Abstract
Objective
Breast cancer survivors (BCS) are at high risk for the development of obesity, type 2 diabetes mellitus, and metabolic syndrome. There is increasing interest in the association between depression and metabolic dysfunction, which is relevant in this population as depression is often present in the chronic phase of cancer recovery. Thus, the aim of this study was to evaluate metabolic risk in BCS with and without depression compared to non-cancer controls.
Methods
African American (46 %) and Caucasian (54 %) postmenopausal BCS (N = 28; age: 60 ± 2 years; mean ± SEM) were matched for race, age (±2 years), and BMI (±2 kg/m2) to non-cancer controls (N = 28). Center for Epidemiologic Studies Depression Scale (CES-D) >16 or antidepressant medication usage was used to classify depression. Metabolic status was defined by 2-hr glucose during an OGTT and classification of metabolic syndrome.
Results
Compared to non-cancer controls, BCS had similar 2-hr glucose, but higher fasting glucose and total cholesterol, and were 2.5 times more likely to have metabolic syndrome (21 vs. 52 %)(P’s < 0.05). Conversely, HDL-C was 16 % higher in BCS (P < 0.05). Forty three % of BCS were on antidepressants compared to 14 % in non-cancer controls, despite similar mean CES-D scores (6 ± 1). Depressed BCS (46 %) had a higher BMI, waist circumference, fasting glucose, and more metabolic syndrome components than non-depressed BCS (P’s < 0.05).
Conclusions
BCS have a heightened prevalence of depression that may be associated with an increased prevalence of metabolic syndrome. These results support the need to monitor weight gain, depression, and the progression of metabolic abnormalities after cancer diagnosis and treatment. Further studies into the mechanistic link between depression and metabolic disease are necessary to identify strategies that can offset their impact on obesity and associated cardiovascular risk following a breast cancer diagnosis.
Similar content being viewed by others
Background
Due to better breast cancer screening modalities and cancer treatment options [1], the number of breast cancer survivors (BCS) in the United States is expected to increase from ~3 million survivors in 2012 to 3.8 million by 2022 [2]. Although more women are surviving this disease, a higher prevalence of depressive symptoms exists among BCS compared to the general female population, often persisting more than 5 years after diagnosis [3]. BCS also are at high risk for the development of obesity and associated cardiovascular disease risk, including metabolic syndrome, type 2 diabetes mellitus (T2DM), and hypertension [4, 5]. Depression and heightened metabolic dysfunction in chronic cancer BCS are often attributed to cancer diagnosis and treatment side effects, including emotional distress, pain, sleep disturbances, and excessive adiposity [6, 7].
Depression may affect the ability to make positive lifestyle changes and comply with medical therapy [8], while antidepressant treatment is associated with weight gain [9], possibly placing BCS at even greater metabolic risk. Indeed, a recent meta-analysis concluded that depression is associated with worse glycemic control, poor adherence to medication and diet regimens, and a reduction in quality of life in adults with T2DM [10]. Large waist circumference and low high-density lipoprotein cholesterol (HDL-C) also show associations with depression in subjects with an unknown cancer history [11]. Thus, determining the presence of depression in BCS may have implications for clinical care in the chronic phase of recovery.
The tendency for weight gain with aging places postmenopausal women at increased risk for metabolic dysfunction [12]. In addition to abdominal obesity, significant risk factors for breast cancer in postmenopausal women are elevated fasting glucose, hypertension, hyperlipidemia [13], all components of the metabolic syndrome. Less is known about whether cancer survivorship increases metabolic risk during long-term recovery. Only a few studies have matched postmenopausal BCS to postmenopausal non-cancer controls [4, 14, 15], finding that BCS are more likely to have metabolic abnormalities than age-matched women without prior breast cancer. However, several limitations are present in these studies, including differences in BMIs between cases and controls and/or lack of consideration of differences in racial profiles between groups, which could affect interpretability of the results. Thus, this study evaluated metabolic risk and depression in postmenopausal BCS compared to postmenopausal women without a prior diagnosis of breast cancer matched for age, race, and BMI. We hypothesized that women with previous breast cancer have worse depression scores and poorer metabolic profiles compared to women without a history of breast cancer, and that this difference is independent of age, race, and obesity. Further, we anticipate that BCS with depression will have greater metabolic dysfunction than BCS without depression.
