Abstract
Background
Homozygous truncating mutations located in the C-terminal region of the desmoplakin gene (DSP) are known to mainly cause Carvajal syndrome, an autosomal recessive syndromic form of arrhythmogenic cardiomyopathy with an extra-cardiac cutaneous phenotype.
Case presentation
Here we describe a female proband with a documented arrhythmogenic left ventricular cardiomyopathy and a syncopal episode at the age of 13, who was found homozygous for the novel DSP variant: NM_004415.4:c.8586delC, p.(Ser2863Hisfs*20) at the extreme C-terminal region of the protein, just 8 amino acids upstream the stop codon. She did not have any of the typical dermatological symptoms that characterize Carvajal syndrome. Her brother had died suddenly at the age of 18 during exercise and was found homozygous for the same variant at the post-mortem, while their parents were heterozygous. The region of origin of both parents was the same geographic area of Greece, but they were not aware of any common ancestor. Detailed clinical examination revealed that the mother displayed a mild arrhythmic phenotype, while the father was asymptomatic.
Conclusion
These observations pinpoint to a significant functional role of the extreme C-terminal tail of the protein.
Similar content being viewed by others
Background
Arrhythmogenic cardiomyopathy (ACM) is a disorder characterized by an early propensity to symptomatic arrhythmia, initially disproportionate to the degree of ventricular dysfunction, with subsequent deterioration of ventricular function [1]. ACM was once viewed as a disease of the right ventricle (RV) with only minor or late-onset involvement of the left ventricle (LV). However, in recent years, the ACM clinical spectrum has grown to include both LV- (i.e., arrhythmogenic left ventricular cardiomyopathy [ALVC]) and biventricular-predominant patterns of disease [2, 3]. Additionally, a growing body of evidence has demonstrated that ACM can present during both childhood and adolescence, although it was once regarded as a disease of young adults (i.e., individuals in their third or fourth decade of life) [4]. Arrhythmogenic cardiomyopathy is mainly associated with mutations in desmosomal genes [5,6,7,8,9].
Desmosomes are major cell adhesion junctions prominent in the epidermis and myocardium, serving as links between intermediate filaments (IFs) and the cell membrane in adjacent cells and contributing to the tissue architecture and integrity [10]. The desmosomes consist of several proteins of whom the most abundant is desmoplakin, a protein encoded by the DSP gene. Its main function is the anchoring of intermediate filaments to desmosomes.
Mutations in DSP (MIM#125647) may manifest as Carvajal syndrome, lethal acantholytic epidermolysis bullosa, skin fragility–wooly hair syndrome, striate palmoplantar keratoderma, and other phenotypes involving hair, nails, and skin, while early cutaneous findings may herald future cardiac involvement [11]. The Carvajal syndrome is an autosomal recessive syndromic form of ACM (MIM#605676) with an extra-cardiac cutaneous phenotype mainly caused by homozygous truncating mutations located in the C-terminal region of DSP [6, 12]. Initially, the cardiac phenotype in Carvajal syndrome resembled to dilated cardiomyopathy [13], but recent clinical data from 107 patients indicate that DSP cardiomyopathy is a distinct form of arrhythmogenic cardiomyopathy characterized by episodic myocardial injury, left ventricular fibrosis that precedes systolic dysfunction and a high incidence of ventricular arrhythmias [14].
In this study, we report for the first time a female patient carrying an homozygous frameshift mutation at the extreme C-terminal region of the protein, just 8 amino acids upstream the stop codon, manifesting a highly arrhythmic profile and the characteristic LV involvement but without any dermatological symptoms.
