Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that involves the death of neurons. ALS is associated with many gene mutations as previously studied. In order to explore the molecular mechanisms underlying ALS with C9orf72 mutation, gene expression profiles of ALS fibroblasts and control fibroblasts were subjected to bioinformatics analysis. Genes with critical functional roles can be detected by a measure of node centrality in biological networks. In gene co-expression networks, highly connected genes called as candidate hubs have been associated with key disease-related pathways. Herein, this method was applied to find the hub genes related to ALS disease.
Methods
Illumina HiSeq microarray gene expression dataset GSE51684 was retrieved from Gene Expression Omnibus (GEO) database which included four Sporadic ALS, twelve Familial ALS and eight control samples. Differentially Expressed Genes (DEGs) were identified using the Student’s t test statistical method and gene co-expression networking. Gene ontology (GO) function and KEGG pathway enrichment analysis of DEGs were performed using the DAVID online tool. Protein-protein interaction (PPI) networks were constructed by mapping the DEGs onto protein-protein interaction data from publicly available databases to identify the pathways where DEGs are involved in. PPI interaction network was divided into subnetworks using MCODE algorithm and was analyzed using Cytoscape.
Results
The results revealed that the expression of DEGs was mainly involved in cell adhesion, cell-cell signaling, Extra cellular matrix region GO processes and focal adhesion, neuroactive ligand receptor interaction, Extracellular matrix receptor interaction. Tumor necrosis factor (TNF), Endothelin 1 (EDN1), Angiotensin (AGT) and many cell adhesion molecules (CAM) were detected as hub genes that can be targeted as novel therapeutic targets for ALS disease.
Conclusion
These analyses and findings enhance the understanding of ALS pathogenesis and provide references for ALS therapy.
Similar content being viewed by others
Background
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that involves the death of neurons [1, 2]. ALS is often called Lou Gehrig’s disease, after the famous baseball player who was diagnosed with it. The occurrence of ALS is two per 100,000 people, and it is estimated that more than 20,000 Americans may be living with ALS at any given time. It is worldwide with no racial, ethnic or socioeconomic boundaries and can affect anyone. It affects the nerve cells in the brain and the spinal cord and is characterized by stiff muscles, muscle twitching and gradually worsening weakness due to muscles decreasing in size. Despite intensive research, the clinical and pathophysiological mechanisms of ALS still remain unclear. There are two types of ALS, sporadic (SALS) and familial (FALS). 90–95 % of ALS cases are sporadic for which the cause is unknown and 5–10 % are familial, which are inherited [3]. Findings on ALS patients have drawn in numerous genes related to ALS pathogenesis and have identified diverse processes, such as excitotoxicity, oxidative stress, cytoskeletol abnormalities, impaired axonal transport, mitochondrial dysfunction and protein aggregation. Presently, ten genes such as SOD1, ALSIN, SETX, SPG11, FUS, VAPB, TARDBP, OPTN, ATXN2 and C9orf72 were found to affect the pathogenesis of ALS [4]. Though SALS and FALS are clinically and pathophysiologically similar, there is a requirement of finding the genes involved in FALS and the molecular mechanism pathways involved in the mutation of these genes. Till date studies were done to know the molecular mechanisms and underlying pathogenesis of ALS disease related to SALS with Superoxide dismutase 1 (SOD1) mutation [5, 6]. As the study of SOD1 has led to great advances in proper illustration of molecular mechanisms underlying in ALS disease, identifying the mutations in other genes and the pathways involved in these mechanisms is utmost important. Till date there is no cure for ALS, however the drug riluzole – the only prescribed drug approved by Food and Drug Administration (FDA) to treat ALS which prolongs the life by 2–3 months but do not relieve the symptoms [7, 8]. Improving diagnoses and treatment of this disease is utmost essential, as currently there exists no cure for ALS.
