained relatively stable (Fig. 7C, F). Therefore, the overall results demonstrated good stability between the small-molecular ligand–protein acceptor complex. (For details on Hbond, molecular docking model, and Align, please refer to Supplementary Fig. 4).
Discussion
LCPD is among the most common hip disabling diseases in children. Endothelial dysfunction is possibly a key part of LCPD pathogenesis. We previously showed that plasma endothelial microparticles phenotype reflected endothelial dysfunction phenotype in children with LCPD and that Perthes-microparticles could lead to endothelial dysfunction. Moreover, IL-6 can promote endothelial dysfunction and stimulate endothelial cell-secreted endothelial microparticles to induce endothelial dysfunction and inhibit angiogenesis [5]. It has been 100 years since the discovery of LCPD, but there is still no effective drug for its clinical treatment. The anti-inflammatory and anti-endothelial dysfunction effects of biochanin A have been previously described [12, 35]. Biochanin A has potential for use as an effective drug treatment of LCPD. However, its specific mechanism needs to be clarified.
We demonstrated in vitro that the expression of ICAM-1 was increased and the expression of ZO-1 was decreased after IL-6 induced endothelial dysfunction. Kim et al. have demonstrated that IL-6 is significantly elevated in the synovial fluid of children with LCPD. Our study also found that IL-6 is also significantly elevated in the plasma of children with LCPD, suggesting that IL-6 may be a key pro-inflammatory factor in LCPD [36]. Therefore, we speculate whether biochanin A can reduce the expression of IL-6, inhibit inflammation, improve IL-6 induced endothelial dysfunction, and promote femoral head vascular reconstruction. ICAM-1 is a cell surface glycoprotein that binds leukocyte adhesion and endothelial function. Under inflammatory stimulation, activated endothelial cells increase the expression of ICAM-1. ICAM-1 recruitment of excessive white blood cells can exacerbate the inflammatory process and, in many cases, exacerbate tissue damage [37] High expression of ICAM-1 can lead to endothelial dysfunction and inflammatory processes [38]. Increased ICAM-1 levels may serve as a molecular marker for the development of atherosclerosis and clinical coronary heart disease [37]. ZO-1 plays a significant role in maintaining the tight connection between the blood–brain barrier and endothelial cells [39, 40], and it is also one of the most commonly used markers in corneal endothelial cells [41], which suggests that it can be used as a marker of endothelial dysfunction. No study has shown that ICAM-1 and ZO-1 can be used clinically as a diagnostic marker of LCPD, but in our in vitro study, the expression of ICAM-1 was decreased and ZO-1 was restored after intervention with biochanin A. Therefore, ICAM-1 and ZO-1 may be used to assist the clinical diagnosis of LCPD. A total of 527 intersecting targets of biochanin A and endothelial dysfunction were identified through network pharmacology. Figure 3B and C present the PPI results. AKT1 is the target with the highest number of interactions. TNF, VCAM-1, ICAM-1, and NOS3 are also key targets in the PPI network. AKT, which is also protein kinase B, affects the expression and/or activity of various angiogenic factors. The AKT subtypes (AKT1, AKT2, and AKT3) were previously identified as potential therapeutic targets for ischemic injury [42]. AKT1 is a major subtype in vascular cells [43] that promotes angiogenesis through phosphorylation and by regulating important downstream targets, e.g., NOS3 [44]. NOS3 is endothelial nitric oxide synthase. It produces NO in endothelial cells, and its metabolic changes usually cause endothelial dysfunction [45]. Progression of endothelial dysfunction is associated with the increase in the expressions of chemokines, cellular adhesion molecules, and cytokines [46], such as VCAM-1, and systemic inflammatory markers, such as TNF-α, among others [47]. VCAM-1 is a known mediator of eosinophils and monocytes. It combines with vascular endothelial cell adhesion factors and is highly expressed in endothelial and smooth muscle cells. VCAM-1 is involved in inflammation and progress, thereby contributing to endothelial dysfunction. Reduction of VCAM-1 gene expression can reduce vascular inflammation and endothelial dysfunction [48, 49]. TNF-α is a cytokine that increases the expression of inflammatory factors. It induces the expressions of ICAM-1 and VCAM-1, helps reduce the interaction among endothelial cells, and accelerates the progression of inflammation and endothelial dysfunction [50]. It has been proved to be the key target of formononetin against atherosclerosis by improving endothelial dysfunction [51].
