Abstract
Background
Long noncoding RNA (lncRNA)-regulated mechanism in acute lung injury (ALI) has attracted special interests in study researches. We planned to disclose whether KCNQ1 overlapping transcript 1 (Kcnq1ot1) is involved in ALI and its mechanism.
Methods
The lipopolysaccharide (LPS)-induced ALI model was established in mice. Kcnq1ot1, microRNA (miR)-7a-5p and Reticulon 3 (Rtn3) levels were measured in lung tissues of mice. The vector that changed Kcnq1ot1, miR-7a-5p and Rtn3 expression was injected into LPS-treated mice, and pathological damage, fibrosis, apoptosis and inflammatory response were subsequently examined in lung tissues. The relation between Kcnq1ot1 and miR-7a-5p, and that between miR-7a-5p and Rtn3 were identified.
Results
Kcnq1ot1 and Rtn3 expression increased while miR-7a-5p expression decreased in LPS-treated mice. Reduced Kcnq1ot1 or elevated miR-7a-5p alleviated pathological damage, fibrosis, apoptosis and inflammatory response in ALI mice, while overexpressed Rtn3 worsened ALI in mice. Downregulation of Rtn3 reversed the exacerbation of miR-7a-5p downregulation in ALI mice. Kcnq1ot1 competitively bound to miR-7a-5p and miR-7a-5p negatively mediated Rtn3 expression.
Conclusion
Our experiments evidence that silencing Kcnq1ot1 upregulates miR-7a-5p to suppress Rtn3 expression, thereby diminishing LPS-induced ALI.
Similar content being viewed by others
Introduction
Acute lung injury (ALI) is a moderate or mild form of acute respiratory distress syndrome (ARDS) [1]. Clinically, the criteria for ALI manifests as progressive hypoxemia, dyspnea and increased work of breathing [2]. ALI is characterized by the recruitment of neutrophils to the lung, accompanied by alveolar and systemic release of chemokines, pro-inflammatory cytokines, acute phase reactants, and matrix remodeling enzymes [3]. The need for positive end-expiratory pressure and high FIO2 mechanical ventilation is a treatment guideline for patients with ALI [4]. The current drugs include surfactants and neuromuscular blockers to limit medical treatment, vasodilators and anticoagulants to optimize alveolar perfusion and diuretics and β2 agonists to reduce pulmonary edema [5]. Although with pharmacological drugs and modern technique care, it is still essential to translate the mechanism of ALI for further exploration of treatment therapeutics.
Long noncoding RNAs (lncRNAs) refer to a family of transcripts that involve in biological processes ranging from housekeeping functions to more specialized functions [6]. A large fraction of lucernes have been implicated in ALI and lncRNAs-based therapy is of importance to control the disease progression. For instance, overexpressing lncRNA cancer susceptibility candidate 2 could improve ALI through suppression of lung epithelial cell apoptosis [7]. Suppression of lncRNA metastasis associated in lung adenocarcinoma transcript 1 [8] or lncRNA maternally expressed gene 3 restrains inflammatory response [9] in ALI. KCNQ1 overlapping transcript 1 (Kcnq1ot1) is a member of lncRNA family that confers oncogenesis in the lung [10]. In lipopolysaccharide (LPS)-induced ARDS, Kcnq1ot1 silencing is of significance in restraining inflammatory insult [11]. LncRNA often exerts functions through acting as a sponge of microRNA (miR). miR-7 protects against pulmonary fibrosis by decreasing epithelial–mesenchymal transition of bronchial epithelial cells [12]. The protective role of miR-7a-5p has been shown in LPS-induced myocardial injury [13] as well as in LPS-mediated microglial inflammation [14]. Reticulon (Rnt) protein family has been implicated in the modification of tissue repair and acute inflammation. For example, Nogo-B could bat against LPS-induced ALI [15]. Of a member of Rnt, Rtn3 was found to have a potential targeting relation with miR-7a-5p online. Definitely, Fu et al. have profiled the pro-inflammatory property of Rtn3 in osteoarthritis [16]. Till now, little few relevant documents have discovered the relative mechanism underlying ALI with attention on Kcnq1ot1-mediated miR-7a-5p/Rtn3 axis. Given that, we started the research to unravel the mechanistic program of Kcnq1ot1/miR-7a-5p/Rtn3 feedback loop in LPS-induced ALI.