C9orf72 mutation is currently the major genetic cause of ALS disease accounting for approximately 34.2 % of the familial ALS cases. C9orf72 mutation is a hexanucleotide repeat expansion of the six letter string of nucleotides GGGGCC. Although nothing about C9orf72 function [9, 10] and the mechanisms by which expanded repeats cause neurodegeneration are known, C9orf72 RNAs levels were found to be reduced as reported in tissue samples from patients with C9orf72 expansions [11–13]. The leading candidate mechanisms by which expanded repeats cause neurodegeneration are RNA-mediated toxicity, loss of C9orf72 gene function, or a combination of the two. In a normal person there would be few repeats of this nucleotide, but in the person with C9orf72 mutation this repeat can occur in the order of hundreds that result in the formation of RNA foci in white blood cells, fibroblasts, glia and multiple neuronal cell types. These RNA foci are not present in the sporadic ALS and familial ALS caused by other mutations. This RNA-mediated toxicity is found to play a crucial role in a variety of repeat expansion disorders by accumulation of expanded transcripts into nuclear RNA foci [14]. These RNA repeats fold into stable structures and sequester RNA binding proteins to set off a molecular cascade leading to neurodegeneration [14]. A loss of C9orf72 gene function is observed in patients with expansion by reported reductions of C9orf72 transcript levels [11–13] Although this reduction to neuronal death is not established, loss of C9orf72 gene function during embryonic development is associated with motor deficits in zebrafish [15].
Lagier-Tourenne et al. have demonstrated the ASO mediated reduction of C9orf72 RNAs by aggravating the loss of C9orf72 function [16]. In this view of finding different molecular mechanisms and pathways related to C9orf72 mutation in ALS disease, we have used systems biology approach for the gene expression data to identify Gene Ontologies (GO) and pathways related to familial ALS. Genome-wide RNA profiling in fibroblasts from patients with C9orf72 expansion was studied in the RNA microarray data [9] used for the present study. This microarray data retrieved from Gene expression omnibus (GEO) [17] was screened to identify differentially expressed genes (DEGs) between ALS and control fibroblasts before and after ASO mediated disease therapy in order to find potential genes for the pathogenesis of ALS. Gene ontology(GO) enrichment analysis and Kyoto encyclopedia of Genes and Genomes(KEGG) pathway analysis was performed, and a protein-protein interaction (PPI) network was constructed by mapping the DEGs to the human PPI data. Our research laid a bridge between the DEGs, the KEGG pathways and the protein-protein interaction networks which will help for the development of novel targets for ALS therapeutic intervention.
Methods
DEGs analysis and Co-expression network
Illumina HiSeq microarray dataset (GSE51684) [16] with their RPKM (Reads per kilo base per million mapped reads) values was retrieved from GEO (http://www.ncbi.nlm.nih.gov/geo/) [17] which was based on the GPL 11154 Illumina HiSeq 2000 platform, having four sporadic ALS, twelve familial ALS and eight control samples. Familial ALS samples and the control samples were used for the present study to identify the hub genes that can act as probable familial ALS targets. Samples were divided into six groups (Table 1) based on a) presence/absence of C9orf72 mutation and b) treatment of the disease with the control/C9orf72 Antisense oligonucleotide (ASO). The four Familial ALS samples having C9orf72 mutation (Group III) and four control samples (Group VI) without ASO treatment were put into network 1 and four familial ALS samples (Group II) and four control samples (Group IV) treated with the C9orf72 ASOs were put into network 2. All the genes with RPKM > 5 in atleast one sample were taken for finding DEGs. The software R v 3.2.2 [18] was used to perform all the statistical analyses. The Student’s t test statistical P-values and fold changes were calculated. Further, each P-value is adjusted with a Benjamini-Hochberg method to account for multiple testing. The Benjamini-Hochberg method provides sufficiently conservative estimates of “significance” among the many statistically detectable scores. Genes with fold change > 2.0 and < 0.5 and the adjusted P-value < 0.05 were identified in both the networks (Additional file 1: Table S1). Gene co-expression network analysis was performed by constructing a matrix of pairwise Pearson correlations between all genes identified by statistical methods across all selected samples. Finally co-expression threshold of > 0.9 was set to find the DEGs in both the networks. This study aimed at obtaining the DEGs for C9orf72 ASO treated samples over ASO untreated samples.
