Abstract
Background
Healthcare facilities have been challenged by the risk of SARS-CoV-2 transmission between healthcare workers (HCW) and patients. During the first wave of the COVID-19 pandemic, infections among HCW were observed, questioning infection prevention and control (IPC) measures implemented at that time.
Aim
This study aimed to identify nosocomial transmission routes of SARS-CoV-2 between HCW and patients in a tertiary care hospital.
Methods
All SARS-CoV-2 PCR positive HCW and patients identified between 1 March and 19 May 2020, were included in the analysis. Epidemiological data were collected from patient files and HCW contact tracing interviews. Whole genome sequences of SARS-CoV-2 were generated using Nanopore sequencing (WGS). Epidemiological clusters were identified, whereafter WGS and epidemiological data were combined for re-evaluation of epidemiological clusters and identification of potential transmission clusters. HCW infections were further classified into categories based on the likelihood that the infection was acquired via nosocomial transmission. Secondary cases were defined as COVID-19 cases in our hospital, part of a transmission cluster, of which the index case was either a patient or HCW from our hospital.
Findings
The study population consisted of 293 HCW and 245 patients. Epidemiological data revealed 36 potential epidemiological clusters, with an estimated 222 (75.7%) HCW as secondary cases. WGS results were available for 195 HCW (88.2%) and 20 patients (12.8%) who belonged to an epidemiological cluster. Re-evaluation of the epidemiological clusters, with the available WGS data identified 31 transmission clusters with 65 (29.4%) HCW as secondary cases. Transmission clusters were all part of 18 (50.0%) previously determined epidemiological clusters, demonstrating that several larger outbreaks actually consisted, of several smaller transmission clusters. A total of 21 (7.2%) HCW infections were classified as from confirmed nosocomial, of which 18 were acquired from another HCW and 3 from a patient.
Conclusion
The majority of SARS-CoV-2 infections among HCW could be attributed to community-acquired infection. Infections among HCW that could be classified as due to nosocomial transmission, were mainly caused by HCW-to-HCW transmission rather than patient-to-HCW transmission. It is important to recognize the uncertainties of cluster analyses based solely on epidemiological data.
Similar content being viewed by others
Background
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) generated a significant burden on healthcare facilities worldwide [1]. Besides the large influx of COVID-19 patients, the nosocomial transmission of SARS-CoV-2 between patients and healthcare workers (HCW) has been a major concern.
Many studies have investigated SARS-CoV-2 outbreaks in healthcare facilities. However, many studies reporting on the nosocomial transmission of SARS-CoV-2 were in the context of either department-specific outbreaks or a select set of samples of the total SARS-CoV-2 positive population in a healthcare facility, whereby HCW data was not always available or analysed [2,3,4,5]. HCW experience community as well as occupational exposure to SARS-CoV-2, therefore HCW can play an important role in hospital outbreaks. It is important to determine what extent of COVID-19 among HCW is community- or hospital-acquired and how much they contribute to in-hospital transmission. Combining epidemiological and whole-genome sequencing (WGS) data can help elucidate the dynamics of SARS-CoV-2 hospital outbreaks, hereby allowing real-time adjustment of targeted infection prevention and control (IPC) measures. However, WGS is a technique not readily available for many healthcare facilities, especially not with a fast turn-around time. Consequently, many healthcare facilities rely on epidemiological data for their initial outbreak response. Therefore, it is necessary to investigate the over- or underestimation of outbreak clusters when using solely epidemiological data.
Here, we describe the transmission of SARS-CoV-2 between HCW and patients within a large tertiary hospital in The Netherlands during the first months of the COVID-19 pandemic. Secondly, we determine the added value of WGS in addition to epidemiological investigations with regard to outbreak investigation and source and contact tracing.