Materials and methods
Ethics statement
The animal research was approved by the Ethics Committee of Daqing Qilfield General Hospital.
Animals
Six-week-old male BALB/c mice (18–22 g) were kept in a specific pathogen-free laboratory (22–26 °C, 40–70% humidity) with day/night cycle. The mice were reared in different cages (4 mice per cage) and fed and drank freely for a week [17].
Modeling of animals
The mice were given intratracheal instillation of LPS at 3 mg/kg. The method of LPS instillation was applied to establish ALI model. At 6 h post LPS instillation, mice were injected intraperitoneally with sh-negative control (NC), sh-Kcnq1ot1, agomiR-NC, miR-7a-5p agomiR, pcDNA-NC, pcDNA-Rtn3, miR-7a-5p antagomiR + sh-NC, and miR-7a-5p antagomiR + sh-Rtn3, respectively. The injection dose was 10 μmol/kg. At 48 h post the injection with vectors, the mice were euthanized 6 h later to obtain lung tissue specimens [18]. All vectors were provided by GenePharma (Shanghai, China).
Hematoxylin–eosin (H&E) staining
The lung tissue was dehydrated by ethanol gradient and embedded in paraffin after permeabilization with xylene. The sectsions (4 μm) were conventionally dehydrated, stained with hematoxylin, differentiated with 1% alcohol hydrochloride and dyed with eosin. Afterwards, gradient alcohol was added in the section for dehydration and xylene for permeabilization. Finally, neutral resin-sealed sections were observed under an optical microscope [19].
Masson staining
The tissue was stained with red algae fuchsin solution, rinsed with glacial acetic acid, immersed in phosphomolybdic acid, and dyed with aniline blue solution. After that, the sample was soaked in glacial acetic acid, treated with xylene and observed by an electron microscope [20].
Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining
The lung tissue was treated with in situ cell death detection kit (Roche, Switzerland) and observed in 5 fields of view to calculate the rate of TUNEL-positive cells [21].
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The lung tissue was processed with Trizol (Invitrogen, CA, USA) to extract total RNA which was reverse-transcribed into cDNA by PrimeScript™ RT kit (Thermo Fisher Scientific, MA, USA) or All-in-One miRNA’s first chain CDNA Synthesis Kit (GeneCopoeia, MD, USA). cDNA was then treated with Lightcycler 480 96-well PCR (Roche, Mannheim, Germany). The internal controls to standardize gene expression were glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6. Additional file 3: Table S1 lists the primer sequences. The gene calculation method was 2−ΔΔCt method [22].
Western blot assay
The lung tissue was lysed by radio-immunoprecipitation assay buffer (Thermo Fisher Scientific) supplemented with protease inhibitors, after which protein level was evaluated by a bicinchoninic acid protein detection kit (Thermo Fisher Scientific). The protein was analyzed with sodium dodecyl sulfate polyacrylamide gel electrophoresis. Subsequently, the protein sample on the polyvinylidene fluoride membrane was blocked, combined with primary antibodies Rtn3 and GAPDH (both from Santa Cruz Biotechnology, CA, USA), and with peroxidase-labeled secondary antibody (Abcam, CA, USA). The protein bands were analyzed with Odyssey 3.0 [23].
Dual luciferase reporter gene assay
The synthesized wild-type (WT) or mutant-type (MUT) Kcnq1ot1 and Rtn3 3′UTR sequences (Generalbio, Anhui, China) containing miR-7a-5p binding site was utilized to form Kcnq1ot1-WT, Rtn3-WT, Kcnq1ot1-MUT and Rtn3-MUT reporters. By Lipofectamine 3000 (Invitrogen), the reporter was co-transfected with miR-7a-5p mimic or miR-7a-5p NC into HEK293T cells to assess cell luciferase activity with dual luciferase reporter gene test kit (Beyotime, Shanghai, China) [24].
RNA immunoprecipitation (RIP) assay
Cells were lysed in RIP lysis buffer (protease inhibitors and RNase inhibitors). Protein-G/A + agarose and Ago2 (Abcam) or rabbit IgG (Abcam) were incubated overnight, the precipitated RNA was subjected to RT-qPCR [25].
