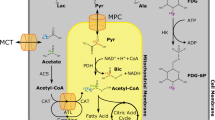
Abstract
Alterations in renal metabolism are associated with both physiological and pathophysiologic events. The existing noninvasive analytic tools including medical imaging have limited capability for investigating these processes, which potentially limits current understanding of kidney disease and the precision of its clinical diagnosis. Hyperpolarized 13C MRI is a new medical imaging modality that can capture changes in the metabolic processing of certain rapidly metabolized substrates, as well as changes in kidney function. Here we describe experimental protocols for renal metabolic [1-13C]pyruvate and functional 13C-urea imaging step-by-step. These methods and protocols are useful for investigating renal blood flow and function as well as the renal metabolic status of rodents in vivo under various experimental (patho)physiological conditions.
This chapter is based upon work from the COST Action PARENCHIMA, a community-driven network funded by the European Cooperation in Science and Technology (COST) program of the European Union, which aims to improve the reproducibility and standardization of renal MRI biomarkers. This experimental protocol is complemented by two separate chapters describing the basic concept and data analysis.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
The causal link between renal blood flow (RBF) and glomerular filtration rate (GFR), tubular reabsorption and thus oxygen/metabolic demand and the development of renal dysfunction is well-known [1]. Therefore, diagnostic modalities able to image RBF, GFR, and the metabolic effects have long been sought for.
One such method is hyperpolarized 13C MR, described in the chapter by von Morze C et al. “Hyperpolarized Carbon (13C) MRI of the Kidneys: Basic Concept.” In short, a transient enhancement of more than 10.000 times in spin polarization of a substrate molecule, such as [1-13C]pyruvate, is achieved by irradiating a supercooled mixture of 13C spins and electron spins with a microwave frequency obeying the nuclear Overhauser condition—and subsequently rapidly dissolved by superheated water [2]. The resulting mixture retains the high polarization and allows injection of metabolic and functional active molecules and subsequent imaging of the substrate and potential metabolic derivatives following cellular uptake and enzymatic conversion [3, 4]. We will describe basic experiments using hyperpolarized 13C MRS and MRI for monitoring of the metabolic conversion of [1-13C]pyruvate and the intra-renal distribution of 13C-urea in the kidney of rodents in a step-by-step experimental protocol. The rationale for choosing acquisition parameters is given in generic terms, together with specific parameter examples for 3 T, 4.7 T and 9.4 T MRI. Several imaging sequences to map renal metabolism and function are described as optional components of the experiment.
This experimental protocol chapter is complemented by two separate chapters describing the basic concept and data analysis, which are part of this book.
This chapter is part of the book Pohlmann A, Niendorf T (eds) (2020) Preclinical MRI of the Kidney—Methods and Protocols. Springer, New York.
2 Materials
2.1 Animals
This experimental protocol is tailored for rats (Wistar, Sprague-Dawley, or Lewis) with a body mass of 200–350 g. Advice for adaptation to mice is given as Notes where necessary.
2.2 Lab Equipment
-
1.
Anesthesia has been shown to impact renal metabolism using hyperpolarized MRI [5]; An appropriate and convenient choice is the use of gas anesthesia. For in-depth description, see the chapter by Kaucsar T et al. “Preparation and Monitoring of Small Animals in Renal MRI.”
-
2.
Gases: O2 with or without additional N2, as well as a gas-mixing system to achieve required blood oxygen saturation level.
-
3.
Hyperpolarizer equipment, typically the commercial preclinical polarizer HyperSense (Oxford Instruments Molecular Biotools, Oxford, United Kingdom) or newer variants of this design [6, 7] as well as the clinical intent polarizer, the so-called SPINLAB (GE Healthcare, Milwaukee, WI, USA).
-
4.
Vein or artery access, typically tail vein access for fast bolus infusion of the hyperpolarized substrate either by manual injection or by infusion pumps [7] (see Note 1).
-
5.
Phantom : A 13C containing phantom is needed to confirm center frequency and power calibration. A typical phantom used: 8 M 13C-urea (Sigma, St. Louis, MO, USA) in 90% H2O and 10% Glycerol with 3 μl/ml Gd contrast agent (Dotarem; Guerbet, Roissy, France) (see Note 2).
