Abstract
Background
Patients with severe obesity [body mass index (BMI) ≥ 40 kg/m2] potentially overload the tibial component after total knee arthroplasty (TKA), risking tibial subsidence. Using a cemented single-radius cruciate-retaining TKA design, this study compared the outcomes of two tibial baseplate geometries in patients with BMI ≥ 40 kg/m2: standard keeled (SK) or universal base plate (UBP), which incorporates a stem.
Methods
This was a retrospective, single-centre cohort study with minimum 2 years follow-up of 111 TKA patients with BMI ≥ 40 kg/m2: mean age 62.2 ± 8.0 (44–87) years, mean BMI 44.3 ± 4.6 (40–65.7) kg/m2 and 82 (73.9%) females. Perioperative complications, reoperations, alignment and patient-reported outcomes (PROMS): EQ-5D, Oxford Knee Score (OKS), Visual Analogue Scale (VAS) pain score and satisfaction were collected preoperatively, and at 1 year and final follow-up postoperatively.
Results
Mean follow-up was 4.9 years. SK tibial baseplates were performed in 57 and UBP in 54. There were no significant differences in baseline patient characteristics, post-operative alignment, post-operative PROMs, reoperations or revisions between the groups. Three early failures requiring revision occurred: two septic failures in the UBP group and one early tibial loosening in the SK group. Five-year Kaplan–Meier survival for the endpoint mechanical tibial failure was SK 98.1 [94.4–100 95% confidence interval (CI)] and UBP 100% (p = 0.391). Overall varus alignment of the limb (p = 0.005) or the tibial component (p = 0.031) was significantly associated with revision and return to theatre.
Conclusions
At early to mid-term follow-up, no significant differences in outcomes were found between standard and UBP tibial components in patients with BMI ≥ 40 kg/m2. Varus alignment of either tibial component or the limb was associated with revision and return to theatre.
Highlights
-
Favourable knee arthroplasty outcomes in BMI ≥ 40 kg/m2 with different tibial baseplates.
-
No overt advantage of UBP over standard keeled tibia in patients with BMI≥40kg/m2, though may be underpowered to demonstrate this.
-
Varus alignment of tibial component or limb associated with increased revision rate.
-
Further research needed to investigate theoretical benefit of longer tibial stem.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is an effective treatment for knee osteoarthritis. Severe obesity, defined as a body mass index (BMI) ≥ 40 kg/m2, is a risk factor for developing osteoarthritis and for requiring TKA [1, 2]. Though complication rates following TKA are higher in patients with increased BMIs [3], TKA remains cost effective in this patient group [4], with no difference in patient-reported outcome measure improvement [4] or survival [5] compared with patients with normal BMIs. Though improved polyethylene engineering has led to an overall reduction in aseptic loosening [6, 7], it remains an important mode of TKA failure [8]. There remains significant concern that overload in severely obese patients may result in early tibial subsidence or loosening [9, 10] and some authors have recommended the routine use of additional tibial stem fixation in patients with high BMIs [10,11,12,13,14].
Complex kinematics at the knee with rollback and rotation during flexion in addition to six degrees of freedom create numerous forces that TKA implants must resist, including compression, tension, axial torque, varus/valgus moments and shear [9]. To reduce shear, micromotion and tibial lift-off, projections of different geometries are added to the under-surface of tibial components including keels, stems or pegs [9]. Whilst stems provide stability, they introduce their own shear forces and as they are load bearing, stress shielding of metaphyseal bone occurs along their length [9, 15, 16]. In turn, stress shielding potentially increases the risk of tibial subsidence, loosening and fracture.
Standard stemless tibial component geometry varies considerably across TKA implants. Though most incorporate a short stem and keels as part of tibial baseplate design, the lengths of these projections vary. The standard keeled tibial baseplate of the Triathlon (Stryker, Mahwah, NJ) TKA consists of anti-torque keels for rotational stability without any stem element (Fig. 1a). Both keel thickness (2.6–3.6 mm) and keel depth (28–39 mm) vary across sizes in SK tibial components. An alternative baseplate, the universal base plate (UBP), is available as part of the system and incorporates a 20 mm keel and a boss/stem of 40 mm depth and 16 mm diameter to which additional stems can be added if desired (Fig. 1b). These dimensions are consistent across all sizes (Fig. 1a, b). Both SK and UBP tibial components are made from cobalt chrome.
