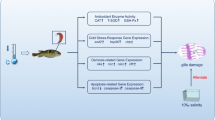
Abstract
Background
Changes in ambient temperature seriously affect physiological regulation and biochemical reactions in ectotherms. However, transient elevation in oceanic temperature occurs naturally during the day. Short-term elevation in the ambient temperature affects different physiological responses in marine fish, including cellular protein stability and osmotic balance of the internal environment. Since fish gills are vital osmoregulatory organ which directly contacts external environment, activation of cytoprotective responses to maintain gill cell viability and biological function is essential for fish survival under challenging environmental conditions. The purpose of this study was to investigate the short-term effects of elevated temperature on physiological regulation in the gills of a marine teleost, blue green damselfish (Chromis viridis).
Results
As part of the stress response, plasma glucose levels were induced by short-term hyperthermic exposure (12 h). Furthermore, upregulation of the levels of gill heat shock proteins (HSPs) and ubiquitinated proteins was essential for preventing the accumulation of protein aggregations in branchial cells of C. viridis under hyperthermic stress. The specific activity of branchial Na+/K+-ATPase (NKA), however, significantly reduced while the amount of protein was similar between normal and high-temperature groups.
Conclusions
The present study provided the evidence to illustrate that activation of the branchial protein quality control mechanism to carry out cytoprotective response was involved in coping with thermal stress. However, plasma osmolality and muscle water content, respectively, that slightly but evidently increased and decreased might result from impaired osmoregulatory ability due to hyperthermia-decreased gill NKA activity.
Similar content being viewed by others
Background
Most organisms on Earth are ectotherms which have to survive and adapt to temperature fluctuations (Hochachka and Somero [2002]; Guschina and Harwood [2006]; Somero [2010]). Temperature fundamentally affects all aspects of physiology by influencing the reactive rates as well as the physical properties of biological molecules (Hochachka and Somero [2002]; Crockett and Londraville [2006]). For marine ectotherms including fish, environmental temperature has the pervasive effects on physiological and biochemical functions at all levels of biological organization, from molecule to organism (Jobling [1995]; Hochachka and Somero [2002]; Hofmann et al. [2002]; Donaldson et al. [2008]). According to the tolerance range of temperature, the fishes can be classified into two groups, eurythermal and stenothermal species. Eurythermal fish can maintain metabolic activity at temperatures as low as Antarctic fish can survive and withstand temperature as high as the avian and mammalian body temperature. In contrast, changes in environmental temperature may lead to the poor maintenance of physiological homeostasis in stenothermal species, resulting in temperature stress (Hochachka and Somero [2002]; Somero [2010]; Long et al. [2012]).
Under adverse conditions, the physiological stress responses of organisms should be promptly activated to correct the disturbance, and cytoprotective mechanisms should be employed for the maintenance of cell viability and functional activity; otherwise, the survival of stressed organism will be in danger (Hofmann and Somero [1995, 1996]; Hofmann et al. [2002]; Kregel [2002]; Cui et al. [2013]). Furthermore, cellular proteins indeed carry out various physiological functions responsible for cell viability. The expression and maintenance of protein quality depends on mechanisms beyond those involved in transcription and translation (Wickner et al. [1999]). Chaperones and proteases mediating the mechanism of protein quality control (PQC) to prevent the accumulation of aggregated proteins and maintain cellular function and activity are highly conserved in organisms from different taxa (Gottesman et al. [1997]; Wickner et al. [1999]; Goldberg [2003]; Bukau et al. [2006]). Therefore, PQC should be a critical cytoprotective mechanism for coping with temperature stress in ectotherms. However, study on the responses of PQC mechanism to temperature challenge in fish is limited. The fish gills are the multifunctional organ which directly contacts the external environment (Evans et al. [2005]; Kaneko et al. [2008]); therefore, it is an excellent model to study stress responses and environmental effects in vivo.
Environmental temperature significantly influences internal electrolyte and osmotic homeostasis in aquatic ectotherms (Christensen [1975]; Amoudi et al. [1996]; Metz et al. [2003]; Sardella et al. [2004, 2008a]). It is due to active ion-transporting mechanisms that are regulated by many proteins, while the cellular proteins of stenothermal species are only marginally stable at a limited range of temperature (Hochachka and Somero [2002]; Metz et al. [2003]; Crockett and Londraville [2006]; Sardella et al. [2008a]). The fundamental transporter proteins responsible for osmoregulation in gill epithelia have been reported in previous studies (see Hirose et al. [2003]; Evans et al. [2005]; Hwang and Lee [2007]; Kaneko et al. [2008]; Hwang et al. [2011]). Among them, Na+/K+-ATPase (NKA) is the most important enzyme that actively transports Na+ out of and K+ into animal cells for sustaining intracellular homeostasis as well as for providing the driving force for ion-transporting systems in fish gills (Hwang and Lee [2007]; Hwang et al. [2011]). Therefore, branchial NKA responses (mRNA and protein expression and specific activity) have been used to assess the osmoregulatory status/ability of teleosts (Epstein et al. [1967]; Hwang and Lee [2007]; Kaneko et al. [2008]). In this regard, it is worth examining the branchial NKA responses to investigate the impact of temperature stress on osmoregulatory responses in stenothermal teleosts and clarify whether the protein expression or specific activity of gill NKA is susceptible to temperature challenge.