2.3 MRI Hardware
The general hardware requirements for renal 1H MRI on mice and rats are described in the chapter by Ramos Delgado P et al. “Hardware Considerations for Preclinical Magnetic Resonance of the Kidney.” The technique described in this chapter was tailored for a 3 T clinical system (GE Healthcare, Milwaukee, WI, USA) and a 9.4 Tesla MR system (Agilent, Yarnton, UK) but advice for adaptation to other field strengths is given where necessary. Additional hardware is required, for excitation and reception of 13C: X-band RF amplifiers and signal preamplifiers supporting the resonance frequency of 13C, 13C transmit and receive RF coils, such as 1H/13C volume RF coils [8,9,10] as well as transmit/receive switches adjusted to the resonance frequency of 13C.
-
1.
A physiological monitoring system that can track the respiration, and which is connected to the MR system such that it can be used to trigger the image acquisition.
3 Methods
3.1 MRI Protocol Setup
3.1.1 Prescan
-
1.
Single pulse and acquire sequence to identify 13C phantom frequency and calibration of the 13C transmitter power for a given pulse shape, width, and amplitude (see Note 3).
3.1.2 2D/3D Slice Selective Chemical Shift Imaging (CSI)
-
1.
Sequence type: 2D or 3D chemical shift imaging sequence . This is a standard sequence on preclinical MRI and clinical MRI systems [9, 11, 12].
-
2.
Repetition time (TR): choose the shortest possible with sufficient spectral resolution (which is inversely related to the duration of the data sampling window), for efficient temporal resolution.
-
3.
Echo time (TE): choose the shortest possible for good signal-to-noise and reduction of first order phase effects (see Note 4).
-
4.
Flip angle (FA): adapt to ensure a good compromise between SNR, T1 relaxation and sufficient signal following repeated excitations. FA is often a low flip angle of Θ = 5–10° leaving a factor of cos(θ)n longitudinal magnetization remaining, with n being the number of excitations (see Note 5).
-
5.
Acquisition bandwidth (BW): set to a value in the typically range of 4000–20,000 Hz, Number of acquisition points (NP): 256–2048 [9, 11, 12]. Choose BW and NP to ensure sufficient resolution and acquisition time of approximately 2.5 × T2*.
-
6.
K-space ordering: use conventional view ordering, which will result in the center of k-space being sampled late in the acquisition (see Note 6).
-
7.
For an example of a specific parameter set, please see Note 7.
3.1.3 2D/3D Echo-Planar Spectroscopic Imaging (EPSI)
-
1.
Sequence type: 2D or 3D echo-planar spectroscopic imaging (EPSI) with or without double spin echo refocusing. This approach superimposes an oscillating readout gradient onto CSI acquisition, replacing one of the phase encoding dimensions needed for CSI. This is not a standard sequence on most preclinical or clinical MRI systems [9, 13, 14].
-
2.
Spectral bandwidth (BW): set spectral BW in the order of 500–600 Hz. The EPSI readout is limited by gradient switching and thus only much lower spectral acquisition bandwidths than CSI are achievable, with outside resonances potentially aliasing into the spectral window. Spectral bandwidth can be traded for spatial resolution but is typically in the order of 500–600 Hz (see Note 8) for a spatial resolution of ~5 mm.
-
3.
Flip angle (FA): adapt to ensure a good compromise between SNR, T1 relaxation, and sufficient signal following repeated excitations. FA is often a low flip of Θ = 5–10° leaving a factor of cos(θ)n longitudinal magnetization remaining, with n being the number of excitations (see Note 5).
-
4.
Respiration trigger: can be added to improve the spatial localization.
-
5.
Geometry: adapt so that animal fits into FOV and both kidneys are covered. Axial or coronal directions are most often used.
-
6.
For an example of a specific parameter set, please see Note 9.
3.1.4 Multi-echo Gradient-Echo Sequence for IDEAL Mapping
-
1.
Sequence type: 2D multi-gradient echo sequence . This is a standard sequence on preclinical MRI systems; alternatively, single timepoint acquisitions such as EPI or spiral readout can be used [15,16,17,18] (see Note 10).