The aim of this study was to compare the outcome of the cemented standard keeled tibial baseplate (SK) with the cemented UBPs in severely obese patients (BMI ≥ 40 kg/m2) undergoing TKA. The outcomes of interest were early mechanical failure of the tibial component requiring revision, patient-reported outcomes (PROMs), pain, complications, reoperation and all-cause revision.
Materials and methods
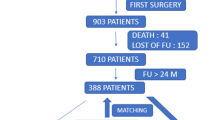
Ethical approval was obtained for this retrospective cohort study (Scotland (A) Research Ethics Committee 16/SS/0026). Following review of a prospectively collected, single-centre arthroplasty database, 111 patients with a BMI ≥ 40 kg/m2 undergoing unilateral Triathlon (Stryker, Mahwah, NJ, USA) TKA for end-stage degenerative joint disease by eight surgeons at a large orthopaedic teaching hospital from 2013 to 2018 were included. The second of bilateral TKAs was excluded.
All patients underwent primary cemented cruciate retaining Triathlon TKAs via a medial parapatellar approach. Patient demographics were recorded. Standard keeled tibial components (SK) and universal base plates (UBPs) were available for all cases and were selected at the surgeons’ discretion. Electronic patient records were reviewed for complications (early, ≤ 30 days and late, > 30 days), reoperations and revision surgeries.
Prior to TKA and at 1 year following surgery, patients completed postal questionnaires including comorbidity scoring and validated patient-reported outcome measures (PROMs): the EuroQol 5-dimension score (EQ-5D) [17], the Oxford Knee Score (OKS) [18], Visual Analogue Scale (VAS) pain scores (0–100), and satisfaction was measured at 1 year using a five-point Likert scale from ‘very dissatisfied’ to ‘very satisfied’ [19]. The EQ-5D is a validated and widely used five-dimension multi-attribute general health questionnaire that defines an overall health index (from −0.594 to 1). The OKS is a validated knee-specific outcome measure, in which 12 questions (five possible answers) give scores from 0 to 48 (higher scores = better function). A minimal important change (MIC) in OKS was defined as seven points [20]. Patients recorded the presence or absence of 18 comorbidities as recorded by the Charlson Comorbidity Index (CCI): myocardial infarction, heart failure, peripheral arterial disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disorder, peptic ulcer, diabetes, kidney disease, hemiplegic stroke, leukaemia, malignant lymphoma, solid tumour, liver disease, acquired immunodeficiency syndrome, back pain, pain from other joints and hypertension [21].
Overall femorotibial alignment (femorotibial angle, FTA), and tibial component alignment in coronal (medial proximal tibial angle, MPTA) and sagittal (posterior tibial slope, PTS) planes were measured on short leg weight-bearing radiographs by the senior author using the method described by Sarmah et al. [22]. An MPTA < 87° was defined as a varus tibia and a femorotibial angle < 177° was defined as varus lower limb alignment.
Follow-up PROMs questionnaires were resent to all patients in January 2021. In addition to the OKS, VAS-pain score, EQ-5D and patient satisfaction scores, patients were asked if they had undergone any reoperations to the operated knee and to select any areas of the knee (as many as applied) that were painful from the following: the front of the knee, the back of the knee, the inside edge, the outside edge, the top of the shin bone, all around the knee or other, with a free text box.
Statistical analysis
Data were analysed using SPSS version 25.0. Variables were tested for normality. Univariate analysis was performed using parametric (unpaired Student’s t-test) and non-parametric (Mann–Whitney U-test) tests as appropriate to assess differences in continuous variables between SK and UBP cohorts. Nominal categorical variables were assessed using Chi squared or Fisher’s exact tests. Pearson’s correlation was used to correlate continuous variables. Survival analysis was undertaken with Kaplan–Meier analysis using the endpoints any revision, mechanical failure and any reoperation. The log rank statistic was used to compare the survival of treatment strategies. A p-value < 0.05 was considered statistically significant.