The blue-green damselfish (Chromis viridis) is a stenothermal teleost that is abundant on coral reefs throughout much of the Indo-Pacific region (Allen [1991]; Lieske and Myers [1994]), including southern Taiwan (Shen et al. [1993]). Previous studies have shown that the average temperature in Nanwan Bay, Kenting National Park, southern Taiwan is 26°C to 27°C and increases to approximately 32°C to 33°C during the day (Meng et al. [2008]; Mayfield et al. [2013]). Accordingly, the normal and hyperthermic temperatures of 26°C and 32°C, respectively, were used in this study. The goal of this study was to investigate the stress responses, PQC mechanism, and osmoregulatory response in the gills of C. viridis exposed to an increase in ambient temperature (32°C) for 12 h to ascertain the physiological strategies employed by stenothermal teleosts under short-term thermal stress.
Methods
Experimental animals and environments
Blue-green damselfish 2.8 ± 0.4 g in weight and 4.1 ± 0.7 cm in length were obtained from husbandry center of National Museum of Marine Biology and Aquarium (NMMBA), Pingtung, Taiwan. Fish were reared in a tank with a 300 L seawater (SW, 33% to 35‰) circulating system at 26 ± 0.5°C with a daily 12-h photoperiod at least 4 weeks for the holding period. The waters were continuously circulated through fabric-floss filters, and the environmental salinity was measured by the refractomter PAL-06S (ATAGO, Tokyo, Japan). Fish were fed daily with commercial pellets (TetraMarin®, Tetra, Melle, Germany) except 48 h prior to the sampling. No mortality was observed during the holding period. For all following experiments, 28 individuals were sacrificed. The facilities and protocols for the experimental fish were approved by the Institutional Animal Care and Use Committee of College of Marine Sciences, Nation Dong Hwa University (i.e., NMMBA).
Short-term exposure of blue-green damselfish to elevated temperature
After the holding period, blue-green damselfish were randomly divided into two different groups for the control and hyperthermic treatment. The temperature was maintained at 26°C ± 0.5°C for control group and 32°C ± 0.5°C for hyperthermic group. A 100 W automatic heater (EBO-JÄGER, El Segundo, CA, USA) was used to maintain the temperature. After 12-h short-term exposure, the experimental animals were randomly selected from two tanks and anesthetized by immersion in MS-222 (50 mg/l) before sampling.
Analysis of plasma glucose levels, plasma osmolality, and muscle water content
Fish blood was collected from the heart using heparinized 1 ml syringes and 21 G needles. After centrifugation at 1,000 × g at 4°C for 10 min, the plasma osmolality and glucose levels were measured immediately using a Wescor 5520 Vapro osmometer (Logan, Utah, USA) and an ACCU-CHEK Go blood glucose meter (Roche, Mannheim, Germany), respectively. The muscle water content (MWC) was measured gravimetrically after drying at 100°C for 48 h. The procedures of analysis of plasma glucose levels, plasma osmolality, and MWC were determined according to Tang and Lee ([2013b]).
Antibodies
The primary antibodies used in the present study included (1) anti-heat shock protein 90 (HSP90) (1:1,500 dilution), a rabbit polyclonal antibody (#4874; Cell Signaling Technology, Beverly, MA, USA) corresponding to human HSP90; (2) anti-HSP70 (1:2500 dilution), a mouse monoclonal antibody (H 5147; Sigma, St. Louis, MO, USA) generated by immunization with purified bovine brain HSP70; (3) anti-HSP60 (1:1,000 dilution), a mouse monoclonal antibody (H3524; Sigma) recognizes an epitope located between amino acid residues 383–419 of the human; (4) anti-ubiquitin (1:2,000 dilution), a rabbit polyclonal antibody (#3933; Cell Signaling Technology) corresponding to the N-terminus of the human ubiquitin protein that detects ubiquitin, polyubiquitin, and ubiquitinated proteins; (5) anti-β-actin (1:5,000 dilution), a monoclonal antibody (ab8226, Abcam, Cambridge, England, UK) against residues 1–100 of human β-actin; and (6) anti-NKA (1:4,000 dilution), a mouse monoclonal antibody (α5; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) raised against the α-subunit of avian NKA. The secondary antibodies for Western blot analyses were horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse IgG (Chemicon, Temecula, CA, USA). A 1:12,000 dilution of secondary antibodies was used in the present study.