Enrichment analysis of GO function and KEGG pathway
The information on the networked molecules and genes is contained in the KEGG. The database for annotation, visualization and integrated discovery (DAVID) was used to analyze list of genes derived from high-throughput genomic experiments. DAVID online tool [19] for Gene ontology (GO) annotations and KEGG pathway analysis were used to perform the enrichment analysis of the biological processes of DEGs in order to identify the enriched genes at the cellular level. The cut-off criteria of more than two genes, FDR and P-values less than 0.05 were chosen.
Construction of gene/protein interaction network and analysis
Human protein – protein interaction network (PPI) data were obtained from public databases MINT [20], BioGrid [21] and HPRD [22]. Potential PPI correlations were demonstrated by mapping all the DEGs on the compiled data set of human interactome for the PPI network construction and microarray data enrichment analysis. The DEGs showed to have 1885 interactions reported in the databases and visualized in CytoHubba [23]. Scale-free property of the protein interaction network was used to find the key hub proteins. PPI network was constructed based on the PPI correlations by the Cytoscape v3.2.0 software platform.
Molecular complex detection analysis
The molecular complex detection (MCODE) algorithm [24] is a well known automated method using the Cytoscape MCODE plug-in to find highly interconnected subgraphs or modules that detects densely connected regions in large PPI networks that may represent molecular complexes. In the present study, Cytoscape MCODE plug-in was used to search clustered subnetworks of highly intraconnected nodes (n > 15). Then the identified modules were used for functional enrichment analysis using the BinGO [25] plug-in of Cytoscape. Validation of molecular mechanism of ALS and finding potentially essential genes can be performed through these analytical results.
Results
DEGs analysis and Co-expression network
The two networks constructed were used to find the DEGs that could be probable targets for familial ALS disease. In the present study network 1 and network 2 were compared to find highly expressed genes before and after ASO treatment of C9orf72 fibroblasts and control fibroblasts. Statistical analysis has yielded 1055 DEGs. Of these, 734 genes were upregulated and 321 genes were downregulated (Additional file 1: Table S1). Statistical methods used in the present study could identify more number of DEGs when compared to the DEGs reported by Lagier-Tourenne et al. [16]. Several important DEGs were identified, including Tumor necrosis factor (TNF), Endothelin 1 (EDN1), Angiotensin (AGT), Apolipoprotein E (APOE), Vitronectin (VTN), Von Willebrand factor (VWF), Thrombospondin receptor (CD36), TNF Receptor Superfamily Member 5 (CD40), Integrin, Alpha 3 (ITGA3), Integrin, Alpha 7 (ITGA7), Transforming growth factor beta 2 (TGFB2), Aggrecan (ACAN),Wingless-Type MMTV Integration site family, member 5A (WNT5A), Neurotrophic tyrosine kinase, receptor, type 2 (NTRK2) and Zinc finger protein GLI1 (GLI1) which were involved in the biological processes related to ALS.
GO function and KEGG pathway enrichment analysis
Functional annotation of large-scale genomic data can be obtained by GO analysis approach. The DEGs were mapped to the DAVID database to investigate the functional changes in the ALS diseased patients. DAVID is the most popular tool in the field of high-throughput functional annotation that is cited in more than 2000 publications. The top GO enrichment analysis results of DEGs based on biological process, cellular components and molecular function are shown in Table 2. According to the biological processes analysis, upregulated DEGs were mainly involved in cell adhesion, biological adhesion, cell-cell adhesion and cell-cell signaling. Based on the molecular function analysis, DEGs were involved in calcium ion binding activity. In addition, upregulated DEGs were located in the extracellular matrix (ECM), extracellular space, extracellular region part. The downregulated DEGs were enriched only in the extracellular region part with FDR 3.3E-08.
Different pathways were identified in the KEGG pathway enrichment analysis as shown in Table 3. Upregulated DEGs were mainly involved in ECM-receptor interaction, focal adhesion, Cell adhesion molecules (CAMs) whereas the downregulated genes were not enriched in any of the KEGG pathways.