Methods
Setting
The Erasmus MC University Medical Center (Erasmus MC) is a large tertiary care hospital in Rotterdam, The Netherlands, with a total of 1100 beds and 39 operating rooms, including the Sophia Children’s Hospital. There are approximately 32,000 clinical admissions per year and 14,000 HCW employed (including physicians, registered nurses and researchers) [6]. The adult clinic primarily consists of single-occupancy rooms with private bathrooms, whereas the pediatric clinic mainly has multiple-occupancy rooms with shared bathrooms [7]. This analysis used data collected from 1 March 2020 until 19 May 2020 during the first wave of the COVID-19 pandemic. The end study date was chosen because no HCW tested positive for SARS-CoV-2 in the six weeks hereafter. At that time diagnostic test availability for SARS-CoV-2 was limited in The Netherlands; testing was only available to clinically suspected patients from COVID-19 risk groups, and hospital HCW. Until 19 May 2020 a total of 44,010 SARS-CoV-2 positive persons were registered in The Netherlands and 5,691 COVID-19 related deaths [8]. In the region of the hospital, Rotterdam-Rijnmond, a total of 4,252 COVID-19 cases were identified and 532 deaths in a population of 1.3 million inhabitants [8].
Study design and data collection
All SARS-CoV-2 positive HCW and admitted patients, patients visiting the outpatient clinic, and patients visiting the emergency department who were tested between 1 March 2020 and 19 May 2020 were included. COVID-19 patients were either patients who tested SARS-CoV-2 positive upon admission (often referred by the general practitioner or transferred from other Dutch hospitals), at their hospital visit, or patients who tested positive during hospitalization. HCW and patients were tested for SARS-CoV-2 by reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal and throat swabs [9].
Epidemiological data collected from SARS-CoV-2 positive HCW included the date of positive SARS-CoV-2 test, age at the date of first positive SARS-CoV-2 PCR, date of symptom onset, symptom description, work location/department, job description, and self-reported source of infection. These data were prospectively collected as part of routine occupational health activity when the positive test result was shared with the HCW. Patient data extracted from electronic health records (EHR) included the date of positive SARS-CoV-2 PCR test, age at the date of the first positive SARS-CoV-2 test, hospital admission date, admission location, and previous contact with SARS-CoV-2 positive persons.
Infection prevention and control measures
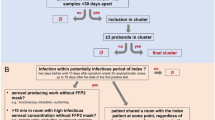
During the first months of the COVID-19 pandemic, national and Erasmus MC guidelines related to SARS-CoV-2 IPC measures were still being developed and adjusted according to new insights from experience and newly available literature. HCW did, however, always use personal protective equipment (PPE) when providing care for (suspected) COVID-19 patients. The recommended use of PPE changed over time, according to updated versions of national guidelines and new insights on transmissibility at the time. The Erasmus MC did not experience any shortage of PPE supply during the first COVID-19 wave. Details on the implemented IPC measures in the Erasmus MC during the study period are described in Fig. 1. For HCW, an occupational health facility for sampling and RT-PCR testing was available from 1 March 2020. Based on the routine occupational health information provided it was decided whether a source and contact investigation (CI) was necessary. These investigations were performed for each HCW that had been working with symptoms and for each patient that had not been cared for in adequate isolation conditions. Contacts were registered starting from the day of symptom onset.
Timeline of infection prevention and control measures implemented in our hospital, per category. AGP = Aerosol generating procedures, ARDS = Acute respiratory distress syndrome, CI = Contact investigation, FFP = filtering face piece, GP = general practitioner, HCW = Healthcare workers, ICU = Intensive care unit, PPE = Personal protective equipment, RTI = Respiratory tract infection, m = meter. (a) Noord-Brabant is a neighboring province with a relatively high COVID-19 prevalence during the first wave. (b) Change from FFP-2 to FFP-1 masks in accordance to the national guideline at the time. (c) HCW who contacted their general practitioner for SARS-CoV-2 testing were referred to the municipal public health authorities. (d) Specific symptoms were: fever, coughing, shortness of breath, sore throat, and loss of sense of smell or taste. (e) Non-specific symptoms were: general malaise, fatigue, muscle ache, joint pain, gastrointestinal complaints and, pain behind the eyes. (f) Healthcare worker with a household member or partner with confirmed COVID-19 or with fever and respiratory symptoms. (g) China, Singapore, South Korea, Iran, Italy, Taiwan, Japan, Malaysia, Thailand, UAE, and Vietnam. From 10 March 2020 also the province of Noord-Brabant in The Netherlands
Whole genome sequencing and cluster identification
WGS was performed on all SARS-CoV-2 PCR positive HCW and patient nasopharyngeal swab samples with a cycle threshold (Ct) value of below 32. Nanopore sequencing was performed on these samples as described previously [10]. Successful sequencing was defined as having more than 90% genome coverage. The generated sequences and all other publicly available sequences from The Netherlands collected before 3 July 2020 were used for downstream analyses. Phylogenetic analysis was performed using IQ-TREE and trees were visualized using FigTree v.1.4.4 [11, 12]. Sequence clusters were identified as sequences from the same epidemiological cluster, same department and having a maximum of two nucleotide differences and sampled within two weeks [13]. Cluster definition for clusters between different departments was set on having a maximum of 1 nucleotide difference.