Cell protein fractionation and isolation of aggregated proteins
The procedures of cell protein fractionation and isolation of aggregated proteins were performed according to published studies (Aufricht et al. [1998]; Chen et al. [2002]; Rinehart et al. [2006]; Tang and Lee [2013a]). The studied tissues were homogenized in chilled extraction buffer containing 0.1% Triton X-100, 60 mM PIPES, 1 mM EDTA, 1 mM ethylene glyco-bis(aminoethyl ether)-N,N,N,N-tetraacetic acid and 100 mM NaCl. In addition, 40 μl of a proteinase inhibitor cocktail (Roche, Mannheim, Germany) was added for each milliliter of chilled extraction buffer. Homogenization was performed in 2 ml tubes with a Polytron PT1200E (Lucerne, Switzerland) at appropriate speed for 10 s. The homogenate was centrifuged at 680 × g for 10 min at 4°C to pellet nuclei and large cellular fragments. The supernatant was assigned to the total cell lysates for the following analyses of HSPs and ubiquitinated proteins. The resulting supernatant (total cell lysate) was centrifuged at 35,000 × g for 14 min at 4°C to separate the Triton-soluble and insoluble protein fractions. Aggregated proteins were isolated by differential centrifugation. The Triton-insoluble fraction was resuspended twice in extraction buffer, sonicated, and pelleted at 17,000 × g for 30 min at 4°C. The resultant pellet was again resuspended in extraction buffer, sonicated, and pelleted at 5,000 × g for 30 min at 4°C. The pellet consisting of aggregated proteins was resuspended in extraction buffer (aggregated protein fraction) and stored at −80°C. Protein concentrations of total cell lysates and aggregated protein fractions were determined with a BCA Protein Assay Kit (Pierce, Hercules, CA, USA) using bovine serum albumin (BSA, Pierce) as a standard.
Preparation of crude membrane fractions
The procedure of preparation of crude gill membrane fractions was performed according to Tang et al. ([2012]). The gills of the fish were excised and blotted dry immediately after the fish were killed by spinal pithing. The samples were immersed in liquid nitrogen and placed into ice-cold homogenization buffer (250 mM sucrose, 1 mM EDTA, 30 mM Tris, pH 7.4). Homogenization was performed in 2 ml tubes using the Polytron PT1200E homogenizer (Lucerne, Switzerland) at appropriate speed for 10 s. Debris, nuclei, and lysosomes were removed by low-speed centrifugation (12,000 × g for 10 min, 4°C). The remaining supernatant was centrifuged at medium speed (20,800 × g for 1 h, 4°C). The resulting pellet was resuspended in homogenization buffer and stored at −80°C. The pelleted fraction contained large fragments of the plasma membrane along with membranes from the Golgi and the endoplasmic reticulum, but no small cytoplasmic vesicles as they typically do not pellet down unless greater forces (100,000 × g for >1 h) are applied (Alberts et al. [1994]). This fraction is therefore referred to as the crude membrane fraction. Aliquots of crude cell membrane fractions were saved for protein determination analysis. Protein concentrations were determined with BCA Protein Assay Kit (Pierce) using bovine serum albumin (Pierce) as a standard. The crude membrane fractions were stored at −80°C until the analysis of Western blot and specific activity of gill NKA.
Western blot analysis
Gill proteins were heated in sample buffer at 90°C for 10 min for detection of HSPs in total cell lysates or at 37°C for 30 min for detection of NKA in crude membrane fractions. The samples were separated by electrophoresis on sodium dodecyl sulfate (SDS) containing 8% polyacrylamide gels for detection of HSPs and NKA. The prestained protein molecular weight marker was purchased from Fermentas (SM0671; Hanover, MD, USA). The separated proteins were then transferred to PVDF membranes (0.45 μm pore size) (Millipore, Bedford, MA, USA) by electroblotting. After preincubation for 3 h in phosphate-buffered saline (PBS) (137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) with 0.075% (v/v) Tween 20, pH 7.4 (PBST) buffer containing 5% (w/v) nonfat dried milk to minimize nonspecific binding, the blots were incubated at room temperature for 3 h with primary antibody diluted in 1% BSA and 0.05% sodium azide in PBST, washed in PBST, and incubated at room temperature for 2 h with secondary antibody. The immunoreactive bands were developed with HRP substrate, Western Blot Enhancer Kit (T-Pro, New Taipei City, Taiwan), and imaged with a Fusion FX7 system (Vilbert Lourmat, Eberhardzell, Germany). β-actin was used as the loading control for HSPs. To verify even the loading of the crude membrane fractions, the protein amount of each lane on the blots was quantified after staining the membranes with Ponceau S (Romero-Calvo et al. [2010]). The developed blots were imported as TIFF files. Immunoreactions were analyzed using a software package (MCID software, Imaging Research, Ontario, Canada). The results were converted to numerical values to compare the relative protein abundance of the immunoreactions.
Dot blot analysis
Levels of ubiquitinated proteins in the gills were measured using an immunochemical analysis modified from the study of Todgham et al. ([2007]). Equal amounts of total protein (10 μg) from each sample were blotted onto pre-wetted nitrocellulose membrane (0.2 μm pore size) (Sartorius, Epsom, Surrey, UK) in triplicates by gravity filtration using a BioDot dot blotter (Bio-Rad, Hercules, CA, USA). Wells were washed twice with 200 μl of PBST and then heat-fixed at 65°C for 20 min. Then, the membrane was blocked in 5% (w/v) nonfat dried milk in PBST for 1.5 h. Following blocking, the membranes were washed three times in PBST (for 5 min each). The membranes were incubated at room temperature for 3 h with primary antibody (anti-ubiquitin antibody, Cell Signaling Technology) diluted in 1% BSA and 0.05% sodium azide in PBST, washed in PBST, and subsequently incubated at room temperature for 2 h with secondary antibody. The immunoreaction was developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore) and imaged with a Fusion FX7 system (Vilbert Lourmat, Eberhardzell, Germany). The developed membranes were imported as TIFF files. The immunoreactive signals were analyzed using a software package (MCID software). The results were converted to numerical values to compare the levels of ubiquitinated proteins of the immunoreactive signals.