Results
Over the study period, 111 patients met the inclusion and exclusion criteria: mean age was 62.2 [standard deviation (SD) 8.0, range 44–87] years; mean BMI 44.3 (SD 4.6, range 40–65.7) kg/m2 and 82 (73.9%) were female. Of the 111, 57 underwent TKA with an SK tibial baseplate and 54 with a UBP. Mean length of follow-up was 4.9 years (SD 1.7 years) with a minimum of 2.1 years. There were no differences in baseline patient characteristics (Table 1) or comorbidities between standard and UBP groups (p > 0.05).
Alignment
There were no significant differences in the mean tibial component alignment achieved after TKA or in overall femorotibial alignment (FTA) between tibial baseplate groups (Table 2), nor were there differences in the number of varus or valgus outliers (Fig. 2).
PROMs
There were no statistically significant differences in improvement in OKS, EQ-5D or pain scores at 1 year or at final follow-up (Table 3; Figs. 3, 4). Pain location did not differ significantly between tibial baseplate designs (p > 0.05, Chi square, Fig. 5). A varus limb alignment with FTA of < 177° (i.e. > 3° varus) was associated with lateral knee pain (p = 0.023 Fisher’s exact test, Fig. 6) but did not significantly affect the prevalence of pain in any other region of the knee (p > 0.05). A varus tibia with MPTA of < 87° did not significantly affect pain location (p > 0.05, Fig. 6). For the entire cohort, a varus FTA of < 177° was significantly associated with revision (2/5 versus 1/104, p = 0.010 Fisher’s exact test) and reoperation (3/7 versus 4/104 p = 0.005 Fisher’s exact test), with a relative risk of revision of 14.4 (4.46–46.5 95% CI) and a relative risk (RR) of any reoperation of 11.1 (3.1–40.3 95% CI). In contrast, a varus MPTA < 87 alone was not significantly associated with revision (1/17 versus 2/94, p = 0.396 Fisher’s exact test) or reoperation (1/17 versus 6/94, p = 1.0 Fisher’s exact test). The study was under-powered to detect a similar trend for mechanical failure.
Complications and revisions
Overall, there were no statistically significant differences in early or late complications (Table 4) or reoperations between baseplate designs.
There was one case of early tibial loosening/subsidence requiring revision. It occurred at 35 months after TKA with a SK baseplate in a 74-year-old female patient with a BMI of 43 kg/m2 and lymphoedema with a tibial alignment of MPTA of 83° and a subsequent FTA of 169°. Two patients from the UBP group underwent revision for infection. Four patients returned to theatre: 2/58 of the SK baseplate group for manipulation under anaesthetic (MUA), and 2/54 of the UBP group where one MUA and one wound washout and closure for dehiscence were performed (p = 0.710 Fisher’s exact test).
Kaplan–Meier estimates of survival and life tables are provided in Tables 5 and 6 and Fig. 7. There were no significant differences in 5 year survival between tibial baseplate designs for the endpoints any reoperation, any revision or tibial failure.
Among SK baseplates, one in five implanted in varus (MPTA < 87°) required revision for mechanical failure of the tibia, and this was significant (p = 0.045, Fisher’s exact test). Three of five standard tibial baseplates implanted in varus with MPTA < 87° required return to theatre, with a relative risk of 9.9 (1.97–49.9 95% CI, p = 0.031, Fisher’s exact test) compared with other knees.
Discussion
This study found no difference in early tibial component subsidence or loosening between standard tibial baseplates and UBP implants in patients with severe obesity when using a cemented cruciate retaining TKA. Similarly, joint-specific function, health-related quality of life, pain severity or pain location did not differ by type of implant. Rates of complications, 5-year survival and reoperations did not differ significantly between implants. The only case of tibial component mechanical failure occurred with a standard baseplate, albeit in varus alignment (MPTA < 87°). Varus alignment of the limb following TKA was associated with increased risk of return to theatre and revision across the entire cohort. Whether tibial implant geometry or alignment is most important in patients with severe obesity remains unclear.