-
2.
Repetition time (TR): choose the shortest possible with sufficient distinct echo times, for highest temporal resolution.
-
3.
Flip angle (FA): adapt to ensure a good compromise between SNR, T1 relaxation and sufficient signal following repeated excitations. FA is often a low flip of Θ = 5–10° utilizing cos(θ)n of excitations of the nonrecoverable magnetization. n being the number of excitations.
-
4.
Echo time (TE): set the number of echoes to N + 1 echoes with N being the number of peaks to be resolved. TE is determined by the ability to separate the a priori known peaks to resolve. This can be calculated using the number of signal averages (NSA) [17]. A rule of thumb is that minimally N + 1 echoes are needed to accurately resolve N peaks.
-
5.
Acquisition bandwidth (BW): use a high BW to shorten ΔTE, to ensure good separation of the peaks according to NSA.
-
6.
Respiration trigger: on (per slice), can be added to improve the spatial localization.
-
7.
Geometry: adapt so that animal fits into FOV and both kidneys are covered. Axial or coronal directions are most often used.
-
8.
For an example of a specific parameter set, please see Note 11.
-
9.
Use double spin echo (DSE) or flow gradients to suppress flowing spins [19, 20] (optional).
3.1.5 Spectral-Spatial Imaging
-
1.
Sequence type: 2D spectral-spatial excitation with Cartesian, EPI or spiral trajectory readout [8, 21,22,23] or 3D sequences with or without slice selection and thus spectrally selective narrow band pulses [24] (see Note 12).
-
2.
Repetition time (TR): adapt to the respiration. If respiration is triggered, the effective TR will be given by the respiration trigger and will be the respiration interval, that is, one excitation per breath. Choose TR to be a little shorter (about 100 ms) than the average respiration interval that is displayed on the physiological monitoring unit (see Note 13).
-
3.
Flip angle (FA): use a low FA (in the range of 3–8°) on the substrate peak ([1-13C]pyruvate) and high FA (in the range of 30–90°) on the metabolic derivatives, to preserve magnetization for metabolic conversions allowing a longer temporal window in dynamic studies, and allowing saturation recovery kinetics to be fitted.
-
4.
For an example of a specific parameter set, please see Note 14.
3.1.6 Perfusion Imaging
-
1.
Sequence type: 2D/3D balanced steady state free precession (bSSFP) sequence [25,26,27].
-
2.
Repetition time (TR): use shortest possible TR for high temporal resolution. If triggering respiration, adapt to the respiration. The effective TR will be given by the respiration trigger and will be the respiration interval, that is, one frame per breath. Ideally choose NP × TR to be shorter (about 100 ms) than the average respiration interval that is displayed on the physiological monitoring unit.
-
3.
For perfusion assessment use full saturation of the inflowing spins or (optional) a lower flip angle (in the range of 10–30°) [28, 29].
-
4.
Use preparation pulses (optional) to improve magnetization utilization; typically, α/2 pulses before and after the bSSFP train to achieve faster pseudo-steady state and to retain the magnetization for subsequent scans [30].
-
5.
For an example of a specific parameter set, please see Table 1.
3.2 In Vivo 13C MR
3.2.1 Preparations
-
1.
Anesthetize the animal and transfer it to the scanner.
-
2.
Place 13C enriched phantom in the FOV (see Note 15).
-
3.
Set up the temperature monitoring (rectal probe) and respiratory monitoring (balloon on chest) unit.
-
4.
Perform anatomical imaging as described in the chapter by Pohlmann A et al. “Essential Practical Steps for MRI of the Kidney in Experimental Research.”
-
5.
Perform localized shimming on the kidney imaging as described in the chapter by Pohlmann A et al. “Essential Practical Steps for MRI of the Kidney in Experimental Research” (see Note 16).
-
6.
Perform 13C frequency calibration and 13C power calibration (see Note 17).
3.2.2 Hyperpolarized 13C Metabolic and Functional Imaging
-
1.
Load the 13C sequence of choice, adapt the slice orientation to provide a coronal or axial view with respect to the kidney (in scanner coordinates this is double-oblique).