Specific Na+/K+-ATPase activity
A method using 96-well microplate to measure the inorganic phosphate concentrations for determination of NKA activity was performed according to Tang et al. ([2010]) with minor modification. Aliquots of the suspension of gill crude membrane fractions, prepared as described above, were used to determine the protein concentration and NKA enzyme activity. The reaction medium (final concentration, 100 mM imidazole-HCl buffer, pH 7.6, 125 mM NaCl, 75 mM KCl, 7.5 mM MgCl2) was prepared according to Tang et al. ([2010]). Then, 10 μl crude membrane fractions, 50 μl 10 mM ouabain (specific inhibitor of NKA) or deionized water, and 100 μl 10 mM Na2ATP were added to 340 μl of the reaction medium. The enzyme activity was defined as the difference between the inorganic phosphate liberated in the presence and absence of ouabain in the reaction mixture. The reaction mixture was incubated at the exposure temperatures for 20 min followed by immediate ice bath for 10 min to stop the reaction (Cheng et al. [1999]). Because the previous studies have demonstrated that the specific NKA activity which was measured at the exposed temperature of fish would correlate with the level of in vivo activity (Metz et al. [2003]; Sardella et al., [2008a]), therefore, the reaction was run at the exposure temperatures in this study. The concentration of inorganic phosphate was measured according to Doulgerakia et al. ([2002]). The colorimetric reagent consisted of 1% Tween-20 and 0.75% ammonium molybdate in 0.9 M H2SO4. The reaction mixtures and colorimetric reagent were mixed in a 1:1 (v/v) ratio, and then, the concentration of inorganic phosphate in each samples was determined by a microplate reader (VERSAmax, Molecular Devices, Sunnyvale, CA, USA) at 405 nm. Each sample was determined in triplicates. Some protocols determine the concentration of inorganic phosphate by measuring the color of molybdenum blue, which is the reduced product of phosphomolybdate. The instability of color formation and reagents, however, were variables in those protocols. The formation of the unreduced phosphomolybdate in the present study is directly proportional to the amounts of inorganic phosphate.
Statistical analysis
In all experiments, statistical significance was determined using Student's t test (P < 0.05) for group data analysis. Values were expressed as means ± S.E.M.
Results
Physiological parameters: plasma glucose, osmolality, and muscle water content
Compared to the normal temperature (NT, 26°C) group, the levels of plasma glucose of blue-green damselfish were significantly elevated after exposure to high temperature (HT, 32°C) condition. In addition, plasma osmolality and MWC which significantly increased and decreased, respectively, were found when blue-green damselfish were exposed to elevated temperature (Table 1).
Changes of the abundance of gill heat shock proteins and ubiquitin-conjugated proteins
In the cellular stress responses, the relative protein abundance of stress proteins (i.e., HSPs) in fish gills was examined. Immunoblotting of the gills (Figure 1) from NT- and HT-exposed blue-green damselfish probed with primary antibodies to HSP90 (Figure 1A,B), HSP70 (Figure 1C,D), and HSP60 (Figure 1E,F) resulted in single immunoreactive bands with molecular weights of approximately 90 (Figure 1A), 70 (Figure 1C), and 60 kDa (Figure 1E), respectively. Protein expression levels of branchial HSPs in HT-exposed fish were significantly higher than those in NT-exposed fish (1.61-fold for HSP90, Figure 1B; 1.69-fold for HSP70, Figure 1D; and 1.64-fold for HSP60, Figure 1F). The response of ubiquitin-conjugated proteins in blue-green damselfish gills to reduced salinity was assayed by using dot-blot analysis. Dot-blot analysis showed the levels of ubiquitin-conjugated proteins in the gills of HT-exposed C. viridis were higher than those in NT-exposed C. viridis (Figure 2). Importantly, the aggregated proteins were maintained at similar levels between HT- (48.4 ± 10.5 μg/mg total protein) and NT-exposed fish (40.1 ± 7.8 μg/mg total protein) (Figure 3).
Protein expression of branchial heat shock protein 90 (HSP90), HSP70, and HSP60 in blue-green damselfish ( Chromis viridis ). The representative immunoblots of HSP90, HSP70, and HSP60 showed a single immunoreactive band with a molecular mass about 90 (A), 70 (C), and 60 kDa (E), respectively. The immunoreactive bands of HT group are more intensive than that in the NT group. The protein amounts of HSP90 (B), HSP70 (D), and HSP60 (F) increased evidently after hyperthermic exposure (n = 5). β-actin was used as the loading control. The asterisk indicated a significant difference (P < 0.05) by unpaired t test. Values were mean ± SEM. M, marker; NT, normal temperature (26°C); HT, high temperature (32°C).
Dot-blot analysis of the levels of ubiquitin-conjugated proteins in the gills of blue-green damselfish ( Chromis viridis ). (A) Ubiquitin-conjugated protein levels were shown as relative values based on dot intensities. (B) Ponceau S total protein stain of blots was used as loading control. (C) Significant elevation of the levels of ubiquitin-conjugated proteins was found after hyperthermic exposure. Unpaired t test was used for the statistics. Values are means ± S.E.M (n = 5). NT, normal temperature (26°C); HT, high temperature (32°C).