Methods
Study design and sample selection
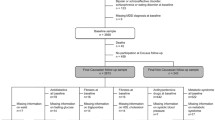
This cohort study was observational in nature. Groups were comprised of 28 postmenopausal BCS and 28 postmenopausal women without prior breast cancer diagnosis matched for race, age (±2 years), and BMI (±2 kg/m2). All women reported being sedentary (<30 min of structured exercise, two times per week) and weight stable (<2 kg weight change) over the prior six months. Women without a history of breast cancer were selected from a larger subset of individuals from a previously published manuscript [16]. BCS were recruited from the Baltimore area and had completed surgical treatment, radiation therapy, and/or chemotherapy at least 6 months prior to enrollment. All women signed University of Maryland Institutional Review Board approved informed consent forms. A medical history, physical examination, resting 12-lead electrocardiogram, and fasting blood profile were performed to determine current metabolic abnormalities.
Cardiorespiratory fitness
VO2 was measured by indirect calorimetry during a graded exercise test on a treadmill as previously described [17]. VO2max was accepted as valid if two of the three following criteria were met: respiratory exchange ratio ≥1.0, maximum heart rate >90 % of age-predicted maximum (220-age), or a plateau in VO2 (<200 ml/min change). If such criteria were met, the highest level of VO2 was defined as VO2max.
Depression assessment
Risk for depression was measured with the Center for Epidemiologic Studies Depression Scale (CES-D). The CES-D is composed of 20 items and assesses risk for depression in the general population. A cut off of 16 was used to indicate “mild” depressive symptomatology [18]. Subjects were classified as having depression if they had a CES-D score >16 and/or were being treated with an antidepression medication.
Clinical parameters and laboratory procedures
Height, body weight, and waist and hip circumferences were measured using standardized protocols [19], and BMI (kg/m2) and waist to hip ratio (WHR) calculated. Blood pressure was measured on the non-affected cancer side for BCS and on the right side in the non-cancer controls after 10 min of resting on three occasions and averaged.
Blood was collected after a 12 h fast. Plasma triglyceride and cholesterol levels were analyzed using enzymatic methods (UniCelDxC880i; Beckman Coulter, Inc., Brea, CA), HDL-C measured in the supernatant after precipitation with dextran sulfate, and low-density lipoprotein cholesterol (LDL-C) calculated as LDL-C = total cholesterol- HDL-C -TG/5 [20]. A oral glucose tolerance test was performed, with glucose measured at fasting and every 30 min. for 2 h following the ingestion of 75 g glucose load. Glucose was measured by the glucose oxidase method (2300-STAT Plus; YSI, Yellow Springs, OH) and insulin measured by radioimmunoassay (Linco Research Inc., St. Charles, MO) [21]. The presence of three or more of the following metabolic syndrome components were used to diagnose metabolic syndrome: central obesity (waist >88 cm), impaired glucose metabolism (fasting glucose >5.6 mmol/L), elevated blood pressure (>130/85 mmHg or antihypertensive treatment), and dyslipidemia (triglyceride (TG) >1.7 mmol/L, HDL-C <1.3 mmol/L, or hypolipidemic treatment) [22].
Statistical analyses
Data were analyzed using SPSS Version 20. Data were analyzed for normality using the Kolmogorov–Smirnov test. Mean ± SEM were calculated for continuous variables and compared using Student’s t test after log transformation of variables (plasma triglycerides), as appropriate. Percentages were calculated for categorical variables (presence vs. absence of medication usage, metabolic syndrome, and depression) and compared using χ2 tests. Pearson correlation coefficients were calculated to determine relationships between variables. All tests were two-tailed, and P < 0.05 were considered statistically significant.
Results
Subject characteristics
The women were 46 % African American and 54 % Caucasian. BCS were an average of 94 ± 18 (range 9–384) months since diagnosis. Treatment was lumpectomy in 54 % and mastectomy in 57 %. Forty-three percent underwent chemotherapy alone, 46 % radiation therapy alone, and 11 % received both. At study entry, 25 % of BCS were taking an aromatase inhibitor, 12 % tamoxifen, and 63 % were not receiving hormone therapy. BCS were well matched to non-cancer controls with regard to age, BMI, waist circumference, and WHR, but VO2max was 25 % higher in BCS (P < 0.05) (Table 1).