Definitions
Epidemiological clusters were defined as two or more SARS-CoV-2 positive HCW or patients with a spatiotemporal link, either by unprotected contact < 14 days before symptom onset at the same hospital department, or known contact determined by source and contact investigations. Contact between patients and HCW whereby all required PPE and appropriate IPC measures were used were not regarded as transmission moments for the cluster analysis based on epidemiological data alone. When the date of symptom onset was not available the date of the first positive PCR test was used.
Transmission clusters were identified by re-evaluation of epidemiological clusters with the addition of WGS data. Hereby, transmission clusters were defined as a group of ≥ 2 SARS-CoV-2 positive HCW or patients with a link in time and place, confirmed by WGS data i.e. belonging to the same sequence cluster. This was done regardless of required PPE and appropriate IPC measures. Sequence clusters without epidemiological links were not regarded as transmission clusters.
For further classification of COVID-19 among HCW, we established definitions for the likelihood that nosocomial transmission had taken place (Table 1). Definitions were developed in a multidisciplinary group of epidemiologists, medical microbiologists and occupational health physicians, and were based upon literature and own experience [14]. For identification of an index case, it was assumed a minimum of 2 days and a maximum of 14 days between symptom onset of the index case and the secondary case was necessary to be able to appoint a definite index case. During instances where multiple indices of both HCW and patients were plausible, the index was classified as indeterminate. For classification a distinction was also made between regional and non-regional clusters. Regional clusters were defined as sequence clusters which were identified in ≥ 5 primary COVID-19 patients, whereby patients with a positive SARS-CoV-2 test upon admission were regarded as primary COVID-19 patients. We assumed primary COVID-19 patients were a good reflection of clusters circulating in the community. All other clusters were defined as non-regional.
Data analyses
Epidemiological data were analyzed with SPSS version 28.0 (IBM, Armonk, NY, USA). Continuous variables were summarized as medians with range, and categorical variables were expressed as median numbers and percentages. For our retrospective cluster analysis, we first identified epidemiological clusters from all patients and HCW with a SARS-CoV-2 positive PCR. In a second analysis, the number and size of epidemiological clusters were determined only for patients and HCW with available WGS results. Thirdly, transmission clusters were established by combining epidemiological clusters and sequence clusters.
The number of secondary cases among HCW resulting from identified clusters was determined by subtracting the total number of index SARS-CoV-2 positive HCW from the total number of HCW in epidemiological or transmission clusters.
Results
Population characteristics: healthcare workers
Between 1 March and 19 May 2020, 4362 HCW were tested for SARS-CoV-2 by RT-PCR at the Erasmus MC, of whom 293 HCW (6.7%) tested positive (Fig. 2). The median age was 36 years (range 18–65) and 73% was female. Of positive HCW, 197 (67.2%) were clinical staff (e.g., nurses and physicians), of whom 11 (3.7%) HCW worked on a COVID-19 ward. The other 96 (32.8%) HCW did not work in direct patient care (e.g., administrative workers and analysts). The median time between symptom-onset and SARS-CoV-2 testing was 3 days (range 0–24 days).
Regarding self-reported sources of infection, 62 of 293 (21.2%) HCW reported a colleague, 33 (11.3%) HCW a patient, 28 (9.6%) HCW reported a family member, 21 (7.2%) HCW recent travel and 151 (51.5%) HCW did not report any possible source. Fourteen (4.8%) HCW reported multiple potential sources of infection.