The levels of aggregated proteins in the gills of blue-green damselfish ( Chromis viridis ) exposed to different temperature. There was no significant difference found in the levels of aggregated proteins between NT and HT groups. Values are means ± S.E.M (n = 5). NT, normal temperature (26°C); HT, high temperature (32°C).
Na+/K+-ATPase responses
The relative protein abundance of branchial NKA was examined. Immunoblotting of the gills from NT- and HT-exposed blue-green damselfish obtained a single immunoreactive band with molecular weight of approximately 105 kDa (Figure 4A). The protein abundance of gill NKA α-subunit in C. viridis was similar between two studied environmental temperatures (Figure 4B). However, reduction of gill NKA specific activities was found in fish exposed to HT condition (Figure 4C).
Impact of changes in temperature on the responses of gill Na + /K + -ATPase (NKA) in blue-green damselfish ( Chromis viridis ). (A) Immunoblots of C. viridis gills probed with a monoclonal antibody (α5; DSHB) to NKA α-subunit. The immunoreactive bands of HT group were more intensive than NT group. (B) Relative abundance of immunoreactive bands of NKA α-subunit in the gills of different temperature groups (n = 5). Expression of NKA α-subunit was similar between NT and HT groups. (C) Specific activity of gill NKA in C. viridis in response to elevation of ambient temperature (n = 5). Downregulation of branchial NKA activity was found after hyperthermic exposure. The asterisk indicated a significant difference (P < 0.05) by unpaired t test. Values were mean ± SEM. M, marker; NT, normal temperature (26°C); HT, high temperature (32°C).
Discussion
Organisms naturally experience diverse environmental challenges throughout their lives. For marine ectothermic organisms, ambient temperature is one of the most significant factors that affects diverse regulation (Hochachka and Somero [2002]; Hofmann et al. [2002]; Crockett and Londraville [2006]; Donaldson et al. [2008]; Somero [2010]). In fish physiological responses, the mechanisms associated with stress and osmoregulatory responses are susceptible to variation of environmental temperature (Hofmann and Somero [1995]; Iwama et al. [1999]; Gonzalez and McDonald [2000]; Metz et al. [2003]; Place et al. [2004]; Sardella et al. [2004]; Fiess et al. [2007]; Sardella et al. [2008a, b]; Cui et al. [2011]; Deane and Woo [2011]; Feidantsis et al. [2012]; Cui et al. [2013]). For ecological relevance, the experimental temperatures used in this study were based on the average (26°C) and daytime (32°C) temperatures in Nanwan Bay, southern Taiwan (Meng et al. [2008]; Mayfield et al. [2013]) because C. viridis is abundant in Nanwan Bay (Shen et al. [1993]).
The stress responses are energy demanding processes, changes in plasma glucose concentrations have widely been used as a stress bioindicator at the organismal level, because glucose is the main fuel source in animals (Basu et al. [2001]; Afonso et al. [2003]; Iwama et al. [2006]). To evaluate whether a short-term increase in environmental temperature would thermally stress C. viridis, its plasma glucose concentrations were measured. Plasma glucose levels increased from 54.0 ± 2.6 to 100.2 ± 8.2 mg/dL after hyperthermic exposure (Table 1). Similar patterns were found in juvenile Chinook salmon (Oncorhynchus tshawytscha) (Mesa et al. [2002]) and two Antarctic nototheniid fish, Pagothenia borchgrevinki and Trematomus bernacchii (Lowe and Davison [2005]), after short-term exposure to thermal stress. Therefore, 32°C should be a stressful temperature for C. viridis and more energy is needed to compensate for the cost of the energy‐demanding processes involved in coping with thermal stress.
At the cellular level, temperature stress affects protein synthesis and conformation, causing protein damage (Hofmann and Somero [1995, 1996]; Hochachka and Somero [2002]; Rinehart et al. [2006]; Todgham et al. [2007]). Once the damaged protein exists, the regulation associated with the repair and degradation of damaged proteins is subsequently triggered to prevent increase in protein aggregation that is harmful to cell viability (Kabakov and Gabai [1993]; Wickner et al. [1999]; Goldberg [2003]; Bukau et al. [2006]). Activation of HSPs and protein ubiquitination which are involved in protein refolding and degradation in response to change in ambient temperature in aquatic animals have been reported in several previous studies (Hofmann and Somero [1995, 1996]; Hochachka and Somero [2002]; Hofmann et al. [2002]; Place et al. [2004]; Iwama et al. [2006]; Todgham et al. [2007]; Cui et al. [2011, 2013]). However, the evidence of protein aggregation level was lack to address the PQC mechanism adequately. In the present study, HSPs and ubiquitin-conjugated proteins evidently elevated in C. viridis exposed to 32°C (Figures 1 and 2), whereas protein aggregation was similar to the normal temperature group at low level (Figure 3). However, elevated protein aggregation levels were found when organisms were cultured in high mortality conditions (Rinehart et al. [2006]; Choe and Strange [2008]). Thus, our findings assumed that the upregulation of HSPs and ubiquitin-conjugated proteins was sufficient to prevent the accumulation of aggregated proteins in C. viridis to adapt to transient elevation of ambient temperature. To our knowledge, this is the first study to examine the expression of HSPs, ubiquitinated proteins, and protein aggregation levels simultaneously in fish, in response to temperature challenge.