PPI network construction
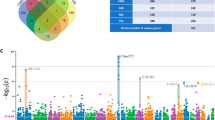
PPI network analysis has been a powerful tool for understanding the biological responses in health and disease. In the PPI network, the protein is defined as the node and the interaction between two nodes is defined as the edge. The DEGs related to ALS were mapped onto the reference network obtained from Mint, HPRD and BioGrid databases (Fig. 1) where the red colour nodes indicate highly connected ‘hub’ nodes in PPI network. The resulting network was divided into subnetworks each of which represented protein subcomplexes or functional modules. This subnetworking would provide more specific and detailed information about the PPI network. The subnetworks were obtained using the MCODE plug-in implementing the MCODE algorithm with the k-score value of 2.0, node score cutoff of 0.2, maximum depth from the seed node of 100 and graphics-processing-unit-based parallelization to find modules efficiently. A total of 19 modules were found, among which five modules were detected with the intra-connection nodes >15 and node score >2.0 (Table 4, Fig. 2). All clustered modules varied in size comprising a total of 103 proteins and 263 PPIs.
Next, the complete PPI network was visualized in Cytohubba to find the hub genes with high degree of connectivity between the nodes. The higher value for the degree indicate highly connected network and likely to be more robust. The degree of each node in the network was calculated by Cytohubba, which identified hubs as nodes with the degree value >25 (Table 5).
Finally, functional annotation of submodules was predicted. Most of the hub genes as mentioned in Table 5 were involved in the subnetworks indicating the reliability of the subnetwork model generated and these hub genes were primarily corresponding to adhesion, signaling and Extra cellular matrix as according to the results obtained from GO enrichment analysis and KEGG pathways with P-values and FDR in the acceptable range.
Subnetworks 1, 2, 4 and 5 were enriched in the GO terms related to the chemical component extracellular region part, extracellular matrix. Subnetworks 1 and 2 were enriched in biological process GO term related to cell-cell signaling. Subnetwork 4 was enriched in the biological process related to cell adhesion and biological adhesion. Subnetwork 3 was not enriched in any of the GO term. The P-values of all the enriched GO terms were in the range of 5.4E-17 to 8.9E-03.
KEGG pathway neuroactive ligand receptor interaction was enriched in Subnetworks 1 and 2. ECM receptor interaction pathway was enriched in subnetworks 3 and 4. Subnetwork 5 was enriched in focal adhesion pathway. The P-values of all the enriched KEGG pathways were in the range of 3.1E-05 to 2.1E-03.
Discussion
In this study, we used the Human Illumina microarray data to find hub genes associated with C9orf72 mutation in FALS disease. C9orf72 expansion was found to be the most common genetic abnormality in FALS [26]. The discovery of the expansion repeat in C9orf72 is too recent [27] to know its complete relationship with the inflammatory system. This repeat expansion has opened new avenues of research in ALS. A total of 1055 DEGs in the C9orf72 patient fibroblasts were identified compared to the control fibroblasts. We could find more number of DEGs compared to the DEGs reported by Lagier-Tourenne et al.[16]. The DEGs were mainly enriched in the GO terms related to cell adhesion, biological adhesion and cell-cell signaling biological processes, extracellular matrix and extracellular space cellular components and the calcium ion signaling molecular function. The pathways enriched by the DEGs are ECM receptor interaction pathway, focal adhesion pathway, cell adhesion molecules. 15 hub genes were identified in the PPI network that was associated with the GO terms and pathways enriched by the DEGs (Table 5).
Tumor necrosis factor (TNF), Endothelin 1 (EDN1) were detected as the main hubs with a degree value of >35. TNF is a pro-inflammatory cytokine produced by monocytes and activated by mast cells, fibroblasts and neurons during acute inflammation. TNF is known to be secreted by the brain resident macrophage i.e. the microglial cell in response to various stimuli and its over production is related to neuronal cell death [28]. Elevated production of TNF is a common feature of several inflammatory diseases including ALS. It is known to play a major role in the central nervous system (CNS) neuroinflammation-mediated cell death in ALS as well as several other CNS complications. As a consequence of innate immune activation, an increased level of TNF is observed in many neurodegenerative diseases as reported earlier [29–32]. Previously, FALS and SALS affected spinal cord of mice was investigated and found increased immunoreactivity for TNF. Inhibiting the expression of TNF was observed in the ALS affected mice treated with thalidomide and lenalidomide drugs. This result gave the confirmation of the hypothesis that TNF plays an important role in the pathogenesis of ALS disease [33, 34]. The overexpression of TNF is observed in the ALS disease fibroblast samples taken for the present study with the fold change of 4.5 compared to the control fibroblast samples. TNF is involved both in immunological pathways and in oxidative stress known in ALS disease [35–37]. Hence modulating the levels of TNF would be worthwhile therapy for various CNS related diseases making TNF as the therapeutic target for neurodegenerative diseases including ALS disease.