Of HCW who tested positive, 162 out of 293 (55.3%) reported to have worked while symptomatic, whereby contact tracing was required for 103 (35.2%) HCW. For 56 HCW (19.1%) contact tracing among both HCW and patients was necessary, for 47 HCW (16.0%) only contact tracing among HCW was needed.
Population characteristics: patients
During the study period, 245 patients tested positive. Patients had a median age of 62 years (range 3–94) and 35.7% were women. Out of 245 patients, 16 patients (6.5%) only had an outpatient visit while the other 229 patients (93.5%) were admitted as inpatients. Patients were admitted to the hospital for a median of 12.6 days (range 1–79 days). Contact tracing was required for 24 patients (9.8%).
Epidemiological cluster analysis
In total, 257 out of 293 HCW (87.7%) and 24 out of 245 patients (9.7%) were potentially part of an epidemiological cluster. Epidemiological data revealed 36 potential epidemiological clusters among the SARS-CoV-2 positive HCW and patients. Cluster size ranged from 2 to 31 cases and contained a median of 5 cases. Epidemiological clusters identified were found in 11 non-clinical departments, 15 inpatient departments, 7 outpatient departments, and 3 clusters in the operation room complex. Eight epidemiological clusters consisted of both patients and HCW, while the remaining 28 consisted of only HCW (Fig. 3A). Out of the 36 epidemiological clusters, only one cluster (cluster 3) had a patient as the index case, in all other clusters a HCW was the index case. Epidemiological cluster investigations resulted in the identification of 222 (75.7%) secondary cases out of 293 HCW.
Identification of epidemiological clusters and transmission clusters. A Epidemiological clusters identified in the complete study population based only on epidemiological data. B Epidemiological clusters identified based only on epidemiological data, excluding cases without WGS results. C Transmission clusters identified in the study population confirmed by WGS. Epidemiological clusters portrayed in Fig. 3B were re-evaluated to form transmission clusters based on the combination of the epidemiological and sequence clusters. Different transmission cluster originating from the same epidemiological cluster are indicated with letters a/b/c/d. Blue = Healthcare workers; Orange = Patients
Sequencing and phylogenetic analysis
WGS results were available for 195 HCW (88.2%) and 20 patients (12.8%) who belonged to an epidemiological cluster (Fig. 3B). Transmission clusters based on epidemiologic data combined with WGS contained a median of 3 persons (range 2–24). A total of 158 (71.5%) HCW were secondary cases. These sequences and all other sequences available from The Netherlands during the time of the study were used for phylogenetic analysis and cluster determination (Fig. 4).
Phylogenetic analysis of all available sequences from the Netherlands on 3 July 2020. The different departments are depicted in different clusters. Thirty-five sequence clusters were identified, which made up 31 transmission clusters. The scale bar represents the number of nucleotide substitutions per site. Different colors represent different departments
A re-evaluation of the epidemiological clusters based on WGS data identified 31 transmission clusters (Fig. 3C). Five epidemiological clusters could not be further analyzed as there was only one person with available WGS data. The WGS determined 31 transmission clusters were part of 18 (50.0%) of the previously determined epidemiological clusters, demonstrating that several larger outbreaks actually consisted, of several smaller transmission clusters. These clusters consisted of 17 patients and 92 HCW. One hundred and thirty of 221 (58.9%) HCW did not belong to a cluster as they had a unique viral strain, indicating acquisition of the infection outside of the hospital. Eleven clusters consisted of both HCW and patients. Combining sequence and epidemiological clusters resulted in a total number of 65 HCW (29.4%) as secondary cases.
Likelihood of nosocomial transmission among HCW
When SARS-CoV-2 positive HCW in transmission clusters were classified based on the likelihood of nosocomial SARS-CoV-2 transmission, the following was found: for 21 HCW (7.2% of all cases) there was confirmed nosocomial transmission, of which 18 acquired the infection from another HCW and 3 infections originated from a patient. For 37 HCW (12.6%) probable transmission was found and for 3 HCW (1.0%) possible transmission (Table 2). In five instances (1.7% of all cases) a patient was the most likely source for nosocomial transmission to a HCW. For the majority of the HCW (78.8% of all cases) the transmission could not be confirmed and/or were most probably infected outside the work setting.