Statistical analysis
SPSS 21.0 (IBM, NY, USA) and GraphPad Prism 6 Software (San Diego, CA, USA) were the data analysis software. Measurement data were expressed as mean ± standard deviation. Two groups of measurement data were compared by t test, while multiple groups of data were analyzed by analysis of variance and Tukey’s post hoc test. P < 0.05 represented statistical significance.
Results
Kcnq1ot1 and Rtn3 expression increases while miR-7a-5p expression decreases in mice with ALI
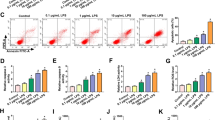
Observation of lung tissue samples by HE staining, Masson staining and TUNEL staining depicted that in LPS-conditioned mice, the alveolar structure was disordered, the alveolar walls were thickened, and a large number of inflammatory cells and erythrocytes were seen in the alveolar cavity (Fig. 1A); diffuse fibrosis and severe damage were manifested (Fig. 1B); more apoptotic cells and brown particles were obviously detected (Fig. 1C). Measurements by ELISA revealed that TNF-α and IL-1β levels were promoted in mice after LPS treatment (Fig. 1D).
Kcnq1ot1 and Rtn3 expression increases while miR-7a-5p expression decreases in mice with ALI. A H&E staining; B Masson staining; C TUNEL staining; D TNF-α and IL-1β levels in LPS-treated mice; E Kcnq1ot1 mRNA expression in LPS-treated mice; F miR-7a-5p expression in LPS-treated mice; G, H Rtn3 protein expression in LPS-treated mice; measurement data are displayed as mean ± standard deviation (n = 6); * P < 0.05 vs. the Control group
Kcnq1ot1, miR-7a-5p, and Rtn3 levels in lung tissues of mice were tested by RT-qPCR and Western blot. After LPS instillation, Kcnq1ot1 and Rtn3 levels went to upregulate and miR-7a-5p expression dropped in mice (Fig. 1E–H).
Reduced Kcnq1ot1 alleviates the pathological degree of ALI in mice
Kcnq1ot1 is a key regulatory factor to prevent myocardial injury [26]. However, it is not clear about the mechanism of Kcnq1ot1 in LPS-induced ALI. LPS-treated mice were injected with sh-Kcnq1ot1 or sh-NC. RT-qPCR confirmed the efficacy of sh-Kcnq1ot1 to lower Kcnq1ot1 expression in the lung tissue (Fig. 2A). In response to Kcnq1ot1 silencing, the structure of the alveoli was improved, the alveolar wall no longer thickened, and the number of inflammatory cells and erythrocytes were reduced (Fig. 2B), the diffuse fibrosis and damage of the lung tissue were improved (Fig. 2C), the apoptosis rate was reduced (Fig. 2D), and TNF-α and IL-1β levels were ablated (Fig. 2E) in LPS-treated mice. In summary, silent Kcnq1ot1 reduces the degree of LPS-induced ALI.
Reduced Kcnq1ot1 alleviates the pathological degree of ALI in mice. A Kcnq1ot1 expression in LPS-treated mice after injection with sh-Kcnq1ot1; B H&E staining; C Masson staining; D TUNEL staining; E TNF-α and IL-1β levels in LPS-treated mice after injection with sh-Kcnq1ot1; measurement data are displayed as mean ± standard deviation (n = 6); * P < 0.05 vs. the LPS + sh-NC group
Kcnq1ot1 competitively binds to miR-7a-5p
On the bioinformatics website DIANA, the presence of binding site between Kcnq1ot1 and miR-7a-5p was confirmed (Additional file 1: Fig. S1A). In the dual luciferase gene reporter detection, miR-7a-5p mimic caused luciferase activity inhibited in the Kcnq1ot1-WT reporter (Additional file 1: Fig. S1B). In RIP analysis, Kcnq1ot1 level Ago was increased by miR-7a-5p mimic (Additional file 1: Fig. S1C).
Besides, the finding of RT-qPCR exhibited that silencing Kcnq1ot1 increased the level of miR-7a-5p in the lung tissue of LPS-treated mice (Additional file 1: Fig. S1D). Therefore, Kcnq1ot1 worked as a molecular sponge for miR-7a-5p.