EDN1 and its receptor B have been previously reported to be associated with ALS pathology [38]. It is a pro-inflammatory vasoconstrictor peptide encoding gene known to exert a variety of ALS-aggravating effects that includes axonal degeneration [39], heightened sensitivity to hypoxic stress [40] and increased excitotoxicity [41]. The expression of EDN1 in astrocytes and microglia is required for their survival under oxidative stress [42] which is a central mechanism by which motor neuron death occurs. EDN1 is found to be abundantly expressed by reactive astrocytes in the spinal cord of mouse model and SALS patients [43]. Several experiments have been performed to understand the toxic effects and lower the levels of EDN1 that would affect the motor neuron cell death. These experiements would suggest EDN1 as the potential target for therapeutic invention in ALS [43].
Furthermore, GO functional analysis also demonstrated that the DEGs were mainly involved in cell adhesion, biological adhesion, cell-cell signaling based on the analysis of biological processes. Adhesion plays an important role in cell signaling, regulation and fundamental in the development and maintenance of tissues [44].
Progression of ALS disease was known to involve cell-to-cell transmission involving motor neurons, microglia and astrocytes [45, 46].
Substantial clinical and pathological characteristics overlap among the common neurodegenerative diseases, ALS, Frontotemporal dementia (FTD) and Alzheimer’s disease (AD). C9orf72 repeat expansions were found to be risk factors for ALS, FTD and AD [47, 48]. Kohli et al. and Beck et al. demonstrated the risk factors of AD to be C9orf72 repeat expansions [49, 50].
Cell adhesion is involved in many aspects of neuronal development, including axon-bundle formation, synapse formation and formation of glial networks that surrounds axons and synapses [51–53]. The mechanical and chemical linkages between the intracellular and extracellular space are formed by cellular adhesions [54]. These adhesion systems forms the major for brain morphology and highly coordinated brain functions, such as memory and learning [51–53]. Various cell adhesion molecules (CAMs) are involved in the synapse formation and neuron-glia interactions [54]. These adhesions makes the interactions between the cells and the ECM through the cell adhesion molecules which also involved in the cell migration and signaling of many biological processes. This functionality of adhesion and adhesion molecules has made this a topic of study in the present scientific community. Previously reports stated cell adhesion molecules (CAMs) as disease biomarkers or pharmaceutical targets of neurological diseases [55].
The upregulated genes with highest hub degree mainly TNF, AGT, VWF, ACAN, VTN, ITGA3, ITGA7 are enriched in the cell adhesion biological process which forms the basis for neuronal damage or repair. The cell adhesion molecules (CAMs) ITGA3, ITGA7, CD36 and CD40 are differentially expressed which makes the links between the cells and ECM for cell migration and signaling cascades of many biological processes. Cell adhesion had previously been shown to be related to neurodegenerative diseases [56] and the CAMs were involved in the pathogenesis of many neurodegenerative diseases as previously reported [57, 58]. These CAMs play an important role in the inflammatory mechanisms associated with neurodegeneration [59]. Specific ECM molecules bind to integrin cell surface receptors and lead to downstream focal adhesion activation involved in the regulation of cell survival signals. Integrins and focal adhesion CAMs’ studies indicate a role for signaling in neurite outgrowth differentiation and in response to the toxic effects associated with neurodegeneration [60, 61].