Discussion
This comprehensive investigation of nosocomial SARS-CoV-2 transmission clusters revealed that the majority of SARS-CoV-2 infections among HCW could be attributed to community-acquired infection. Infections among HCW that could be classified as due to nosocomial transmission, were mainly caused by HCW-to-HCW transmission rather than patient-to-HCW transmission. Furthermore, we demonstrated that analyses based on epidemiological data alone largely overestimated the number of nosocomial transmissions, as well as the size of nosocomial transmission clusters.
SARS-CoV-2 has been widely recognized as an occupational health hazard for HCW [15, 16]. HCW have been shown to have a higher seroprevalence than the general population and a higher risk of severe COVID-19 [17, 18]. Especially during the initial phase of the pandemic, there were major concerns for nosocomial transmission of SARS-CoV-2 from patient-to-HCW. Because of these concerns, HCW testing was prioritized over community testing. Even though numerous IPC measures were in place, SARS-CoV-2 infections still occurred in HCW. Our analysis showed that only a very minor proportion of HCW infections (1.7%) were likely caused by patient-to-HCW transmission and limited nosocomial transmission took place from HCW-to-HCW. These results are in line with another Dutch study which identified multiple introductions of the virus among HCW through community-acquired infections [2]. Other studies reporting on hospital transmission dynamics have also pinpointed many different sources of infection for HCW outside the hospital, such as SARS-CoV-2 positive household members or contact with a potential case outside of work [17, 19, 20]. The findings of the study of Lindsey et al. similarly suggested that the majority of HCW were infected by another HCW [21]. In long-term care facilities, studies have shown that HCW posed a greater risk for patients rather than vice versa [22]. Contrary to our findings, Lumley et al. suggest that nosocomial transmission is underestimated [23]. This study however, focused on nosocomial acquisition of SARS-CoV-2 by inpatients rather than HCW and did not classify the likelihood of nosocomial transmission in HCW. Additionally, our setting with mainly single-occupancy rooms is different compared to settings with multiple occupancy rooms possibly resulting in different transmission dynamics.
A couple of explanations for HCW-to-HCW transmission can be listed; more than half of HCW (55%) reported working whilst symptomatic, reflected in the delay between the date of symptom onset and median test date three days later. Prior studies have noted that sickness presenteeism behavior among HCW is common for influenza-like illness and that HCW are known to be vectors for infectious diseases [24, 25]. Furthermore, the criteria for SARS-CoV-2 testing eligibility were quite stringent in our hospital in March 2020, partially due to the scarcity of tests and limited knowledge of the extent of COVID-19 symptoms. This could have contributed to the high number of HCW who remained working while symptomatic. When HCW in patient care were not working in patient rooms, for instance during coffee breaks or small meetings, masks were often not worn as universal masking was not implemented at our hospital during the first wave. Physical distancing and universal masking of HCW are IPC measures that can be implemented to assure fewer transmission events can take place [26, 27]. Masking should also be accompanied by proper hand hygiene and adequate doffing and donning [28]. Physical distancing was implemented in our hospital at the end of March 2020, however, implementation in practice took time. As HCW are essential workers, especially during a pandemic response, preventive measures for HCW-to-HCW transmission are important in addition to measures during contact with patients.
The comparison of cluster analyses demonstrated that identification of secondary cases through epidemiological data alone can result in substantial overestimation. These findings highlight once more the importance of investigating potential nosocomial transmission through a combination of detailed epidemiological investigation combined with WGS data [14, 21, 29]. Knowing the extent of overestimation of nosocomial transmission will help us understand and put the findings of epidemiologic outbreak investigations into perspective. One of the studies which clearly presented both epidemiological clusters and clusters with combined epidemiological and WGS data, is the study of Watt et al. [30]. Contrary to our findings, this study identified more nosocomial transmission compared to classical epidemiology using WGS data [30]. This discrepancy may be due to the extent of epidemiological data available for the initial epidemiological cluster analysis, difference in genomic cluster definition and difference in the community prevalence of SARS-CoV-2 during the study period.