Elevated miR-7a-5p reduces ALI in mice
LPS-treated mice were injected with miR-7a-5p agomiR or miR-7a-5p NC. RT-qPCR detected that miR-7a-5p agomiR successfully raised miR-7a-5p expression in ALI mice (Fig. 3A). After miR-7a-5p overexpression, the pathological conditions were improved (Fig. 3B), the degree of lung fibrosis (Fig. 3C), the number of apoptotic cells and the level of inflammatory indices presented a decrease (Fig. 3D, E). It was indicated that overexpression of miR-7a-5p can improve ALI in mice.
Elevated miR-7a-5p reduces ALI in mice. A miR-7a-5p expression in LPS-treated mice after injection with miR-7a-5p agomiR; B H&E staining; C Masson staining; D TUNEL staining; E TNF-α and IL-1β levels in LPS-treated mice after injection with miR-7a-5p agomiR; measurement data are displayed as mean ± standard deviation (n = 6); * P < 0.05 vs. the LPS + agomiR-NC group
miR-7a-5p negatively mediates Rtn3 expression
On the starBase, the binding site of miR-7a-5p and Rtn3 was predicted (Additional file 2: Fig. S2A). In the dual luciferase gene reporter test, Rtn3-WT luciferase activity could be suppressed by miR-7a-5p mimic (Additional file 2: Fig. S2B). In the RIP experiment, the enrichment of Rtn3 was induced by miR-7a-5p mimic (Additional file 2: Fig. S2C). Additionally, in RT-qPCR and Western blot, Rtn3 expression was decreased in mice after upregulating miR-7a-5p (Additional file 2: Fig. S2D, E). Distinctly, miR-7a-5p and Rtn3 have a negative targeting relation.
Overexpressed Rtn3 worsens ALI in mice
LPS-suffered mice were treated with pcDNA-Rtn3 or pcDNA-NC. RT-qPCR and Western blot identified that Rtn3 expression was substantially elevated by pcDNA-Rtn3 (Fig. 4A, B). As a result of Rtn3 overexpression, LPS-suffered mice presented deteriorated pathological condition of the lung tissue, more serious lung tissue fibrosis (Fig. 4C, D), increased number of apoptotic cells (Fig. 4E) and aggravated inflammatory response (Fig. 4F). Markedly, overexpressed Rtn3 worsens ALI in mice.
Overexpressed Rtn3 worsens ALI in mice. A, B. Rtn3 mRNA and protein expression in LPS-treated mice after injection with pcDNA-Rtn3; C H&E staining; D Masson staining; E TUNEL staining; F TNF-α and IL-1β levels in LPS-treated mice after injection with pcDNA-Rtn3; measurement data are displayed as mean ± standard deviation (n = 6); * P < 0.05 vs. the LPS + pcDNA-NC group
miR-7a-5p suppresses Rtn3 expression to alleviate ALI
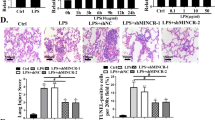
Rescue tests were performed in LPS-treated mice. On the basis miR-7a-5p antagomiR, sh-Rtn3 was injected into mice to lower Rtn3 expression (Fig. 5A, B). Moreover, downregulating Rtn3 weakened the negative role of inhibited miR-7a-5p in ALI mice (Fig. 5D–F). Plainly, Kcnq1ot1 worsens ALI in mice by suppressing miR-7a-5p to promote Rtn3 expression.
miR-7a-5p suppresses Rtn3 expression to alleviate ALI. A, B Rtn3 mRNA and protein expression in LPS-treated mice after injection with miR-7a-5p antagomiR and sh-Rtn3; C H&E staining; D Masson staining; E TUNEL staining; F TNF-α and IL-1β levels in LPS-treated mice; measurement data are displayed as mean ± standard deviation (n = 6); * P < 0.05 vs. the LPS + miR-7a-5p antagomiR + sh-NC group
Discussion
ALI represents a serious heterogenous pulmonary disorder with high mortality. In experimental trails, direct intratracheal LPS instillation is a commonly used method to induce ALI in mice [27]. In mice with ALI induced by LPS, the manifestation and functions of Kcnq1ot1/miR-7a-5p/Rtn3 axis were partly explored. The main outcome elaborated that silencing Kcnq1ot1 made a relief of ALI in mice by upregulating miR-7a-5p to downregulate Rtn3.