Results of GO biological function analysis and KEGG pathway enrichment analysis indicated that biochanin A is involved in the treatment of endothelial dysfunction through many different biological processes and signaling pathways. The KEGG pathway enrichment analysis involves PI3K-Akt, TNF, and AGE-RAGE signaling pathways. When induced, the pharmacological effects may eventually be evident in various cell biological phenotypes, such as inflammation, vascular smooth muscle function, prostaglandin production, and vascular tension, as well as cell migration, survival, and apoptosis. Literature suggests that biochanin A alleviates endothelial dysfunction through a complex network structure that has many targets and pathways. The molecular docking between small molecules and coding proteins of biochanin A and its key targets has been verified in the present study, thereby providing a strong foundation for the future validation and transformation of clinical results.
Network pharmacology was used to construct a drug-target-critical pathway diagram (Fig. 4B). AKT1 is involved in PI3K-Akt, VEGF, and TNF signaling pathways and is a key target in the complex drug–disease interaction network. PI3K-Akt signaling pathway activation in endothelial cells regulates endothelial cell survival and migration, as well as capillary-like structure formation, which are key steps in angiogenesis [52]. LEE indicated that PI3K-AKT1-NO signaling pathway is needed for vascular tone regulation and adaptive vascular remodeling [53]. In Somanath et al.’s study, results of gene knockout mouse experiments showed that AKT1 deficiency affects VEGF expression, wound angiogenesis, and subsequent vascular system maturation [54]. VEGF in turn acts on endothelial cell surface receptors, thereby triggering downstream signals, such as those of PI3K-Akt and NOS3, to promote angiogenesis [55]. TNF signaling pathway has a relationship with immune response and inflammation It mediates pathways by activating various receptors, such as MAPK and NF-κB signaling through the TNFR1 receptor and PI3K-Akt signaling through the TNFR2 receptor [56, 57]. VCAM1 and ICAM1 are potential targets of biochanin A that are involved in AGE-RAGE signaling pathways and are associated with endothelial dysfunction. Advanced glycation end products (AGEs) interact with their cellular receptors (RAGE) to activate NF-κB by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. TNF-α, cell adhesion molecule, tissue factor, cytokine, and monocyte chemotactic protein-1 (McP-1) expressions were induced [58, 59]. Therefore, slowing AGE-RAGE signaling pathway expression can alleviate endothelial dysfunction [60]. In network pharmacology, NOS3, as a potential target of biochanin A, is involved in AGE-RAGE, VEGF, GMP-PKG, PI3K-Akt, platelet activation, and HIF-1 signaling pathways. NOS3 plays a role in the pathophysiology of femoral head necrosis through vasodilation, angiogenesis, platelet activation, and other mechanisms. NOS3, as an enzyme complex, is a key molecule involved in the occurrence of endothelial dysfunction. It promotes nitric oxide (NO). Oxidative stress can lead to the dysregulation of NOS3 and endothelial dysfunction [61, 62]. The 27-bp VNTR polymorphism in intron 4 of NOS3 gene and the G894T polymorphism in exon 7 in children with LCPD were possibly associated with the disease’s etiology, as we mentioned previously [63].
Of course, the clinical transformation and application of biochanin A is still not mature at present. Although some studies have shown that its side effects are less than other anti-inflammatory drugs, biochanin A still has shortcomings such as poor absorption, rapid systemic clearance, and accelerated metabolism, which can reduce its bioavailability. This may cause certain limitations in clinical treatment [64]. Meanwhile, our current research still has certain limitations, such as the lack of clinical proof of the diagnostic efficacy of ICAM-1 and ZO-1, and the specific mechanism of action of biochanin A is not yet clear. In the future, we will further explore the mechanism by which biochanin A can improve endothelial dysfunction by binding to its target proteins AKT1 and TNF-α, to provide theoretical support for the possibility of biochanin A as a clinical therapeutic drug.