Strengths and limitations
Results of our study were obtained through a retrospective in-depth analysis combining epidemiologic and WGS data. However, for IPC and outbreak management WGS is often not readily available, requiring decision-making of real-time outbreak interventions to rely on epidemiological data alone. While many studies have previously highlighted the added value of WGS for in-depth cluster analysis, fewer studies have presented the disparity in results after adding WGS to the cluster analyses [30,31,32]. Factors which distinguish our study from others are our inclusion of the full (SARS-CoV-2 positive) hospital patient and HCW population and the provision of definitions on the likelihood of nosocomial transmission among HCW. Multiple outbreak investigations combining epidemiological and WGS data have been described, however, the majority of studies focus on hospital-acquired COVID-19 among patients rather than HCW. Therefore, studies often only describe definitions for hospital-acquired COVID-19 for patients and exclude these definitions for HCW, making it unclear what proportion of SARS-CoV-2 positive HCW is attributable to community transmission [26]. Studies that focus on SARS-CoV-2 transmission to HCW often describe risk factors for a positive SARS-CoV-2 PCR among HCW, but do not classify the likelihood of nosocomial transmission among HCW, nor pinpoint the actual number of SARS-CoV-2 positive HCW that can be attributed to nosocomial transmission [33].
Limitations of this study include missing WGS data due to low viral loads, which could have resulted in missing links. Another challenge in identifying and confirming nosocomial transmission is the relatively low genetic diversity of SARS-CoV-2 strains [22]. This can affect the cluster analysis in a way that separate community introductions or nosocomial transmission are indistinguishable based on WGS data. We regarded these cases as possible transmission and more information via detailed epidemiological data was crucial for the interpretation of the WGS data in outbreak investigation. By classifying the likelihood of nosocomial transmission among HCW, this factor of uncertainty due to regional clusters should be taken into account. This is especially true in the beginning of a pandemic when testing and subsequent sequencing of positive cases is biased towards high-risk groups (i.e., HCW) and hospitalized patients, and community surveillance is not yet performed, possibly leading to an overestimation of the identified clusters. Our study only comprises data from the first COVID-19 wave in 2020, and different factors have changed during the course of time such as SARS-CoV-2 variants, immune status and differences in community prevalence. However, the results of this study still highlight the challenges of pandemic preparedness and outbreak investigations when a new virus emerges.
Moreover, information regarding SARS-CoV-2 positive visitors was not registered and asymptomatic HCW and patients were not tested and therefore could not be taken into account. Up to 33% of SARS-CoV-2 infections in adults are estimated to be asymptomatic, therefore this could have resulted in missing links and clusters [34]. Only a fraction of all regional COVID-19 cases were tested and sequenced. This might have resulted in an underestimation of sequence diversity in the community and thus, regional clusters. Additionally, regional clusters were defined as having ≥ 5 primary patients, which is an arbitrary cut-off.
Conclusion
The findings of this study highlight the contribution of SARS-CoV-2 community-acquired infections in HCW settings, the limited number of patient-to-HCW transmissions as well as the added value of WGS to epidemiological data. The COVID-19 pandemic has emphasized the importance of real-time outbreak management for pandemic preparedness. While epidemiological data such as source and contact tracing is important in hospital outbreak management and investigation, it may not suffice in scenarios of high community prevalence. Since WGS is not readily available for many healthcare facilities it is important to recognize the uncertainties of cluster analyses based solely on epidemiological data as well as to recognize the contribution of HCW-to-HCW transmission. The collaboration between the IPC team and occupational health services, together with the use of complementary techniques like epidemiological cluster analysis and WGS is essential to provide knowledge on nosocomial SARS-CoV-2 transmission dynamics. During this first wave of the pandemic, HCW testing was prioritized over community testing. Our study shows the importance of surveillance in the community in order to understand sequence clusters. Our study population originated from a single tertiary care center with single occupancy rooms, which could result in different transmission dynamics compared to healthcare facilities with multiple occupancy rooms as more contact occurs between patients. Future studies should investigate this difference.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AGP:
-
Aerosol generating procedures
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Contact investigation
- COVID-19:
-
Coronavirus disease 2019
- Ct:
-
Cycle threshold
- EHR:
-
Electronic health record
- Erasmus MC:
-
Erasmus MC University Medical Center
- GP:
-
General practitioner
- HCW:
-
Healthcare worker
- ICU:
-
Intensive care unit
- IPC:
-
Infection prevention and control
- M:
-
Meter
- NA:
-
Not applicable
- PPE:
-
Personal protective equipment
- RTI:
-
Respiratory tract infection
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- WGS:
-
Whole genome sequencing
References
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Sikkema RS, Pas SD, Nieuwenhuijse DF, O’Toole Á, Verweij J, van der Linden A, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis. 2020;20(11):1273–80.