To start with, Kcnq1ot1 expression was calculated to increase in the lung tissue of mice with ALI. Then, targeted silencing Kcnq1ot1 was performed to minimize injury in the lung of mice with ALI by alleviating alveolar injury and fibrosis, and reducing the secretion of inflammatory indices (TNF-α and IL-1β) and cell apoptosis. Exactly, knocking down Kcnq1ot1 in mice with ARDS is prerequisite to reduce inflammatory response through decreasing the level of pro-inflammatory actors and increasing anti-inflammatory parameter in ARDS induced by LPS [11]. It is of interest that Kcnq1ot1 expression reaches to a high level in neurons after stimulation with LPS, while repression of Kcnq1ot1 blocks apoptosis and inflammation to facilitate the survival of neurons [28]. High Kcnq1ot1 expression has been once measured in oxygen–glucose deprivation and reperfusion-conditioned neurons, but artificially restraining Kcnq1ot1 expression subsides inflammation and neuronal apoptosis [29]. Remarkably, cardiomyocytes subjected to hypoxic injury have high Kcnq1ot1 expression which notoriously foments cardiomyocyte injury and apoptosis [30]. Yang et al. have checked that loss of Kcnq1ot1 in mice with diabetic cardiomyopathy could rescue cardiac function and relieve fibrosis and pyroptosis [31]. An increase has been detected in Kcnq1ot1 expression in clinical cartilage tissues of patients with osteoarthritis, and depletion of Kcnq1ot1 is fundamental for obstructing the release of inflammatory cytokines and the degradation of extracellular matrix [32]. Generally, it is therapeutically potential to manage disease progression by taking control of the aberrant expression of Kcnq1ot1.
Subsequently, miR-7a-5p expression was inspected to downregulate in the lung tissue of mice with ALI. In fact, miR-7a-5p expression is limited to a relative low level in LPS-suffered cardiomyocytes [13]. Based on the binding relation with Kcnq1ot1, miR-7a-5p was speculated to involve in Kcnq1ot1-mediated ALI. Actually, the experimental outcomes displayed that restoring miR-7a-5p alone defensed against ALI in mice, but inhibiting miR-7a-5p lessened Kcnq1ot1 silencing-eased ALI. Du et al. have profiled that miR-7a-5p is downregulated in the case of diabetic kidney disease, but experimentally elevating miR-7a-5p expression in mice could prevent renal fibrosis to progress in the disease [33]. In a similar fashion, miR-7a-2-3p expression is prone to downregulate in oxygen–glucose deprivation-treated neurons, but the survival of cortical neurons is appreciative of the induction of miR-7a-2-3p [34]. Meng et al. have recognized an interesting fact that overexpressed miR-7a-5p detains the activation and inflammatory reaction of microglial [14]. To the best of our knowledge, inducing miR-7a/b in the heart of mice with myocardial infarction is the cornerstone of fibrosis and apoptosis inhibition [35].
The miRNA–mRNA network was also observed between miR-7a-5p and Rtn3 in ALI. Regarding the results, Rtn3 was upregulated in the lung of mice with ALI. Manipulated overexpression of Rtn3 deteriorated ALI in mice independently, whereas downregulating Rtn3 reduced the effect of miR-7a-5p silencing on mice with ALI. Regarding the biological functions of Rtn3, a study report on osteoarthritis has offered evidence that Rtn3 augments apoptosis and inflammatory response of chondrocytes [16]. Besides, in the setting of ischemia/reperfusion injury, the abnormal elevation of Rtn3 expression is an active stimuli for cardiomyocyte apoptosis [36].
Conclusion
Overall, the present research proves that Kcnq1ot1 and Rtn3 are overexpressed, whereas miR-7a-5p is under-expressed in LPS-induced ALI. Suppression of Kcnq1ot1 elevates miR-7a-5p and reduces Rtn3 expression, so as to induce protection against ALI. The main finding in the present research provides a theoretical basis, promisingly carrying forward the development of molecule-targeted therapy for ALI. Meaningfully, evaluation of Kcnq1ot1/miR-7a-5p/Rtn3 axis discloses a novel direction for carrying forward treatments in ALI.