Patients with a normal BMI have been shown to have comparable rates of aseptic loosening with both non-stemmed and stemmed tibial components [23]. The use of non-stemmed components is considered safe in this population and is utilised to reduce costs, shorten operative time and preserve bone-stock [23]. However, in patients with higher BMIs, some studies have demonstrated a benefit to using stemmed tibial components in terms of reduced revision rates [11,12,13,14]. Parratte et al. performed a randomised controlled trial of standard versus stemmed (10 × 100 mm stem) TKA in patients with BMI > 30 kg/m2, also stratifying a subgroup of BMI > 35 kg/m2 patients [24]. They reported a modest, clinically insignificant difference in Knee Injury and Osteoarthritis Outcome Score (KOOS) scores at 2 years in favour of stems, stating that they could not recommend the routine use of additional stems for obesity alone [24]. A retrospective study of TKA with or without a short additional 30 mm stem, found no difference between groups in terms of radiographic tibial failures [25]. Other studies in support of stems are retrospective, with some including implants of different designs [14, 26], relatively low BMI thresholds [14] or small sample sizes of patients with both elevated BMI and stems [13]. All involve adding stems to tibial components rather than investigating keeled and stemmed primary tibial baseplates.
Over 3 million Triathlon TKAs have been implanted worldwide to date, with 5- and 10-year studies of survival and PROMs published [27, 28]. To the authors knowledge, outcomes specific to the UBP have not previously been reported. Although no UBP implants were revised for mechanical failure, two were revised for infection. This may be related to the increased risk of periprosthetic joint infection in this severely obese patient group [29].
Cox et al. demonstrated that the most common mechanisms of TKA mechanical failure were failure of the cement–implant interface and tibial varus collapse, defined as a change in component position of > 10° [30]. They advocated for use of stemmed tibial implants in high-risk patients. Our findings similarly showed that degree of varus implantation (FTA < 177°or MPTA < 87°) was associated with increased complications, specifically lateral knee pain and an increased rate of revision. The one case of mechanical tibial failure in the current study occurred in a tibial component with varus malalignment and this, rather than tibial baseplate design, may have contributed to its early loosening. Using longer stems may actually aid alignment and it may be that the beneficial effect of stems in obese patients [12, 13, 25] is in optimising alignment rather than implant–bone integration. Using intramedullary referencing may help to reduce tibial alignment outliers. Therefore, caution should be used if moving away from mechanical alignment philosophies in severe obesity.
The current study reports early outcomes, with follow-up at a mean of 4.9 years (minimum 2.1 years). Aseptic loosening is most commonly observed as a late complication, with an average time from implantation to revision of 5.5 years (range 0.03–24.2 years) [6]. Although obesity is associated with an increased risk of early failure [3, 31], with 40% of aseptic loosening occurring in the first 2 years in these patients [26], longer term follow-up is required to detect differences between implants in the medium-to-longer term. Though longer-term follow-up is required, it is also important to look for and explore early failures.
Similar to Steere et al. [25], the current study demonstrated no difference in early tibial failures between implants. The sample size of 111 meant the study was under-powered to show statistical significance when comparing rare outcomes between implant groups. Only one patient (0.9%) in the study required reoperation for aseptic loosening, though this is at an early stage of follow-up (minimum 2 years, mean 4.9 years). Further cases of loosening or subsidence may occur with longer follow-up. Whether the theoretical mechanical advantages of a stemmed geometry translates into superior longer-term survival requires longer follow-up and a larger sample size. Though statistically it was not possible to demonstrate tibial baseplate design superiority, the tibial loosening rate shown by both implants compares favourably at this timepoint in this patient group to that reported by other studies: non-stemmed 71.4% versus stemmed 100% at 4 years [13]; non-stemmed 93.4% versus stemmed 100% at minimum 2 years [14]. Other limitations of this study include its retrospective nature. The use of the surgeon’s discretion presents room for bias in the study. Since there were no significant differences between patient groups in terms of demographic or comorbidities, it is uncertain what factors influenced the surgeons to choose each respective implant. It remains unclear whether tibial component geometry or alignment is a more important risk factor for tibial failure in obesity as varus alignment of the limb and of the tibial component were associated with increased risk of return to theatre and revision.
Conclusions
This study suggests favourable early-to-mid-term outcomes for both standard keel and short stemmed tibial baseplate geometries as part of a cemented cruciate retaining TKA in patients with a BMI ≥ 40 kg/m2. Using this TKA system in severely obese patients, there was a single case of early tibial loosening, giving a 5-year Kaplan–Meier survival of 98.1% using the standard tibial baseplate and 100% using the universal baseplate.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality but are available from the corresponding author on reasonable request.