The internal ionic and osmotic balance of fish is affected by ambient temperature (Gonzalez and McDonald [2000]; Metz et al. [2003]; Sardella et al. [2004]; Fiess et al. [2007]; Sardella et al. [2008a, b]). After exposure of C. viridis to hyperthermic condition, significant increase of plasma osmolality as well as decrease of muscle water content were found (Table 1). This might be explained by the marked depression of branchial NKA activity at 32°C, even though the protein expression of gill NKA was not affected (Figure 4). Moreover, in this study, NKA activity was assayed at the exposure temperature of the fish to show the apparent NKA activity to provide a physiological interpretation of our results. This is because temperature affects the reactivity of molecules by affecting protein conformation, kinetic properties, and assembly. On the other hand, activation of ion transporter system is energy-required while the rate of cellular respiration the main process for energy providing is temperature-dependent (Hochachka and Somero [2002]). Therefore, the decrease in gill NKA activity reflected that metabolically-dependent ion transporter proteins are more susceptible to temperature change than is passive ion diffusion (Christensen [1975]; Hochachka and Somero [2002]). Furthermore, temperature inhibited the specific activity of NKA was found in the common carp (Cyprinus carpio) and the Mozambique tilapia (Oreochromis mossambicus). By using biochemical and immunohistochemical approaches, it was found that a lower apparent NKA activity was compensated for by strongly enhanced NKA expression (Metz et al. [2003]; Sardella et al. [2008a]). The present study was difficult to rule out the possibility that the other compensatory responses were enhanced in C. viridis only based on protein expression of gill NKA.
Conclusions
A local species and recorded in situ water temperature were used in this study to understand the impacts of short-term increases in temperature on stress responses, cellular protein stability, and osmoregulatory status in a reef-associated fish by using physiological and molecular approaches. The results provided the implication for elucidation that C. viridis possesses the molecular mechanisms for coping with thermal stress to maintain protein stability, but inhibitory effects on osmoregulatory ability resulted in slight changes of plasma osmolality and muscle water content.
References
Afonso L, Basu N, Nakano K, Devlin R, Iwama G: Sex-related differences in the organismal and cellular stress response in juvenile salmon exposed to treated bleached kraft mill effluent. Fish Physiol Biochem 2003, 29: 173–179. 10.1023/B:FISH.0000035939.81588.09
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD: Molecular biology of the cell. Garland Science, New York; 1994.
Allen GR: Damselfishes of the world. Mergus, Germany; 1991.
Amoudi MA, El-Sayed A-FM, El-Ghobashy A: Effects of thermal and thermo-haline shocks on survival and osmotic concentration of the tilapias Oreochromis mossambicus and Oreochromis aureus × Oreochromis niloticus hybrids. J World Aquacult Soc 1996, 27: 456–461. 10.1111/j.1749-7345.1996.tb00630.x
Aufricht C, Lu E, Thulin G, Kashgarian M, Siegel NJ, Van Why SK: ATP releases HSP-72 from protein aggregates after renal ischemia. Am J Physiol-Renal Physiol 1998, 274: F268-F274.
Basu N, Kennedy C, Hodson P, Iwama G: Altered stress responses in rainbow trout following a dietary administration of cortisol and β-napthoflavone. Fish Physiol Biochem 2001, 25: 131–140. 10.1023/A:1020566721026
Bukau B, Weissman J, Horwich A: Molecular chaperones and protein quality control. Cell 2006, 125: 443–451. 10.1016/j.cell.2006.04.014
Chen Q, Ma E, Behar KL, Xu T, Haddad GG: Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster . J Biol Chem 2002, 277: 3274–3279. 10.1074/jbc.M109479200
Cheng SXJ, Aizman O, Nairn AC, Greengard P, Aperia A: [Ca2+]i determines the effects of protein kinases A and C on activity of rat renal Na+, K + −ATPase. J Physiol 1999, 518: 37–46. 10.1111/j.1469-7793.1999.0037r.x
Choe KP, Strange K: Genome-wide RNAi screen and in vivo protein aggregation reporters identify degradation of damaged proteins as an essential hypertonic stress response. Am J Physiol Cell Physiol 2008, 295: C1488-C1498. 10.1152/ajpcell.00450.2008
Christensen HN: Biological transport. W. A. Benjamin, Inc., Reading, MA; 1975.
Crockett EL, Londraville RL: Temperature. In The physiology of fishes. 3rd edition. Edited by: Evans DH, Claiborne JB. CRC Press, Boca Raton, FL; 2006:231–269.
Cui M, Zhang QZ, Yao ZJ, Zhang ZH: Molecular cloning and expression analysis of heat-shock protein 70 in orange-spotted grouper Epinephelus coioides following heat shock and Vibrio alginolyticus challenge. J Fish Biol 2011, 79: 486–501. 10.1111/j.1095-8649.2011.03045.x
Cui Y, Liu B, Xie J, Xu P, Tsion HMH, Zhang Y: The effect of hyperthermia on cell viability, oxidative damage, and heat shock protein expression in hepatic cells of grass carp ( Ctenopharyngodon idellus ). J Therm Biol 2013, 38: 355–361. 10.1016/j.jtherbio.2013.04.007
Deane EE, Woo NYS: Advances and perspectives on the regulation and expression of piscine heat shock proteins. Rev Fish Biol Fish 2011, 21: 153–185. 10.1007/s11160-010-9164-8
Donaldson MR, Cooke SJ, Patterson DA, Macdonald JS: Cold shock and fish. J Fish Biol 2008, 73: 1491–1530. 10.1111/j.1095-8649.2008.02061.x
Doulgerakia A, Papadopoulou-Daifoti Z, Tsakiris S: Effects of L-phenylalanine on acetylcholinesterase and Na + , K + -ATPase activities in suckling rat frontal cortex, hippocampus and hypothalamus. Z Naturforsch [C] 2002, 57: 182–188.