Paltansing S, Sikkema RS, de Man SJ, Koopmans MPG, Oude Munnink BB, de Man P. Transmission of SARS-CoV-2 among healthcare workers and patients in a teaching hospital in the Netherlands confirmed by whole-genome sequencing. J Hosp Infect. 2021;110:178–83.
Wong RCW, Lee MKP, Siu GKH, Lee LK, Leung JSL, Leung ECM, et al. Healthcare workers acquired COVID-19 disease from patients? An investigation by phylogenomics. J Hosp Infect. 2021;115:59–63.
Chan ER, Jones LD, Redmond SN, Navas ME, Kachaluba NM, Zabarsky TF, et al. Use of whole genome sequencing to investigate a cluster of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections in emergency department personnel. Infect Control Hosp Epidemiol. 2021:1–10.
Jaarverslag 2019. Rotterdam. The Netherlands: Erasmus MC University Medical Center. 2020.
van der Schoor AS, Severin JA, van der Weg AS, Strepis N, Klaassen CHW, van den Akker JPC, et al. The effect of 100% single-occupancy rooms on acquisition of extended-spectrum beta-lactamase-producing Enterobacterales and intra-hospital patient transfers: a prospective before-and-after study. Antimicrob Resist Infect Control. 2022;11(1):76.
National Institute for Public Health and the Environment (RIVM). COVID-19 aantallen per gemeente per publicatiedatum 2021. https://data.rivm.nl
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045.
Oude Munnink BB, Nieuwenhuijse DF, Stein M, O’Toole Á, Haverkate M, Mollers M, et al. Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat Med. 2020;26(9):1405–10.
Rambaut A. FigTree v1.4.4 2018. http://tree.bio.ed.ac.uk/software/figtree/.
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2014;32(1):268–74.
Taiaroa G, Rawlinson D, Featherstone L, Pitt M, Caly L, Druce J, et al. Direct RNA sequencing and early evolution of SARS-CoV-2. bioRxiv. 2020:2020.03.05.976167.
Meredith LW, Hamilton WL, Warne B, Houldcroft CJ, Hosmillo M, Jahun AS, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20(11):1263–71.
Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–83.
Carlsten C, Gulati M, Hines S, Rose C, Scott K, Tarlo SM, et al. COVID-19 as an occupational disease. Am J Ind Med. 2021;64(4):227–37.
Eyre DW, Lumley SF, O'Donnell D, Campbell M, Sims E, Lawson E, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9.
Mutambudzi M, Niedzwiedz C, Macdonald EB, Leyland A, Mair F, Anderson J, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78(5):307.
Løvestad AH, Jørgensen SB, Handal N, Ambur OH, Aamot HV. Investigation of intra-hospital SARS-CoV-2 transmission using nanopore whole-genome sequencing. J Hosp Infect. 2021;111:107–16.
Gordon CL, Trubiano JA, Holmes NE, Chua KYL, Feldman J, Young G, et al. Staff to staff transmission as a driver of healthcare worker infections with COVID-19. Infect Dis Health. 2021;26(4):276–83.
Lindsey BB, Villabona-Arenas CJ, Campbell F, Keeley AJ, Parker MD, Shah DR, et al. Characterising within-hospital SARS-CoV-2 transmission events using epidemiological and viral genomic data across two pandemic waves. Nat Commun. 2022;13(1):671.