References
Zheng H, Chen C (2015) Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open 5:e007568. https://doi.org/10.1136/bmjopen-2014-007568
Weir CB, Jan A (2019) BMI classification percentile and cut off points. StatPearls, USA
Kerkhoffs GMMJ, Servien E, Dunn W, Dahm D, Bramer JAM, Haverkamp D (2012) The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review. J Bone Jt Surg 94:1839–1844. https://doi.org/10.2106/JBJS.K.00820
Elcock KL, Carter TH, Yapp LZ, MacDonald DJ, Howie CR, Stoddart A et al (2022) Total knee arthroplasty in patients with severe obesity provides value for money despite increased complications. Bone Joint J 104-B:452–463. https://doi.org/10.1302/0301-620X.104B4.BJJ-2021-0353.R3
Spicer D, Pomeroy D, Badenhausen W, Schaper JL, Curry J, Suthers K et al (2001) Body mass index as a predictor of outcome in total knee replacement. Int Orthop 25:246–249. https://doi.org/10.1007/s002640100255
Postler A, Lützner C, Beyer F, Tille E, Lützner J (2018) Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord 19:55. https://doi.org/10.1186/s12891-018-1977-y
Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J (2014) Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty 29:1774–1778. https://doi.org/10.1016/j.arth.2013.07.024
Le DH, Goodman SB, Maloney WJ, Huddleston JI (2014) Current modes of failure in TKA: infection, instability, and stiffness predominate. Clin Orthop Relat Res 472:2197–2200. https://doi.org/10.1007/s11999-014-3540-y
Scott CEH, Biant LC (2012) The role of the design of tibial components and stems in knee replacement. J Bone Joint Surg Br 94-B:1009–1015. https://doi.org/10.1302/0301-620X.94B8.28289
Abdel MP, Bonadurer GF, Jennings MT, Hanssen AD (2015) Increased aseptic tibial failures in patients with a BMI ≥ 35 and well-aligned total knee arthroplasties. J Arthroplasty 30:2181–2184. https://doi.org/10.1016/j.arth.2015.06.057
Elzohairy MM, Elaidy SM, Attia ME (2021) A comparative prospective study between stemmed versus an unstemmed tibial component in total knee arthroplasty in obese patients. Eur J Orthop Surg Traumatol 31:695–703. https://doi.org/10.1007/s00590-020-02816-x
Schultz BJ, DeBaun MR, Huddleston JI (2019) The use of stems for morbid obesity in total knee arthroplasty. J Knee Surg 32:607–610. https://doi.org/10.1055/s-0039-1681078
Garceau SP, Harris NH, Felberbaum DL, Teo GM, Weinblatt AI, Long WJ (2020) Reduced aseptic loosening with fully cemented short-stemmed tibial components in primary cemented total knee arthroplasty. J Arthroplasty 35:1591-1594.e3. https://doi.org/10.1016/J.ARTH.2020.01.084
Fournier G, Yener C, Gaillard R, Kenney R, Lustig S, Servien E (2020) Increased survival rate in extension stemmed TKA in obese patients at minimum 2 years follow-up. Knee Surg Sport Traumatol Arthrosc 28:3919–3925. https://doi.org/10.1007/s00167-020-05860-6
Completo A, Simões JA, Fonseca F (2006) The influence of stem design on strains and micromotion in revision total knee arthroplasty: finite element analysis. III Eur Conf Comput Mech 2008:182–182. https://doi.org/10.1007/1-4020-5370-3_182
Abraham R, Malkani AL, Lewis J, Beck D (2007) An anatomical study of tibial metaphyseal/diaphyseal mismatch during revision total knee arthroplasty. J Arthroplasty 22:241–244. https://doi.org/10.1016/j.arth.2006.06.001
Rabin R, de Charro F (2001) EQ-SD: a measure of health status from the EuroQol group. Ann Med 33:337–343. https://doi.org/10.3109/07853890109002087
Dawson J, Fitzpatrick R, Murray D, Carr A (1998) Questionnaire on the perceptions of patients about total knee replacement. J Bone Jt Surg 80:63–69. https://doi.org/10.1302/0301-620X.80B1.7859
Scott CEH, Howie CR, MacDonald D, Biant LC (2010) Predicting dissatisfaction following total knee replacement. J Bone Joint Surg Br 92-B:1253–1258. https://doi.org/10.1302/0301-620X.92B9.24394
Beard DJ, Harris K, Dawson J, Doll H, Murray DW, Carr AJ et al (2015) Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 68:73–79. https://doi.org/10.1016/j.jclinepi.2014.08.009