Conclusion
We have demonstrated through in vitro experiments that IL-6 intervention in HUVECs can cause changes in the expression levels of endothelial dysfunction markers ICAM-1 and ZO-1 ——an increase in ICAM-1 expression and a decrease in ZO-1 expression. However, the use of biochanin A improved IL-6 induced endothelial dysfunction. Biochanin A possibly prevents endothelial dysfunction via a complex network structure with multiple targets and pathways, among which AKT1, TNF-α, NOS3, ICAM-1, and VCAM-1 may be the key targets, according to network pharmacological analysis results. The most probable binding targets of biochanin A were AKT1 and TNF-α according to molecular docking screening results. This study introduced new ideas and potential therapeutic drugs for the treatment of LCPD and provided a basis for clinical transformation.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated and/or analysed during the current study are available in the Worldwide Protein Data Bank (wwPDB) repository, RCSB PDB: Homepage.
Abbreviations
- LCPD:
-
Legg-Calve-Perthes disease
- IL-6:
-
Interleukin-6
- PPI:
-
Protein–protein interaction
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomics
- ZO-1:
-
Zonula occludens-1
- ICAM1:
-
ICAM-1 Intercellular adhesion molecule 1
- AKT1:
-
Protein kinase B
- TNF-α:
-
Tumor necrosis factor-α
- VCAM1:
-
VCAM-1 Vascular cell adhesion molecule 1
- NOS3:
-
Nitric oxide synthase 3
- PI3K:
-
Phosphoinositide-3-kinase
- NF-κB:
-
Nuclear factor kappa-B
- MAPK:
-
Mitogen-activated protein kinase
References
Pavone V, Chisari E, Vescio A, Lizzio C, Sessa G, Testa G. Aetiology of Legg-Calvé-Perthes disease: a systematic review. World journal of orthopedics. 2019;10(3):145–65.
Hailer Y, Hailer N. Is Legg-Calvé-Perthes disease a local manifestation of a systemic condition? Clin Orthop Relat Res. 2018;476(5):1055–64.
Aksoy M, Aksoy D, Haznedaroglu I, Sayinalp N, Kirazli S, Alpaslan MJH. Thrombomodulin and GFC levels in Legg-Calve-Perthes disease. 2008;13(6):324-8.
Perry D, Green D, Bruce C, Pope D, Dangerfield P, Platt M, et al. Abnormalities of vascular structure and function in children with Perthes disease. Pediatrics. 2012;130(1):e126–31.
Li B, Huang Q, Lin C, Lu R, Wang T, Chen X, et al. Increased circulating CD31+/CD42b-EMPs in Perthes disease and inhibit HUVECs angiogenesis via endothelial dysfunction. Life Sci. 2021;265:118749.
Dong Q, Li Q, Duan L, Yin H, Wang X, Liu Y, et al. viaBiochanin A Inhibits Glioblastoma Growth Restricting Glycolysis and Mitochondrial Oxidative Phosphorylation. Front Oncol. 2021;11:652008.
Zhang S, Niu Y, Yang Z, Zhang Y, Guo Q, Yang Y, et al. Biochanin A alleviates gingival inflammation and alveolar bone loss in rats with experimental periodontitis. Exp Ther Med. 2020;20(6):251.
Hsu Y, Shyu H, Hu T, Yeh J, Lin Y, Lee L, et al. Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem Toxicol. 2018;112:194–204.
Hanski L, Genina N, Uvell H, Malinovskaja K, Gylfe Å, Laaksonen T, et al. Inhibitory activity of the isoflavone biochanin A on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation. PLoS ONE. 2014;9(12):e115115.
Liang F, Cao W, Huang Y, Fang Y, Cheng Y, Pan S, et al. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. BioFactors (Oxford, England). 2019;45(4):563–74.
Guo M, Qu S, Lu H, Wang W, He M, Su J, et al. In VivoBiochanin A Alleviates Cerebral Ischemia/Reperfusion Injury by Suppressing Endoplasmic Reticulum Stress-Induced Apoptosis and p38MAPK Signaling Pathway and. Front Endocrinol. 2021;12:646720.
Ming X, Ding M, Zhai B, Xiao L, Piao T, Liu M. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 2015;136:36–41.
Wu W, Wu Y, Huang H, He C, Li W, Wang H, et al. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int J Mol Med. 2015;35(2):391–8.