Availability of data and materials
Not applicable.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- lncRNAs:
-
Long noncoding RNAs
- Kcnq1ot1:
-
KCNQ1 overlapping transcript 1
- LPS:
-
Lipopolysaccharide
- miR:
-
MicroRNA
- Rnt:
-
Reticulon
- NC:
-
Negative control
- H&E:
-
Hematoxylin–eosin
- TUNEL:
-
Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling
- ELISA:
-
Enzyme-linked immunosorbent assay
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- WT:
-
Wild type
- MUT:
-
Mutant type
- RIP:
-
RNA immunoprecipitation
References
Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–50.
Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg. 2020;57(5):100777.
Parekh D, Dancer RC, Thickett DR. Acute lung injury. Clin Med. 2011;11(6):615–8.
Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc. 2015;12(Suppl 1):S3-8.
Sweeney RM, Griffiths M, McAuley D. Treatment of acute lung injury: current and emerging pharmacological therapies. Semin Respir Crit Care Med. 2013;34(4):487–98.
Charles Richard JL, Eichhorn PJA. Platforms for investigating LncRNA functions. SLAS Technol. 2018;23(6):493–506.
Li H, et al. Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell Biosci. 2018;8:15.
Liang WJ, et al. Long non-coding RNA MALAT1 sponges miR-149 to promote inflammatory responses of LPS-induced acute lung injury by targeting MyD88. Cell Biol Int. 2019;44(1):317–26.
Liao H, Zhang S, Qiao J. Silencing of long non-coding RNA MEG3 alleviates lipopolysaccharide-induced acute lung injury by acting as a molecular sponge of microRNA-7b to modulate NLRP3. Aging. 2020;12(20):20198–211.
Kang Y, et al. Long noncoding RNA KCNQ1OT1 promotes the progression of non-small cell lung cancer via regulating miR-204-5p/ATG3 axis. Onco Targ Ther. 2019;12:10787–97.
Jiang X, et al. Kcnq1ot1/miR-381-3p/ETS2 axis regulates inflammation in mouse models of acute respiratory distress syndrome. Mol Ther Nucleic Acids. 2020;19:179–89.
Yao W, et al. The CDR1as/miR-7/TGFBR2 axis modulates EMT in silica-induced pulmonary fibrosis. Toxicol Sci. 2018;166(2):465–78.
Liang D, et al. Down-regulation of Xist and Mir-7a-5p improves LPS-induced myocardial injury. Int J Med Sci. 2020;17(16):2570–7.
Meng J, et al. LncRNA-Meg3 promotes Nlrp3-mediated microglial inflammation by targeting miR-7a-5p. Int Immunopharmacol. 2021;90:107141.
Xu W, et al. Nogo-B protects mice against lipopolysaccharide-induced acute lung injury. Sci Rep. 2015;5:12061.
Fu Q, et al. LINC02288 promotes chondrocyte apoptosis and inflammation through miR-374a-3p targeting RTN3. J Gene Med. 2021;23(5):e3314.
Lu ZB, et al. Forsythoside a inhibits adhesion and migration of monocytes to type II alveolar epithelial cells in lipopolysaccharide-induced acute lung injury through upregulating miR-124. Toxicol Appl Pharmacol. 2020;407:115252.
Lu Z, et al. MiR-122–5p protects against acute lung injury via regulation of DUSP4/ERK signaling in pulmonary microvascular endothelial cells. Life Sci. 2020;256:117851.
Li T, et al. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-kappaB signaling pathway. Mol Immunol. 2020;120:13–22.
Kong D, et al. Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway to reduce lipopolysaccharide-induced acute lung injury by inhibiting TLR2. J Cell Physiol. 2019;234(4):4597–607.
Zhang Q, et al. Dexmedetomidine alleviates hyperoxia-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Cell Physiol Biochem. 2017;42(5):1907–19.
Xiao Y, et al. The POU2F1/miR-4490/USP22 axis regulates cell proliferation and metastasis in gastric cancer. Cell Oncol. 2020;43(6):1017–33.
Nan CC, et al. Knockdown of lncRNA MALAT1 alleviates LPS-induced acute lung injury via inhibiting apoptosis through the miR-194-5p/FOXP2 axis. Front Cell Dev Biol. 2020;8:586869.