Epstein FH, Katz AI, Pickford GE: Sodium- and potassium-activated adenosine triphosphatase of gills: role in adaptation of teleosts to salt water. Science 1967,156(3779):1245–1247. 10.1126/science.156.3779.1245
Evans DH, Piermarini PM, Choe KP: The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 2005, 85: 97–177. 10.1152/physrev.00050.2003
Feidantsis K, Pörtner HO, Markou T, Lazou A, Michaelidis B: Involvement of p38 MAPK in the induction of Hsp70 during acute thermal stress in red blood cells of the gilthead sea bream, Sparus aurata . J Exp Zool Part A 2012, 317: 303–310. 10.1002/jez.1725
Fiess JC, Kunkel-Patterson A, Mathias L, Riley LG, Yancey PH, Hirano T, Grau EG: Effects of environmental salinity and temperature on osmoregulatory ability, organic osmolytes, and plasma hormone profiles in the Mozambique tilapia ( Oreochromis mossambicus ). Comp Biochem Physiol A-Mol Integr Physiol 2007, 146: 252–264. 10.1016/j.cbpa.2006.10.027
Goldberg AL: Protein degradation and protection against misfolded or damaged proteins. Nature 2003,426(6968):895–899. 10.1038/nature02263
Gonzalez RJ, McDonald DG: Ionoregulatory responses to temperature change in two species of freshwater fish. Fish Physiol Biochem 2000, 22: 311–317. 10.1023/A:1007837214327
Gottesman S, Wickner S, Maurizi MR: Protein quality control: triage by chaperones and proteases. Genes Dev 1997, 11: 815–823. 10.1101/gad.11.7.815
Guschina IA, Harwood JL: Mechanisms of temperature adaptation in poikilotherms. FEBS Lett 2006, 580: 5477–5483. 10.1016/j.febslet.2006.06.066
Hirose S, Kaneko T, Naito N, Takei Y: Molecular biology of major components of chloride cells. Comp Biochem Phys B-Biochem Mol Biol 2003, 136: 593–620. 10.1016/S1096-4959(03)00287-2
Hochachka PW, Somero GN: Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York; 2002.
Hofmann G, Somero G: Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus . J Exp Biol 1995, 198: 1509–1518.
Hofmann GE, Somero GN: Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis : evidence from the heat-shock response and protein ubiquitination. Mar Biol 1996, 126: 65–75. 10.1007/BF00571378
Hofmann GE, Buckley BA, Place SP, Zippay ML: Molecular chaperones in ectothermic marine animals: biochemical function and gene expression. Integr Comp Biol 2002, 42: 808–814. 10.1093/icb/42.4.808
Hwang PP, Lee TH: New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A-Mol Integr Physiol 2007, 148: 479–497. 10.1016/j.cbpa.2007.06.416
Hwang PP, Lee TH, Lin LY: Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol-Regul Integr Comp Physiol 2011, 301: R28-R47. 10.1152/ajpregu.00047.2011
Iwama GK, Vijayan MM, Forsyth RB: Heat shock proteins and physiological stress in fish. Am Zool 1999, 39: 901–909.
Iwama GK, Afonso LOB, Vijayan MM: Stress in fishes. In The physiology of fishes. Edited by: Evans DH, Claiborne JB. CRC Press, Boca Raton, FL; 2006:319–335.
Kabakov AE, Gabai VL: Protein aggregation as primary and characteristic cell reaction to various stresses. Experientia 1993, 49: 706–713. 10.1007/BF01923956
Kaneko T, Watanabe S, Lee KM: Functional morphology of mitochondrion-rich cells in euryhaline and stenohaline teleosts. Terrapub, Tokyo; 2008.
Kregel KC: Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002, 92: 2177–2186.
Lieske E, Myers RF: Coral reef fishes: Indo-Pacific and Caribbean. HarperCollins, London; 1994.
Long Y, Li L, Li Q, He X, Cui Z: Transcriptomic characterization of temperature stress responses in larval zebrafish. PLoS ONE 2012, 7: e37209. 10.1371/journal.pone.0037209
Lowe CJ, Davison W: Plasma osmolarity, glucose concentration and erythrocyte responses of two Antarctic nototheniid fishes to acute and chronic thermal change. J Fish Biol 2005, 67: 752–766. 10.1111/j.0022-1112.2005.00775.x
Environmental biology of fishes. Chapman and Hall Publishers, London; 1995.