Oh J, Cho I, Kang K, You J, Yu S, Lee G, et al. Biochanin-A antagonizes the interleukin-1β-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes. Biochem Biophys Res Commun. 2016;477(4):723–30.
Vitali F, Mulas F, Marini P, Bellazzi R. Network-based target ranking for polypharmacological therapies. J Biomed Inform. 2013;46(5):876–81.
Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discovery. 2012;11(3):191–200.
Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1.
Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123.
Maddox JF, Luyendyk JP, Cosma GN, Breau AP, Bible RH Jr, Harrigan GG, et al. Metabonomic evaluation of idiosyncrasy-like liver injury in rats cotreated with ranitidine and lipopolysaccharide. Toxicol Appl Pharmacol. 2006;212(1):35–44.
de Liyis B, Nolan J, Maharjana M. Fibroblast growth factor receptor 1-bound extracellular vesicle as novel therapy for osteoarthritis. Biomedicine. 2022;12(2):1–9.
Jiao X, Jin X, Ma Y, Yang Y, Li J, Liang L, et al. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput Biol Chem. 2021;90:107402.
Yamanishi Y, Kotera M, Kanehisa M, Goto S. Drug-target interaction prediction from chemical, genomic and pharmacological data in an integrated framework. Bioinformatics. 2010;26(12):i246–54.
Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–64.
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(W1):W356–60.
Yao ZJ, Dong J, Che YJ, Zhu MF, Wen M, Wang NN, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des. 2016;30(5):413–24.
Stelzer G, Plaschkes I, Oz-Levi D, Alkelai A, Olender T, Zimmerman S, et al. VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC Genomics. 2016;17(Suppl 2):444.
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27(1):29–34.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7.
Yun W, Dan W, Liu J, Guo X, Li M, He Q. Investigation of the mechanism of traditional Chinese medicines in angiogenesis through network pharmacology and data mining. Evid Based Complement Alternat Med. 2021;2021:5539970.
Hu P, Sun N, Khan A, Zhang X, Sun P, Sun Y, et al. Network pharmacology-based study on the mechanism of scutellarin against zearalenone-induced ovarian granulosa cell injury. Ecotoxicol Environ Saf. 2021;227:112865.
Sneha P, Doss C. Molecular dynamics: new frontier in personalized medicine. Adv Protein Chem Struct Biol. 2016;102:181–224.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–92.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Verma A, Sharma S. Beneficial effect of protein tyrosine phosphatase inhibitor and phytoestrogen in dyslipidemia-induced vascular dementia in ovariectomized rats. J Stroke Cerebrovasc Dis. 2015;24(11):2434–46.
Kamiya N, Yamaguchi R, Adapala N, Chen E, Neal D, Jack O, et al. Legg-Calvé-Perthes disease produces chronic hip synovitis and elevation of interleukin-6 in the synovial fluid. J Bone Miner Res. 2015;30(6):1009–13.
Calabriso N, Massaro M, Scoditti E, Carluccio C, Verri T, Carluccio M. Epigenetic mechanisms in vascular inflammation: modulation of endothelial adhesion molecules and endothelium-leukocyte adhesion. Front Biosci (Landmark edition). 2023;28(9):194.
Cox M, Barnum S, Bullard D, Zajac A. ICAM-1-dependent tuning of memory CD8 T-cell responses following acute infection. Proc Natl Acad Sci USA. 2013;110(4):1416–21.
Li C, Zhang Y, Liu R, Mai Y. Anagliptin protected against hypoxia/reperfusion-induced brain vascular endothelial permeability by increasing ZO-1. ACS Omega. 2021;6(11):7771–7.
Pimentel E, Sivalingam K, Doke M, Samikkannu T. Effects of drugs of abuse on the blood-brain barrier: a brief overview. Front Neurosci. 2020;14:513.
Bogerd BVD, Zakaria N, Adam B, Matthyssen S, Dhubhghaill SN. Corneal endothelial cells over the past decade: are we missing the mark(er)? Transl Vision Sci Technol. 2019;8(6):13.
Somanath P, Razorenova O, Chen J, Byzova T. Akt1 in endothelial cell and angiogenesis. Cell cycle (Georgetown, Tex). 2006;5(5):512–8.