Case presentation
Patients
The proband was a 13-years old female athlete who was evaluated due to a syncopal event. Her ECG showed T-wave inversion in V1-V4 leads and low QRS voltages in the limb leads. The echocardiogram was within normal limits and no further evaluation was requested at the time. She was re-evaluated at the age of 15, after the sudden cardiac death (SCD) of her brother. She reported no further symptoms. Her resting ECG showed extensive repolarization abnormalities with T-wave inversion in leads V1-V6, flattened T waves and low QRS voltages in the limb leads, as well as ventricular premature beats (VPBs) (Fig. 1). Her echocardiogram revealed mild biventricular dilatation with borderline biventricular function (LV ejection fraction of 50%). There were no RV dyskinetic areas or aneurysms. Cardiac magnetic resonance (CMR) showed extensive circumferential subepicardial LV late gadolinium enhancement (LGE) compatible with a ring-like myocardial fibrosis that affected 35% of the LV myocardial mass (Fig. 2). There was LGE in the inferior RV wall. There was no evidence of inflammation. She displayed a highly arrhythmic profile with 7000 polymorphic VPBs with both a left bundle branch block (LBBB) and right bundle branch block (RBBB) morphology in a 12-lead 24-hours Holter monitoring. In the exercise test, she developed a symptomatic sustained ventricular tachycardia with an LBBB morphology, inferior axis and late precordial transition suggesting an RV outflow tract origin (Fig. 3). Based on these findings she was diagnosed with ALVC according to the Padua Criteria [15, 16] (Table 1). She was classified at high risk of SCD and an ICD was implanted. One week after the implantation, the ICD was appropriate discharged.
Her brother was an athlete without any symptoms who died suddenly during exercise at the age of 18-years. In the athletic pre-participation cardiovascular screening he had a pathological resting ECG with an isolated negative T wave in lead V3 and polymorphic VPBs originating from both ventricles (Fig. 4). His echocardiogram was within normal range and no further cardiac evaluation was requested. The post-mortem examination revealed areas of LV fibrosis.
ECG of the proband’s brother who died suddenly during exercise (II1): Non-specific T wave abnormalities with T-wave inversion in lead V3 and polymorphic VPBs. Two different morphologies are noted: RBBB - indicating origin from the left ventricle (blue arrow) and LBBB - indicating origin from the right ventricle (red arrow)
The mother was 51 years old and complained of palpitations. Her ECG showed an isolated T negative T wave in lead aVL and RVOT originated VPBs. Upon cardiovascular imaging there was mild RV dilatation without any regional wall motion abnormalities or evidence of myocardial fibrosis. She exhibited more than 20,000 VPBs per 24 h without evidence of complex ventricular arrhythmias. The father, 52 years old, was asymptomatic with a normal ECG and echocardiogram and with no evidence of arrhythmias.
Genetic analysis
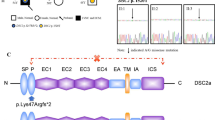
The molecular basis of the disease was identified for the proband with next generation sequencing technology, using Illumina’s Trusight Cardio sequencing panel, covering 174 genes clinically relevant to cardiac diseases. Alignment, quality filtering, variant calling and variant annotation were performed using in parallel the standard MiSeq Reporter (Illumina) and the Sophia Genetics pipeline. The variant calling files were filtered using the Sophia Genetics DDM platform and the detected variants were characterized according to the recommendations of the American College of Medical Genetics and Genomics (ACMG) [17]. All benign or likely benign variants were filtered out and the retained variants (Table 2) were then evaluated according to the relevance of the gene to the observed phenotype resulting in one plausible candidate variant which was located in the DSP gene: NM_004415.4:c.8586delC,p.(Ser2863Hisfs*20). The proband was homozygous for the variant (https://databases.lovd.nl/shared/individuals/00424916), which was novel and characterized as VUS (variant of unknown significance) (according to ACMG criteria). It was a frameshift variant located in the last exon of the gene producing a transcript that was not predicted to undergo nonsense-mediated mRNA decay. Specifically, the variant resulted in the substitution of the last 9 amino acids of the protein and the prolongation of the C-terminal tail by 10 additional amino acids (PVS1 criterion reduced to moderate; [18]). Furthermore, it was absent from the population databases of Exome Sequencing Project and Genome Aggregation Database (PM2 criterion). The parents of the proband underwent DSP genetic testing by targeted Sanger sequencing for the detection of the variant and were found both heterozygous for the same variant. Material from the autopsy subjected to genetic testing revealed that the proband’s brother was also homozygous for the same variant. Following these genetic results and during the course of a second and more detailed interview, the parents revealed a common origin from the same geographic region of Greece, but they were not aware of any common ancestor (Fig. 5).