Elevated metabolic abnormalities and depression in breast cancer survivors (Table 2)
On average, BCS had slightly elevated fasting glucose, with 9 % higher fasting glucose than non-cancer controls (P < 0.05). BCS also had higher HDL cholesterol (P < 0.05), tended to have higher total cholesterol (P = 0.08), and 39 % more required a lipid lowering medication (P < 0.01). Diastolic and systolic blood pressure and hypertension medication usage was similar between groups. BCS were 2.5 times more likely to have metabolic syndrome (21 vs. 52 %; P < 0.05), with blood pressure (64 %) and waist circumference (61 %) being the most prevalent component in BCS.
CES-D scores >16 were observed in ~14 % of non-cancer control and BCS subjects. Despite similar mean CES-D scores, three times more BCS were being treated with an antidepressant medication (P < 0.05; 58 % of BCS were on a selective serotonin reuptake inhibitor, 25 % on an atypical antidepressant, and 17 % on a tricyclic antidepressant). Twenty-six % of non-cancer controls and 46 % of BCS were classified as depressed (CES-D >16 and/or on an antidepressant) (P = NS).
Comparisons of metabolic abnormalities by depression status in breast cancer survivors
As anticipated, BCS classified with depression had higher mean CES-D scores than BCS without depression (4.1 ± 0.9 vs. 8.5 ± 2.0, P < 0.05). Despite similar age, racial profiles, fitness levels, and time from cancer diagnosis (data not shown), BCS with depression also had a higher BMI, waist circumference, fasting glucose, and more metabolic syndrome components (P’s < 0.05; Fig. 1) than non-depressed BCS. Further, there was a trend for fasting insulin to be higher (P = 0.07) and HDL-C (P = 0.09) to be lower in BCS with depression (Fig. 1). Blood pressure and all other lipid variables were similar between groups, including the usage of hypertension and lipid lowering medications (data not shown).
Discussion
While numerous studies have identified weight gain as a consequence of breast cancer treatment extending into the chronic phase of recovery [23, 24], fewer studies have examined the prevalence of metabolic dysfunction in BCS. Although studies have reported that postmenopausal BCS have more metabolic abnormalities than non-cancer controls [4, 14], ours is the first to show that this is independent of age, race, and obesity. We find that fasting glucose is higher, with a trend for higher cholesterol, in BCS compared to non-cancer controls. We also determined that BCS are more likely to be treated for depression than non-cancer controls and that BCS with depression have more metabolic abnormalities. Thus, understanding the consequences of breast cancer diagnosis and treatment on risk for depression and metabolic dysfunction may aid in medical monitoring to optimize health during the survivorship phase of cancer recovery.
The pathophysiological response linking depression and metabolic dysfunction is inconclusive with regard to causal factors. It is postulated that depression is associated with increased stress and activation of the hypothalamic–pituitary–adrenal (HPA) axis, which is involved in the pathogenesis of central adiposity and metabolic syndrome [25]. Greater metabolic dysfunction is associated with higher proinflammatory cytokines [26], many of which are elevated in BCS [27, 28]. Conversely, there is evidence that many cancer treatments, including radiation and chemotherapy may activate an immune response [29, 30], leading to dysregulation of the HPA axis and, ultimately, depression [31]. Further, development of central adiposity also increases secretion of endogenous steroid hormones in obese, postmenopausal women [32]. High levels of circulating estrogen and testosterone are associated with an increased risk of breast cancer in peri- and postmenopausal women [33, 34]. Although, we do not believe estrogen concentrations have been compared between BCS and non-cancer controls, no differences in hyperandrogenic status (testosterone concentrations >1.2 ng/ml) were found between these groups previously, despite observed metabolic differences [15]. Studies, including ours, suggest that as many as 50–55 % of BCS are depressed [35–37], which is ~20–25 % more than that observed in postmenopausal women without a history of cancer [38]. Further, our results agree with previous reports [14, 39] that metabolic syndrome is prevalent in ~50 % of postmenopausal BCS. This is ~1.5 higher than what is observed in postmenopausal women of comparable age and BMI in NHANES [40] and our postmenopausal non-cancer controls. The presence of metabolic abnormalities and depression following breast cancer diagnosis are associated with elevated cancer recurrence and mortality rates [39, 41, 42], highlighting the importance of understanding the mechanism linking depression and metabolic dysfunction in BCS.