Chen J, Somanath P, Razorenova O, Chen W, Hay N, Bornstein P, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–96.
Lee M, Luciano A, Ackah E, Rodriguez-Vita J, Bancroft T, Eichmann A, et al. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci USA. 2014;111(35):12865–70.
Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discovery. 2011;10(4):277–91.
Al-Isa A, Thalib L, Akanji A. Circulating markers of inflammation and endothelial dysfunction in Arab adolescent subjects: reference ranges and associations with age, gender, body mass and insulin sensitivity. Atherosclerosis. 2010;208(2):543–9.
Endemann D, Schiffrin E. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–92.
van den Oever I, Raterman H, Nurmohamed M, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393.
Funk S, Yurdagul A, Orr A. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vascular Med. 2012;2012:569654.
Kong D, Kim Y, Kim M, Jang J, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19(4):1057.
Zhang B, Hao Z, Zhou W, Zhang S, Sun M, Li H, et al. Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPAR-γ signaling based on network pharmacology and experimental validation. 2021;12(1):4887-98.
Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90(12):1243–50.
Lee M, Gamez-Mendez A, Zhang J, Zhuang Z, Vinyard D, Kraehling J, et al. Endothelial cell autonomous role of Akt1: regulation of vascular tone and ischemia-induced arteriogenesis. Arterioscler Thromb Vasc Biol. 2018;38(4):870–9.
Somanath P, Chen J, Byzova T. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11(3):277–88.
Kang Z, Zhu H, Jiang W, Zhang S. Protocatechuic acid induces angiogenesis through PI3K-Akt-eNOS-VEGF signalling pathway. Basic Clin Pharmacol Toxicol. 2013;113(4):221–7.
Chen G, Goeddel D. TNF-R1 signaling: a beautiful pathway. Science (New York, NY). 2002;296(5573):1634–5.
Xu J, Zhang Z, Zhou K, Li Y, Wan J, Mao T, et al. Integration of network pharmacology and molecular docking technology reveals the mechanism of the herbal pairing of Codonopsis Pilosula (Franch.) Nannf and Astragalus Membranaceus (Fisch.) Bge on chronic heart failure. Ann Palliative Med. 2021;10(7):7942–59.
Wautier J, Schmidt A. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95(3):233–8.
Prasad K, Bhanumathy K. AGE-RAGE axis in the pathophysiology of chronic lower limb ischemia and a novel strategy for its treatment. Int J Angiology. 2020;29(3):156–67.
Pertynska-Marczewska M, Diamanti-Kandarakis E, Zhang J, Merhi Z. Advanced glycation end products: a link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metabolism. 2015;64(11):1564–73.
Obrosova I, Minchenko A, Marinescu V, Fathallah L, Kennedy A, Stockert C, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44(9):1102–10.
Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107(7):1017–23.
Zhao Y, Liao S, Lu R, Dang H, Zhao J, Ding X. Endothelial nitric oxide synthase gene polymorphism is associated with Legg-Calvé-Perthes disease. Exp Ther Med. 2016;11(5):1913–7.
Felix FB, Vago JP, Amorim.Beltrami V, Dantas.Araújo JM, Grespan R, Teixeira MM, et al. Biochanin A as a modulator of the inflammatory response: an updated overview and therapeutic potential. Pharmacol Res. 2022;180:106246.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82060396 and No. 82160809), the Natural Science Foundation of Guangxi Province (No. 2017GXNSFAA198305, No. 2018GXNSFBA281090) and the Youth Talent Training Program of Guangxi-Collaborative Innovation Center for Biomedicine (0240622005C).
Author information
Authors and Affiliations
Contributions
Xiaofei Ding and Chengsen Lin conceived and designed research. Jianhong Liu and Zhirui Hua conducted cell experiments and network analysis. Boxiang Li, Shengping Tang and Zhendi Wei analyzed data. Jianhong Liu and Zhirui Hua wrote the manuscript. Shijie Liao, Qian Huang and Rongbin Lu revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Hua, Z., Liao, S. et al. Prediction of the active compounds and mechanism of Biochanin A in the treatment of Legg-Calvé-Perthes disease based on network pharmacology and molecular docking. BMC Complement Med Ther 24, 26 (2024). https://doi.org/10.1186/s12906-023-04298-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04298-w