(A) The family pedigree is depicted. The proband (shown by arrow, II2) was homozygous for the NM_004415.4:c.8586delC. Her brother (II1) died suddenly during exercise and was also homozygous for the same variant. The proband’s parents (I1 and I2) were found heterozygous for the same variant. Circle = female. Square = male. Filled symbols = affected individuals. (B) Electropherogram of the involved sequence fragment of the DSP for the members of the family
Discussion and conclusions
Desmosomes are intercellular junctions found in epithelial and cardiac tissue. They mediate the connection of IFs of neighboring cells, creating a network of adhesive structural interactions conferring strength and durability to these tissues. Cardiac IFs consist mainly of desmin and mutation in DES gene can cause ACM [19]. Desmoplakin is an essential component of desmosomes as it serves the connection of the inner desmosomal plaque comprised of the cytoskeletal IFs to the plakoglobin and plakophilin molecules of the outer desmosomal plaque [20]. A modular structure with distinct domains of the protein interacting with neighboring molecules is the hallmark of desmosomal proteins. In the case of desmoplakin, the plakin domain in the N-terminal region of the protein interact with plakophilin and plakoglobin [21], the central coiled-coil region is responsible for desmoplakin dimerization [22], while three plakin repeat domains (PRDs A, B, and C) in the C-terminal region of the protein are reported to bind to IFs [21, 23,24,25].
Beyond PRD-C, in the carboxyl extremity of the protein, several amino acid residues, comprising the Gly-Ser-Arg (GSR) repeats, undergo post-translational modifications (Fig. 6). Such modifications of cell junction proteins by phosphorylation and methylation represent a specific, rapid mechanism for regulating their function and association with neighboring molecules [26,27,28]. Furthermore, the carboxyl extremity has been proposed to form an arginine claw [28], a recently identified structural element of serine-rich regions. This was first characterized in the C-terminal region of ASF/SF2 [29], a protein involved in mRNA splicing, spliceosome assembly, and mRNA nuclear trafficking. This structure is affected by the post-translational modifications observed in the region and this structural interplay may function as a molecular switch for the binding affinity of desmoplakin to the IFs [28].
Schematic diagram of arginine methylation sites (bold and underlined) and serine/threonine sites (red) phosphorylated by PKC-ɑ and GSK3 within the C-terminal extremity of desmoplakin (aa 2823–2871). The change introduced by the mutation of the proband p.(S2863fs) is depicted along with two other frameshift mutation that have been observed in this region
In this context, an amino acid residue that has been shown to undergo post-translational modification is serine 2849 (Fig. 6), which is phosphorylated by PKC-ɑ [30]. This phosphorylation reportedly induce conformational changes that permit the recruitment of GSK3, a second kinase, that further phosphorylates upstream located serine residues (positions 2833–2845, [27]). Other nearby residues that have been found phosphorylated are Thr2853 and Ser2868 [31]). So far, two frameshift mutations have been reported in ClinVar downstream of Ser2849:p.(Ser2859fs) (rs727504909) and p.(Tyr2862fs) (rs765683790) in patients with clinical phenotype of ACM and have been characterized as VUS, since they are not anticipated to result in nonsense mediated decay and due to the lack of evidence on the functional role of the extreme C-terminal 20 residues (database assessed April 11, 2023). Camors et al. [32] described an homozygous case for the p.(Ser2859fs) variant presenting infantile epidermolysis bullosa and severe ACM, who died at the age of 12 months. It was the child of a consanguineous marriage, whose mother reportedly died prematurely from hypertrophic cardiomyopathy and whose father had lifelong lesions on his feet but refuted genetic testing. Our patient carried a homozygous frameshift mutation at position 2863 altering the last 9 amino acids of the protein and prolongating the C-terminal tail by 10 additional amino acids (Fig. 6). She displayed a malignant cardiac phenotype with significant biventricular fibrosis, extensive T wave inversion, diffuse and a highly arrhythmic profile but without any dermatological symptoms. Her homozygous brother had VBPs in his resting ECG, died suddenly during exercise and the post-mortem revealed LV fibrosis. The heterozygous mother (aged 51) displayed milder arrhythmia symptoms, while the heterozygous father was asymptomatic (Table 1). This phenotype is suggestive of a functional role of this extreme C-terminal region, as the mutation might perturb some of the post-translational modifications observed in the region or may infer conformational changes that perturb the normal function of the protein.