Often the failure to recover after breast cancer treatment (i.e. low quality of life persisting for years following completion of cancer treatment [43]) is attributed to lifestyle choices, including low activity levels and suboptimal nutrient intake. However, our results suggest that the metabolic abnormalities and depression observed in BCS may not be attributable to low cardiorespiratory fitness, as these risk factors were present even though a higher VO2max was observed in BCS than non-cancer controls. Despite this finding, many studies suggest that depressive symptoms and metabolic dysfunction improve with exercise in BCS [44–46].
This study is limited by a small sample size, which prevented stratification by breast cancer treatments and the control of length of time post-cancer treatment. As there is evidence that breast cancer treatments, including chemical variations in drugs used for chemotherapy, may have varying effects on metabolism [47], future studies should attempt to stratify by treatment. There is limited data available on the impact of duration of survivorship on the observed relationships; however, there is evidence that subjective outcomes (i.e. quality of life and depression) remain lower than pre-diagnosis or may even continue to decline during the survivorship phase [3, 48]. The cause of this continued failure to recover is not elucidated and may be influenced by factors other than the cancer diagnosis and treatment (i.e. financial and emotional support) during the survivorship phase. Although the gold standard for depression evaluation is the use of a clinician rated interview technique, such as the Structured Clinical Interview for DSM-IV Axis I Disorders and the Hamilton Depression Rating Scale, these methods require substantial subject time and trained clinical interviewers [49]. Self-report questionnaires, such as the CES-D, have strong validity and reliability [50] and are less burdensome [51] when compared to interview techniques. Further, because of the pilot nature of this study, body composition assessments of the BCS were limited to anthropometric measurements. While more sophisticated measures of body composition may provide additional insight as to the role of cancer survivorship on metabolism, these tests can be expensive and often require radiation exposure, which may decrease participant interest in and compliance to the study protocol. However, some of these limitations are balanced by our strong study design, where BCS were matched for age, race, and BMI to non-cancer controls. BMI of the subjects spanned from normal weight to morbidly obese, while most studies examining metabolic dysfunction in BCS only includes those who are obese; thus, these findings may be more representative of the general BCS population.
In summary, our results support the need to monitor weight gain, depression, and the progression of metabolic abnormalities during treatment and longitudinally in BCS, as their development may affect long-term survivorship. Further studies into the mechanistic links between depression and metabolism are necessary to identify strategies that can offset their impact on survivorship following a breast cancer diagnosis.
References
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71.
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41.
Maass SW, Roorda C, Berendsen AJ, Verhaak PF, de Bock GH. The Prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas. 2015;82:100–8.
Jones DH, Nestore M, Henophy S, Cousin J, Comtois AS. Increased Cardiovascular risk factors in breast cancer survivors identified by routine measurements of body composition, resting heart rate and arterial blood pressure. Springerplus. 2014;3:150.
Sheean P, Liang H, Schiffer L, Arroyo C, Troy K, Stolley M. Assessing the prevalence of compromised bone health among overweight and obese African-American breast cancer survivors: a case-control study. J Cancer Surviv. 2015;10:21–30.
Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol. 2014;5:272–82.
Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol. 2002;13:589–98.
Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23.
Paige E, Korda R, Kemp-Casey A, Rodgers B, Dobbins T, Banks E. A record linkage study of antidepressant medication use and weight change in australian adults. Aust N Z J Psychiatry. 2015;49:1029–39.
Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19:113–22.
Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31:2368–73.
Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull. 2011;97:169–96.
Rosato V, Bosetti C, Talamini R, Levi F, Montella M, Giacosa A, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol. 2011;22:2687–92.
Buttros Dde A, Nahas EA, Vespoli Hde L, Uemura G, de Almeida Bda R, Nahas-Neto J. Risk of metabolic syndrome in postmenopausal breast cancer survivors. Menopause. 2013;20:448–54.
Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National cancer institute of naples experience. Cancer Biol Ther. 2010;10:1240–3.
Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese post-menopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2011;302:E145–52.
Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–8.
Radloff LS. The Ces-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401.
Ferrara CM, Goldberg AP, Nicklas BJ, Sorkin JD, Ryan AS. Sex differences in insulin action and body fat distribution in overweight and obese middle-aged and older men and women. Appl Physiol Nutr Metab. 2008;33:784–90.
Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc. 2014;62:607–14.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/National heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–52.
Sedjo RL, Byers T, Ganz PA, Colditz GA, Demark-Wahnefried W, Wolin KY, et al. Weight gain prior to entry into a weight-loss intervention study among overweight and obese breast cancer survivors. J Cancer Surviv. 2014;8:410–8.
Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21:1260–71.
Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015:341583.
Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10:e0121971.
Thomson CA, Thompson PA, Wright-Bea J, Nardi E, Frey GR, Stopeck A. Metabolic syndrome and elevated c-reactive protein in breast cancer survivors on adjuvant hormone therapy. J Womens Health (Larchmt). 2009;18:2041–7.
Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44.
Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15:927–43.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73.
Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62.
Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–35.
Rock CL, Flatt SW, Laughlin GA, Gold EB, Thomson CA, Natarajan L, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:614–20.
Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR, Study of Osteoporotic Fractures Research Group. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Ann Intern Med. 1999;130:270–7.
Begovic-Juhant A, Chmielewski A, Iwuagwu S, Chapman LA. Impact of body image on depression and quality of life among women with breast cancer. J Psychosoc Oncol. 2012;30:446–60.
Rabin EG, Heldt E, Hirakata VN, Bittelbrunn AC, Chachamovich E, Fleck MP. Depression and perceptions of quality of life of breast cancer survivors and their male partners. Oncol Nurs Forum. 2009;36:E153–8.
Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–6.
Unsal A, Tozun M, Ayranci U. Prevalence of depression among postmenopausal women and related characteristics. Climacteric. 2011;14:244–51.
Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–8.
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the us population from the third national health and nutrition examination survey, 1988–1994. Arch Intern Med. 2003;163:427–36.
Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: a review of the recent literature. Curr Nutr Rep. 2014;3:9–15.
Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354:1331–6.
Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8:330.
Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: an overview. World J Clin Oncol. 2014;5:406–11.
Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise Effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19.
Bao PP, Zheng Y, Nechuta S, Gu K, Cai H, Peng P, et al. Exercise after diagnosis and metabolic syndrome among breast cancer survivors: a report from the Shanghai breast cancer survival study. Cancer Causes Control. 2013;24:1747–56.
Braddock M, Heilbraun J, Mendzelevski B. Cardiovascular safety and hemodynamic considerations in oncology drug development—webinar highlights october 10th 2012. Expert Opin Drug Saf. 2013;12:783–91.
Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–99.
Vares EA, Salum GA, Spanemberg L, Caldieraro MA, Fleck MP. Depression dimensions: integrating clinical signs and symptoms from the perspectives of clinicians and patients. PLoS One. 2015;10:e0136037.
Stuart AL, Pasco JA, Jacka FN, Brennan SL, Berk M, Williams LJ. Comparison of self-report and structured clinical interview in the identification of depression. Compr Psychiatry. 2014;55:866–9.
Pessin H, Galietta M, Nelson CJ, Brescia R, Rosenfeld B, Breitbart W. Burden and benefit of psychosocial research at the end of life. J Palliat Med. 2008;11:627–32.
Authors’ contributions
MCS was involved in study design, data collection and analysis, and writing the manuscript and ASR and APG in the study design, data interpretation, and manuscript review. All authors read and approved the final manuscript.
Acknowledgements
Our appreciation is extended to the women who participated in this study. We are grateful to the nurses and exercise physiologists of the University of Maryland School of Medicine, Division of Gerontology and Geriatric Medicine and Baltimore VA GRECC for their assistance in this project. This study was supported by funds from: National Institute on Aging (NIA) Grants: R01-AG19310, R01-AG20116, Maryland Claude D. Pepper Older Americans Independence Center (P30 AG028747), and 5T32AG000219-18; NIDDK Mid-Atlantic Nutrition Obesity Research Center (NIH P30 DK072488); Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC); a VA Senior Research Career Scientist Award to ASR and a VA Career Development Award to MCS.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Serra, M.C., Goldberg, A.P. & Ryan, A.S. Increased depression and metabolic risk in postmenopausal breast cancer survivors. Diabetol Metab Syndr 8, 44 (2016). https://doi.org/10.1186/s13098-016-0170-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-016-0170-4