Li L, et al. Circ_100565 promotes proliferation, migration and invasion in non-small cell lung cancer through upregulating HMGA2 via sponging miR-506-3p. Cancer Cell Int. 2020;20:160.
Cao L, et al. Linc02349 promotes osteogenesis of human umbilical cord-derived stem cells by acting as a competing endogenous RNA for miR-25-3p and miR-33b-5p. Cell Prolif. 2020;53(5):e12814.
Yao Y, Wang X, Gao J. LncRNA KCNQ1OT1 sponges miR-206 to ameliorate neural injury induced by anesthesia via up-regulating BDNF. Drug Des Devel Ther. 2020;14:4789–800.
Ehrentraut H, et al. Inducing acute lung injury in mice by direct intratracheal lipopolysaccharide instillation. J Vis Exp. 2019;149:e59999.
Song A, et al. Inhibition of long non-coding RNA KCNQ1OT1 attenuates neuroinflammation and neuronal apoptosis through regulating NLRP3 expression via sponging miR-30e-3p. J Inflamm Res. 2021;14:1731–42.
Yi M, et al. KCNQ1OT1 exacerbates ischemia–reperfusion injury through targeted inhibition of miR-140-3P. Inflammation. 2020;43(5):1832–45.
Pan F, et al. Gingerol protects cardiomyocytes against hypoxia induced injury by regulating the pathway. Panminerva Med. 2020. https://doi.org/10.23736/s0031-0808.20.03956-7.
Yang F, et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018;9(10):1000.
Aili D, et al. Knockdown of long non-coding RNA KCNQ1OT1 suppresses the progression of osteoarthritis by mediating the miR-211-5p/TCF4 axis in vitro. Exp Ther Med. 2021;21(5):455.
Du Y, et al. Butyrate alleviates diabetic kidney disease by mediating the miR-7a-5p/P311/TGF-beta1 pathway. FASEB J. 2020;34(8):10462–75.
Zhang ZB, et al. miRNA-7a-2-3p inhibits neuronal apoptosis in oxygen-glucose deprivation (OGD) model. Front Neurosci. 2019;13:16.
Li R, et al. miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci Rep. 2016;6:29082.
Chen Z, et al. Knockdown of long non-coding RNA AFAP1-AS1 promoted viability and suppressed death of cardiomyocytes in response to I/R in vitro and in vivo. J Cardiovasc Transl Res. 2020;13(6):996–1007.
Acknowledgements
We thank the associate editor and the reviewers for their useful feedback that improved this paper.
Funding
None.
Author information
Authors and Affiliations
Contributions
DW contributed to study design; SY contributed to manuscript editing; FL contributed to experimental studies; DW contributed to data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal research was approved by the Ethics Committee of Daqing Qilfield General Hospital.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Additional file 1: Figure S1.
Kcnq1ot1 competitively binds to miR-7a-5p. A The binding sites of Kcnq1ot1 and miR-7a-5p on DIANA; B–C Targeting relationship between Kcnq1ot1 and miR-7a-5p verified by dual luciferase detection and RIP; D miR-7a-5p expression in LPS-treated mice after injection with sh-Kcnq1ot1; measurement data were displayed as mean ± standard deviation (n = 6); repetitions = 3; * P < 0.05 vs. the LPS + sh-NC group.

Additional file 2: Figure S2.
miR-7a-5p negatively mediates Rtn3 expression. A The binding sites of miR-7a-5p and Rtn3 on StarBase; B–C Targeting relationship between miR-7a-5p and Rtn3 verified by dual luciferase detection and RIP; D–E Rtn3 mRNA and protein expression in LPS-treated mice after injection with miR-7a-5p agomiR; measurement data were displayed as mean ± standard deviation (n = 6); repetitions = 3; * P < 0.05 vs. the LPS + agomiR-NC group.
Additional file 3: Table S1.
Primer sequences for genes in our article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Liu, F. & Wang, D. Long noncoding RNA Kcnq1ot1 prompts lipopolysaccharide-induced acute lung injury by microRNA-7a-5p/Rtn3 axis. Eur J Med Res 27, 46 (2022). https://doi.org/10.1186/s40001-022-00653-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00653-8