Neuro active ligand receptor interaction KEGG pathway is highly enriched in the subnetworks. Many GPCRs are involved in this pathway. Signal transduction pathways activated by GPCRs are known to regulate variety of neuronal functions, plasticity and synaptic transmission, and control of various behaviours including memory, learning, emotions and motor functions. Most of the neurological disorders involve degeneration and death of neurons in brain and spinal cord. For example, ALS is characterized by selective degeneration of motor neurons in the spinal cord. The causes of neurodegenerative conditions are different in different diseases, but they do share mechanisms that include metabolic compromise, oxidative stress and disruption of cellular calcium homeostasis [62–64]. GPCRs are expressed in neurons and/or glial cells in central nervous system affected with neurodegenerative diseases. Increasing evidences suggest that signaling pathways play an important role in modifying the neurodegenerative processes.
Previous studies have reported TNF as the therapeutic target for neurodegenerative [65] and also CAMs have been reported as targets for many neurodegenerative diseases [59]. All these forms the important aspects of the central nervous system hence could be important therapeutic targets for neurodegenerative diseases including ALS.
Conclusion
In the present study, systems biology approach was used to examine the relationships between the importance of genes and several topological characteristics in the human PPI network. There are many studies previously reported with the mutations and pathways associated with SOD1 gene mutation and the pathways involved in its pathogenesis. We have uncovered gene-expression changes that occurred in familial ALS with C9orf72 mutation with ASO mediated reduction of C9orf72 RNAs. In view of this, gene expression data of C9orf72 fibroblasts and control fibroblasts were used to identify the DEGs related to ALS. Various bioinformatic tools were used to find the hub genes, enriched GO terms and KEGG pathways. GO terms related to adhesion, signaling were the main terms enriched by the DEGs. TNF, EDN1, AGT along with many other CAMs were identified to be the possible potential genes as targets for ALS disease. However, further studies are required to determine the clinical utility of these observations in the therapeutic management of ALS related neurological disease.
Abbreviations
- ACAN:
-
Aggrecan
- AD:
-
Alzheimer’s disease
- AGT:
-
Angiotensin
- ALS:
-
Amyotrophic lateral sclerosis
- APOE:
-
Apolipoprotein E
- ASO:
-
Antisense oligonucleotide
- CAM:
-
Cell adhesion molecule
- CD36:
-
Thrombospondin receptor
- CD40:
-
TNF receptor superfamily member 5
- CNS:
-
Central nervous system
- DAVID:
-
Database for annotation, visualization and integrated discovery
- DEG:
-
Differentially expressed genes
- ECM:
-
Extracellular matrix
- EDN1:
-
Endothelin 1
- FALS:
-
Familial amyotrophic lateral sclerosis
- FDA:
-
Food and drug administration
- FTD:
-
Frontotemporal dementia
- GEO:
-
Gene expression omnibus
- GLI1:
-
Zinc finger protein GLI1
- GO:
-
Gene ontology
- ITGA3:
-
Integrin, alpha 3
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- MCODE:
-
Molecular complex detection
- NTRK2:
-
Neurotrophic tyrosine kinase, receptor, type 2
- PPI:
-
Protein-protein interaction
- SALS:
-
Sporadic amyotrophic lateral sclerosis
- SOD1:
-
Superoxide dismutase 1
- TGFB2:
-
Transforming growth factor beta 2
- TIGA7:
-
Integrin, alpha 7
- TNF:
-
Tumor necrosis factor
- VTN:
-
Vitronectin
- VWF:
-
Von willebrand factor
- WNT5A:
-
Wingless-type MMTV integration site family, member 5A.
References
de Carvalho M. Tackling diagnostic delays in ALS. Lancet Neurol. 2015;14(5):457–8.
Kiernan MC. ALS and neuromuscular disease: in search of the Holy Grail. Lancet Neurol. 2014;13(1):13–4.
Ling SC, Polymenidou M, Cleveland DW. Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis. Neuron. 2013;79(3):416–38.
Beleza-Meireles A, AL-Chalabi A. Genetic studies of Amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph Lateral Scler. 2009;10(1):1–14.
Oh YK, Shin KS, Yuan J, Kang SJ. Superoxide dismutase 1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells. J Neurochem. 2008;104(4):993–1005.
Aronica E, Baas F, Iyer A, ten Asbroek AL, Morello G, Cavallaro S. Molecular classification of amyotrophic lateral sclerosis by unsupervised clustering of gene expression in motor cortex. Neurobiol Dis. 2015;74:359–76.
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425–31.
Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–91.
Levine TP, Daniels RD, Gatta AT, Wong LH, Hayes MJ. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics. 2013;29(4):499–503.
Zhang D, Iyer LM, He F, Aravind L. Discovery of Novel DENN Proteins: Implications for the Evolution of Eukaryotic Intracellular Membrane Structures and Human Disease. Front Genet. 2012;3:283.
DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–56.
Renton AE, et al. ITALSGEN Consortium, A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68.
Gijselinck I, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: A gene identification study. Lancet Neurol. 2012;11(1):54–65.
Wojciechowska M, Krzyzosiak WJ. Cellular toxicity of expanded RNA repeats: focus on RNA foci. Hum Mol Genet. 2011;20(19):3811–21.
Xi Z, et al. Hypermethylation of the CpG Island Near the G4C2 Repeat in ALS with a C9orf72 Expansion. Am J Hum Genet. 2013. doi:10.1016/j.ajhg.2013.04.017.
Lagier-Tourenne C, Baughn M, Rigo F, Sun S, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013;110(47):4530–9.
Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10.
RStudio Team. RStudio: Integrated Development for R. Boston: RStudio, Inc.; 2015. http://www.rstudio.com/.
Da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Licata L, Briganti L, Peluso D, Perfetto L, Iannuccelli M, Galeota E, Sacco F, Palma A, Nardozza AP, Santonico E, Castagnoli L, Cesareni G. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 2012;40(Database issue):857–61.
Chatr-Aryamontri A, Breitkreutz BJ, Henicke S, Boucher L, Winter A, Stark C, Nixon J, Ramage L, Kolas N, O’Donnell L, Reguly T, Breitkreutz A, Sellam A, Chen D, Chang C, Rust J, Livstone M, Oughtred R, Dolinski K, Tyers M. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41:D816–23.
Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009;37(Database issue):D767–72.
Chung-Yen L, Chia-Hao C, Hsin-Hung W, SHu-Hwa C, Chin-Wen H, Ming-Tat K. Hubba: hub objects analyser- a framework of interactome hubs identification for network biology. Nucleic Acids Res. 2008;36:W438–43.
O’Driscoll P, Merenyi E, Karmonik C, Grossman R. SOM and MCODE methods of defining functional clusters in MRI of the brain. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:734–7.
Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–9.
Mercedes P, Veronique VB, Ranjan B, et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS, Nat Neurosci. 2015;18(8):1175–82.
Marka VB, Mariely DH, Rosa R. How do C9orf72 repeat expansions cause ALS and FTD: can we learn from other non-coding repeat expansion disorders?, Curr Opin Neurol. 2012;25(6):689–700.
Greig NH, Mattson MP, Perry T, et al. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-α inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;1035:290–315.
Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–8.
Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–7.
Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–12.
Fillit H, Ding WH, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett. 1991;129:318–20.
Cereda C, Boiocchi C, Bongioanni P, Cova E, Guareschi S, Metelli MR, Rossi B, Sbalsi I, Cuccia MC, Ceroni M. TNF and sTNFR1/2 plasma levels in ALS patients. J Neuroimmunol. 2008;194:123–31.
Kiaiei M, et al. Thalidomide and Lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2006;26(9):2467–73.
Mir M, Asensio VJ, Tolosa L, et al. Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants. Neuroscience. 2009;162(4):959–71.
Hensley K, Mhatre M, Mou S, et al. On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8(11–12):2075–87.
Mhatrea M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis. 2004;6(2):147–57.
Lederer CW, et al. Pathways and genes differentially expressed in the motor cortex of patients with sporadic amyotrophic lateral sclerosis. BMC Genomics. 2007;8:26.
Uesugi M, Kasuya Y, Hayashi K, Goto K. SB209670, a potent endothelin receptor antagonist, prevents or delays axonal degeneration after spinal cord injury. Brain Res. 1998;786:235–9.
Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, Tsang MC, Chung SS, Chung SK. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005;25:998–1011.
Rozyczka J, Figiel M, Engele J. Endothelins negatively regulate glial glutamate transporter expression. Brain Pathol. 2004;14:406–14.