Abbas M, Nunes TR, Cori A, Cordey S, Laubscher F, Baggio S, et al. Explosive nosocomial outbreak of SARS-CoV-2 in a rehabilitation clinic: the limits of genomics for outbreak reconstruction. J Hosp Infect. 2021;117:124–4.
Lumley SF, Constantinides B, Sanderson N, Rodger G, Street TL, Swann J, et al. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J Infect. 2021;83(4):473–82.
Huttunen R, Syrjänen J. Healthcare workers as vectors of infectious diseases. Eur J Clin Microbiol Infect Dis. 2014;33(9):1477–88.
Tartari E, Saris K, Kenters N, Marimuthu K, Widmer A, Collignon P, et al. Not sick enough to worry? “Influenza-like” symptoms and work-related behavior among healthcare workers and other professionals: results of a global survey. PLoS ONE. 2020;15(5): e0232168.
Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10(1):7.
Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290–1.
Klompas M, Morris CA, Sinclair J, Pearson M, Shenoy ES. Universal masking in hospitals in the covid-19 era. N Engl J Med. 2020;382(21): e63.
Czech-Sioli M, Günther T, Robitaille A, Roggenkamp H, Büttner H, Indenbirken D, et al. Integration of sequencing and epidemiological data for surveillance of SARS-CoV-2 infections in a tertiary-care hospital. Clin Infect Dis. 2023;76(3):e263-e273.
Watt AE, Sherry NL, Andersson P, Lane CR, Johnson S, Wilmot M, et al. State-wide genomic epidemiology investigations of COVID-19 in healthcare workers in 2020 Victoria, Australia: Qualitative thematic analysis to provide insights for future pandemic preparedness. The Lancet Regional Health – Western Pacific. 2022;25.
Meijer SE, Harel N, Ben-Ami R, Nahari M, Yakubovsky M, Oster HS, et al. Unraveling a nosocomial outbreak of COVID-19: the role of whole-genome sequence analysis. Open Forum Infect Dis. 2021;8(10):120.
Lucey M, Macori G, Mullane N, Sutton-Fitzpatrick U, Gonzalez G, Coughlan S, et al. Whole-genome sequencing to track severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in nosocomial outbreaks. Clin Infect Dis. 2020;72(11):e727–35.
Mo Y, Eyre DW, Lumley SF, Walker TM, Shaw RH, O’Donnell D, et al. Transmission of community- and hospital-acquired SARS-CoV-2 in hospital settings in the UK: a cohort study. PLoS Med. 2021;18(10): e1003816.
Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174(5):655–62.
Acknowledgements
We would like to thank all persons involved in the surveillance of SARS-CoV-2 positive patients and HCW at the Erasmus MC, especially efforts from the Unit infection prevention of the department of Medical Microbiology and Infectious Diseases, the occupational health services and the COVID-19 call center. We would also like to thank the laboratory staff from the department of Viroscience and the Unit diagnostics of the department of Medical Microbiology and Infectious Diseases for performing the SARS-CoV-2 laboratory analyses.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement numbers 874735 (VEO), 848096 (SHARP JA) and 101003589 (RECoVER).
Author information
Authors and Affiliations
Contributions
This paper was conceptualized by CH, BO, RS, AV, HJ, RM, MV, MK and JS. Data collection was performed by MA, RB, HK, MB, IC, KO & AL. Data was analyzed by CH, AV, BO, RS, JS. The manuscript was drafted by CH & AV. RS, BO, KO and JS edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The medical ethical research committee from the Erasmus MC gave written approval to conduct this study (MEC-2021-0845). Passive informed consent was received from patients meaning they had the right to opt-out against the use of their medical data and patient material in research. Furthermore, patient data was pseudonymized and HCW data was anonymized.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Preliminary data of this study were presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2021 in an online oral presentation (abstract 02575)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haanappel, C.P., Oude Munnink, B.B., Sikkema, R.S. et al. Combining epidemiological data and whole genome sequencing to understand SARS-CoV-2 transmission dynamics in a large tertiary care hospital during the first COVID-19 wave in The Netherlands focusing on healthcare workers. Antimicrob Resist Infect Control 12, 46 (2023). https://doi.org/10.1186/s13756-023-01247-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01247-7