Data Availability
All data from this study that do not pertain to identifiable patient information are available and can be provided by contacting the corresponding author on reasonable request. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The identified mutation has been submitted to the corresponding LOVD database (https://databases.lovd.nl/shared/individuals/00424916).
Abbreviations
- ACM:
-
Arrhythmogenic cardiomyopathy
- ACMG:
-
American College of Medical Genetics and Genomics
- ALVC:
-
Arrhythmogenic left ventricular cardiomyopathy
- CMR:
-
Cardiac magnetic resonance
- ECG:
-
Electrocardiogram
- GSR:
-
Gly-Ser-Arg repeats
- IF:
-
Intermediate filaments
- LBBB:
-
Left bundle branch block
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- PRD:
-
Plakin repeat domains
- RBBB:
-
Right bundle branch block
- RV:
-
Right ventricular
- SCD:
-
Sudden cardiac death
- VPB:
-
Ventricular premature beats
- VUS:
-
Variant of unknown significance
References
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–14.
Elliott PM, Anastasakis A, Asimaki A, Basso C, Bauce B, Brooke MA, et al. Definition and treatment of arrhythmogenic cardiomyopathy: an updated expert panel report. Eur J Heart Fail. 2019;21:955–64.
Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B, et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–97.
DeWitt ES, Chandler SF, Hylind RJ, Beausejour Ladouceur V, Blume ED, VanderPluym C, et al. Phenotypic manifestations of arrhythmogenic cardiomyopathy in children and adolescents. J Am Coll Cardiol. 2019;74:346–58.
McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet. 2000;355:2119–24.
Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–6.
Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–4.
Brodehl A, Weiss J, Debus JD, Stanasiuk C, Klauke B, Deutsch MA, et al. A homozygous DSC2 deletion associated with arrhythmogenic cardiomyopathy is caused by uniparental isodisomy. J Mol Cell Cardiol. 2020;141:17–29.
Brodehl A, Meshkov A, Myasnikov R, Kiseleva A, Kulikova O, Klauke B et al. Hemi- and Homozygous Loss-of-Function Mutations in DSG2 (Desmoglein-2) Cause Recessive Arrhythmogenic Cardiomyopathy with an Early Onset. Int J Mol Sci. 2021;22.
Patel DM, Green KJ. Desmosomes in the heart: a review of clinical and mechanistic analyses. Cell Commun Adhes. 2014;21:109–28.
Polivka L, Bodemer C, Hadj-Rabia S. Combination of palmoplantar keratoderma and hair shaft anomalies, the warning signal of severe arrhythmogenic cardiomyopathy: a systematic review on genetic desmosomal diseases. J Med Genet. 2016;53:289–95.
Castelletti S, Vischer AS, Syrris P, Crotti L, Spazzolini C, Ghidoni A, et al. Desmoplakin missense and non-missense mutations in arrhythmogenic right ventricular cardiomyopathy: genotype-phenotype correlation. Int J Cardiol. 2017;249:268–73.
Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:418–21.
Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, et al. Desmoplakin Cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–84.
Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–14.
Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–29.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39:1517–24.
Protonotarios A, Brodehl A, Asimaki A, Jager J, Quinn E, Stanasiuk C, et al. The novel desmin variant p.Leu115Ile is Associated with a unique form of Biventricular Arrhythmogenic Cardiomyopathy. Can J Cardiol. 2021;37:857–66.