Ho MC, Lo AC, Kurihara H, Yu AC, Chung SS, Chung SK. Endothelin-1 protects astrocytes from hypoxic/ischemic injury. FASEB J. 2001;15:618–26.
Ranno E, D’Antoni S, Spatuzza M, Berretta A, Laureanti F, Bonaccorso CM, Pellitteri R, Longone P, Spalloni A, Iyer AM, Aronica E, Catania MV. Endothelin-1 is over expressed in Amyotrophic lateral sclerosis and induces motor neuron cell death. Neurobiol Dis. 2014;65:160–71.
Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–8.
Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One. 2010;5(5):10627.
Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl AcadSci U S A. 2011;108(9):3548–53.
Smith BN, Newhouse S, Shatunov A, et al. The C9ORF72 expansion mutation is a common cause of ALS+/−FTD in Europe and has a single founder. Eur J Hum Genet. 2013;21(1):102–8.
Stewart H, Rutherford NJ, Briemberg H, et al. Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathologica. 2012;123(3):409–17.
Kohli MA, John-Williams K, Rajbhandary R, et al. Repeat expansions in the C9ORF72 gene contribute to Alzheimer’s disease in Caucasians. Neurobiol Aging. 2013;34(5):1519.e5–1519.e12.
Beck J, Poulter M, Hensman D, et al. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet. 2013;92(3):345–53.
Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr Opin Neurobiol. 1999;9:79–87.
Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–32.
Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, et al. Cell adhesion molecules in synapse formation. J Neurosci. 2004;24:9244–9.
Murase S. The role of cell adhesion molecules in synaptic plasticity and memory. Curr Opin Cell Biol. 1999;11(5):549–53.
Wennström M, Nielsen HM. Cell adhesion molecules in Alzheimer’s disease. Degenerative Neurol Neuromuscul Dis. 2012;2:65–77.
Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–69.
Klevanski M, Herrmann U, Weyer SW, et al. The APP intracellular domain is required for normal synaptic morphology, synaptic plasticity, and hippocampus-dependent behavior. J Neurosci. 2015;35(49):16018–33.
IrynaLeshchyns’ka, Vladimir Sytnyk. Synaptic cell adhesion molecules in Alzheimer’s disease. Neural Plasticity. 2016;2016:1-9.
Berezin V, Walmod P. Cell Adhesion Molecules. Implications in Neurological Diseases. Eur 160.49, ISBN: 978-1-4614-8089- 1. Springer Science + Business Media, New York, NY, USA. 2014. p. 409.
Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32.
de Curtis I, Malanchini B. Integrin-mediated tyrosine phosphorylation and redistribution of paxillin during neuronal adhesion. Exp Cell Res. 1997;230:233–43.
Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5(5):291–309.
Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 2003;4(1–2):21–36.
Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3(2):65–94.
Cheng X, Shen Y, Li R. Targeting TNF: a therapeutic strategy for Alzheimer’s disease. Drug Discov Today. 2014;19(11):1822–7.
Acknowledgements
Part of our computations were carried out at the High Performance Computing Center of Shanghai Jiaotong University.
Funding
Dong-Qing Wei is supported by grants from the National High-Tech R&D Program (863 Program Contract No. 2012AA020307), the National Basic Research Program of China (973 Program) (Contract No. 2012CB721000), the Key Research Area Grant 2016YFA0501703 from the Ministry of Science and Technology of China, and Ph.D. Programs Foundation of Ministry of Education of China (Contract No., 20120073110057).
Mingzhu Zhao is supported by grants from National Natural Science Foundation of China (Contract No. 61503244).
Availability of data and materials
Not applicable.
Authors’ contributions
KMK has made contribution in the acquisition, analysis and interpretation of the data and drafted the manuscript. MZ is involved in the design of the study. DW is the academic supervisor and involved in the supervision of the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Differentially Expressed Genes in C9orf72 patients compared to control individuals fibroblasts. (XLSX 74 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kotni, M., Zhao, M. & Wei, DQ. Gene expression profiles and protein-protein interaction networks in amyotrophic lateral sclerosis patients with C9orf72 mutation. Orphanet J Rare Dis 11, 148 (2016). https://doi.org/10.1186/s13023-016-0531-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-016-0531-y