Mayfield AB, Chen MN, Meng PJ, Lin HJ, Chen CS, Liu PJ: The physiological response of the reef coral Pocillopora damicornis to elevated temperature: results from coral reef mesocosm experiments in Southern Taiwan. Mar Environ Res 2013, 86: 1–11. 10.1016/j.marenvres.2013.01.004
Meng PJ, Lee HJ, Wang JT, Chen CC, Lin HJ, Tew KS, Hsieh WJ: A long-term survey on anthropogenic impacts to the water quality of coral reefs, southern Taiwan. Environ Pollut 2008, 156: 67–75. 10.1016/j.envpol.2007.12.039
Mesa MG, Weiland L, Wagner P: Effects of acute thermal stress on the survival, predator avoidance, and physiology of juvenile fall chinook salmon. Northwest Sci 2002, 76: 118–128.
Metz JR, van den Burg EH, Bonga SE, Flik G: Regulation of branchial Na + /K + -ATPase in common carp Cyprinus carpio L. acclimated to different temperatures. J Exp Biol 2003, 206: 2273–2280. 10.1242/jeb.00421
Place SP, Zippay ML, Hofmann GE: Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am J Physiol-Regul Integr Comp Physiol 2004, 287: R429–436. 10.1152/ajpregu.00223.2004
Rinehart JP, Hayward SA, Elnitsky MA, Sandro LH, Lee RE Jr, Denlinger DL: Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc Natl Acad Sci U S A 2006, 103: 14223–14227. 10.1073/pnas.0606840103
Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS: Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 2010, 401: 318–320. 10.1016/j.ab.2010.02.036
Sardella BA, Cooper J, Gonzalez RJ, Brauner CJ: The effect of temperature on juvenile Mozambique tilapia hybrids ( Oreochromis mossambicus x O. urolepis hornorum ) exposed to full-strength and hypersaline seawater. Comp Biochem Physiol A-Mol Integr Physiol 2004, 137: 621–629. 10.1016/j.cbpb.2003.12.003
Sardella BA, Kültz D, Cech J Jr, Brauner C: Salinity-dependent changes in Na + /K + -ATPase content of mitochondria-rich cells contribute to differences in thermal tolerance of Mozambique tilapia. J Comp Physiol B 2008, 178: 249–256. 10.1007/s00360-007-0211-2
Sardella BA, Sanmarti E, Kültz D: The acute temperature tolerance of green sturgeon ( Acipenser medirostris ) and the effect of environmental salinity. J Exp Zool Part A 2008, 309A: 477–483. 10.1002/jez.477
Shen SC, Shao KT, Chen CT, Chen CH, Lee SC, Mok H: Fishes of Taiwan. National Taiwan University, Taipei, Department of Zoology; 1993.
Somero GN: The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 2010, 213: 912–920. 10.1242/jeb.037473
Tang CH, Lee TH: Early response of protein quality control in gills is associated with survival of hypertonic shock in Mozambique tilapia. PLoS ONE 2013, 8: e63112. 10.1371/journal.pone.0063112
Tang CH, Lee TH: Freshwater acclimation induces stress responses and expression of branchial Na + /K + -ATPase and proliferating cell nuclear antigen in Takifugu niphobles . J Exp Zool Part A 2013, 319: 409–421. 10.1002/jez.1804
Tang CH, Wu WY, Tsai SC, Yoshinaga T, Lee TH: Elevated Na + /K + -ATPase responses and its potential role in triggering ion reabsorption in kidneys for homeostasis of marine euryhaline milkfish ( Chanos chanos ) when acclimated to hypotonic fresh water. J Comp Physiol B 2010, 180: 813–824. 10.1007/s00360-010-0458-x
Tang C-H, Lai D-Y, Lee T-H: Effects of salinity acclimation on Na + /K + –ATPase responses and FXYD11 expression in the gills and kidneys of the Japanese eel ( Anguilla japonica ). Comp Biochem Physiol A-Mol Integr Physiol 2012, 163: 302–310. 10.1016/j.cbpa.2012.07.017
Todgham A, Hoaglund E, Hofmann G: Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation of proteins in vivo. J Comp Physiol B 2007, 177: 857–866. 10.1007/s00360-007-0183-2
Wickner S, Maurizi MR, Gottesman S: Posttranslational quality control: folding, refolding, and degrading proteins. Science 1999, 286: 1888–1893. 10.1126/science.286.5446.1888
Acknowledgements
The α5 monoclonal antibody were purchased from the Developmental Studies Hybridoma Bank (DSHB) maintained by the Department of Pharmacology and Molecular Sciences, John Hopkins University School of Medicine, Baltimore, MD 2120521205, and the Department of Biological Sciences, University of Iowa, Iowa City, IA 52242, under Contract N01-HD-6-2915, NICHD, USA. This study was supported by the grants from the National Science Council of Taiwan (NSC 102-2313-B-259-001 to C.H.T.) and the National Museum of Marine Biology and Aquarium (to C.H.T.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The work presented here was carried out in collaboration among all authors. CHT, MYL, and KS designed and carried out the experiments. CHT drafted the manuscript. LYH and WBC helped for rearing the experimental animals. All authors made comments on the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tang, CH., Leu, MY., Shao, K. et al. Short-term effects of thermal stress on the responses of branchial protein quality control and osmoregulation in a reef-associated fish, Chromis viridis . Zool. Stud. 53, 21 (2014). https://doi.org/10.1186/s40555-014-0021-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40555-014-0021-7