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251. https://doi.org/10.1016/0895-4356(94)90129-5
Sarmah SS, Patel S, Hossain FS, Haddad FS (2012) The radiological assessment of total and unicompartmental knee replacements. J Bone Joint Surg Br 94-B:1321–1329. https://doi.org/10.1302/0301-620X.94B10.29411
Morcos MW, Howard JL, Lanting B, MacDonald S, Naudie D, McCalden R et al (2021) Outcomes of stemmed versus un-stemmed varus-valgus constrained components in primary total knee arthroplasty. Orthop Res Rev 13:9–13. https://doi.org/10.2147/ORR.S290015
Parratte S, Ollivier M, Lunebourg A, Verdier N, Argenson JN (2017) Do stemmed tibial components in total knee arthroplasty improve outcomes in patients with obesity? Clin Orthop Relat Res 475:137–145. https://doi.org/10.1007/s11999-016-4791-6
Steere JT, Sobieraj MC, Defrancesco CJ, Israelite CL, Nelson CL, Kamath AF (2018) Prophylactic tibial stem fixation in the obese: comparative early results in primary total knee arthroplasty. Knee Surg Relat Res 30:227–233. https://doi.org/10.5792/ksrr.18.022
Garceau SP, Pivec R, Teo G, Chisari E, Enns PA, Weinblatt AI et al (2022) Increased rates of tibial aseptic loosening in primary cemented total knee arthroplasty with a short native tibial stem design. J Am Acad Orthop Surg 30:e640–e648. https://doi.org/10.5435/JAAOS-D-21-00536
Scott CEH, Clement ND, MacDonald DJ, Hamilton DF, Gaston P, Howie CR et al (2015) Five-year survivorship and patient-reported outcome of the Triathlon single-radius total knee arthroplasty. Knee Surgery, Sport Traumatol Arthrosc 23:1676–1683. https://doi.org/10.1007/s00167-014-2922-8
Scott CEH, Bell KR, Ng RT, MacDonald DJ, Patton JT, Burnett R (2019) Excellent 10-year patient-reported outcomes and survival in a single-radius, cruciate-retaining total knee arthroplasty. Knee Surg Sport Traumatol Arthrosc 27:1106–1115. https://doi.org/10.1007/s00167-018-5179-9
Xu J-L, Liang Z-R, Xiong B-L, Zou Q-Z, Lin T-Y, Yang P et al (2020) Correlation between body mass index and periprosthetic joint infection following total joint arthroplasty. Medicine (Baltimore) 99:e20549. https://doi.org/10.1097/MD.0000000000020549
Cox ZC, Green CC, Otero JE, Mason JB, Martin JR (2022) Varus collapse in total knee arthroplasty: does fixation or bone fail first? J Arthroplasty 37:162–167. https://doi.org/10.1016/j.arth.2021.09.012
Gunst S, Fessy M-H (2015) The effect of obesity on mechanical failure after total knee arthroplasty. Ann Transl Med 3:310. https://doi.org/10.3978/j.issn.2305-5839.2015.10.37
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
The authors received no funding for this research.
Author information
Authors and Affiliations
Contributions
K.E. generated the database, carried out the literature review for the background and discussion sections and wrote these sections. D.M. sought ethical approval, managed the database and provided technical support. N.C. reviewed the manuscript and provided valuable feedback and suggestions. C.S. designed the study, carried out the statistical analysis and wrote the methods and results sections of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Granted by Scotland A Research Committee. Reference number 16/SS/0026.
Consent for publication
Not applicable.
Competing interests
C.S. is a consultant for Stryker (Mahwah, New Jersey, USA) delivering education as part of courses. No funding has been received from Stryker for this project nor have they influenced it in any way. All other authors have no conflicts of interest to declare relating to any matters discussed or materials used in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elcock, K.L., MacDonald, D.J., Clement, N.D. et al. Total knee arthroplasty in patients with severe obesity: outcomes of standard keeled tibial components versus stemmed universal base plates. Knee Surg & Relat Res 35, 9 (2023). https://doi.org/10.1186/s43019-023-00184-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43019-023-00184-4