Kowalczyk AP, Green KJ. Structure, function, and regulation of desmosomes. Prog Mol Biol Transl Sci. 2013;116:95–118.
Stappenbeck TS, Bornslaeger EA, Corcoran CM, Luu HH, Virata ML, Green KJ. Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J Cell Biol. 1993;123:691–705.
Green KJ, Stappenbeck TS, Parry DA, Virata ML. Structure of desmoplakin and its association with intermediate filaments. J Dermatol. 1992;19:765–9.
Choi HJ, Park-Snyder S, Pascoe LT, Green KJ, Weis WI. Structures of two intermediate filament-binding fragments of desmoplakin reveal a unique repeat motif structure. Nat Struct Biol. 2002;9:612–20.
Fontao L, Favre B, Riou S, Geerts D, Jaunin F, Saurat JH, et al. Interaction of the bullous pemphigoid antigen 1 (BP230) and desmoplakin with intermediate filaments is mediated by distinct sequences within their COOH terminus. Mol Biol Cell. 2003;14:1978–92.
Lapouge K, Fontao L, Champliaud MF, Jaunin F, Frias MA, Favre B, et al. New insights into the molecular basis of desmoplakin- and desmin-related cardiomyopathies. J Cell Sci. 2006;119:4974–85.
Stappenbeck TS, Lamb JA, Corcoran CM, Green KJ. Phosphorylation of the desmoplakin COOH terminus negatively regulates its interaction with keratin intermediate filament networks. J Biol Chem. 1994;269:29351–4.
Albrecht LV, Zhang L, Shabanowitz J, Purevjav E, Towbin JA, Hunt DF, et al. GSK3- and PRMT-1-dependent modifications of desmoplakin control desmoplakin-cytoskeleton dynamics. J Cell Biol. 2015;208:597–612.
McAnany CE, Mura C. Claws, disorder, and Conformational Dynamics of the C-Terminal region of human desmoplakin. J Phys Chem B. 2016;120:8654–67.
Hamelberg D, Shen T, McCammon JA. A proposed signaling motif for nuclear import in mRNA processing via the formation of arginine claw. Proc Natl Acad Sci U S A. 2007;104:14947–51.
Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, et al. Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J Cell Biol. 2008;181:605–13.
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–7.
Camors EM, Purevjav E, Jefferies JL, Saffitz JE, Gong N, Ryan TD, et al. Early Lethality due to a novel desmoplakin variant causing Infantile Epidermolysis Bullosa Simplex with Fragile skin, Aplasia Cutis Congenita, and arrhythmogenic cardiomyopathy. Circ Genom Precis Med. 2020;13:e002800.
Acknowledgements
We would like to thank the proband and her family for allowing us to present their results.
Funding
This work was supported by GR i CARDIAC NET: Greek National Network of Precision Medicine in Cardiology and the Prevention of Sudden Death in the Young.
Author information
Authors and Affiliations
Contributions
MP and PG participated in research design, data analysis and interpretation and writing of the manuscript, VV, EP and AT participated in sample collection, patient management and follow-up and provided clinical data, EN provided cardiac magnetic resonance imaging data, DD participated in research design and supervised the course of the project, AA participated in research design and supervised clinical management of the family.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent for molecular genetic testing was obtained from all members of the family. For the proband who was minor and her deceased brother the informed consent was obtained from their parents. The study has been performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Onassis Cardiac Surgery Center.
Consent for publication
Written informed consent was obtained from the patients for the publication of this report. For the proband who was minor the informed consent for the publication of identifying information in an online open-access publication was obtained from her parents.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pantou, M.P., Gourzi, P., Vlagkouli, V. et al. A truncating variant altering the extreme C-terminal region of desmoplakin (DSP) suggests the crucial functional role of the region: a case report study. BMC Med Genomics 16, 95 (2023). https://doi.org/10.1186/s12920-023-01527-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01527-6