-
2.
Triggering (13C): in the monitoring unit set the trigger delay so that the trigger starts at the beginning of the expiratory plateau (no chest motion) and the duration such that it covers the entire expiratory phase, that is, until just before inhalation starts.
-
3.
Run the 13C imaging scan (see Note 18). Examples of images are shown in Figs. 1, 2, 3, and 4.
3.3 Emerging Sequences
Several novel alternative strategies have been proposed for various imaging applications and just to mention a few there are control of the inflowing spins with bolus tracking [31, 32], spatiotemporal encoding [33], improved resolution by spatially shaped excitations [32, 34]. Variations of the balanced steady state sequences [35, 36] hold potential for further improvement by optimally designing the imaging strategy toward the long T2’s seen in most 13C molecules. Relaxation contrast is another potential renal contrast mechanism, showing substantial promise to improve the diagnostic potential of hyperpolarized MR [37,38,39,40].
4 Notes
-
1.
In order to allow optimal usage of the substrate, keep dead-volume as small as possible for injection line; injection speed is typically 0.1 ml/s and up to 2 ml for a rat. If the sequence is single timepoint imaging method or flow artifacts are corrupting the images with a minimum of 20 s time delay from start of injection it is an appropriate choice for intrarenal metabolism and function of the rodent kidneys.
-
2.
Ensure short enough T1 of the 13C phantom to allow rapid frequency and power calibration. Caution, due to B0 variation the reference 13C peak is not always representative of the in vivo position.
-
3.
Frequency calibration and power calibration can be done in a similar manner as any 1H experiment with a centering on the phantom peak and running a power calibration using a standard nutation experiment. Calibrating with a 180 pulse is desirable as the signal changes more rapidly as a function of applied power than with a 90 pulse. Alternatively, a Bloch–Siegert shift method can be used [41].
-
4.
As the RF pulse length contributes to the TE and the RF flip often is very low, it is often possible to shorten the TE by reducing the RF pulse length.
-
5.
Signal decay over the phase encoding steps, either due to T1 decay or consumption of magnetization due to RF pulses (i.e., T2). Constant flip angle excitation constitutes a k-space filter that can introduce significant image blurring. Alternatively, an increasing flip angle can be employed to compensate for such effects [42].
-
6.
A variety of CSI sampling patterns can be adopted to optimize temporal constraints, SNR, and partial volume effects [9].
-
7.
Example for a 300 g rat at 9.4 T: TR = 70 ms; TE = 0.68 ms, flip angle = 10°; pulse length 500 us; receiver bandwidth = 8 kHz; number of points = 512 complex points (i.e., 1024), averages = 1; slice orientation = axial oblique; FOV = (60 × 60) mm; matrix size = 16 × 16 zero-filled to 32 × 32; 1 slice with 1–2 cm thickness; TA = 17 s (without triggering).
-
8.
The low bandwidth will typically require prior knowledge of the peaks of interest and their folding patterns to fully recover the spectral information [9].
-
9.
Example 3D EPSI for a 300 g rat at 3 T: An EPSI sequence with double spin echo refocusing (13). Images are acquired with (8 × 8 × 8) mm3 voxel size and a matrix size of 18 × 10 × 8, coronal view, with a TR of 215 ms, TE of 140 ms, and a spectral width 543 Hz.
-
10.
The multispectral signals are resolved by fitting to a signal model with unknown components at known frequency offsets. B0 offset is typically an important component of the model and is fitted along with the spectral components or estimated separately (see https://github.com/LarsonLab/hyperpolarized-mri-toolbox).
-
11.
Example: 2D IDEAL-spiral for a 300 g rat at 3 T (18): TR = 100 ms; TE = 0.9 ms; flip angle = 10°; 11 IDEAL echoes with ΔTE values 0.9 ms, determined by the NSA formalism to resolve [1-13C]pyruvate, [1-13C]lactate, [1-13C]alanine, [1-13C]pyruvate hydrate, [13C]bicarbonate. Slice orientation = axial oblique; FOV = (80 × 80) mm2; matrix size = 32 × 32; 5 mm × 5 mm real spatial resolution; 1 slice with 1–2 cm thickness covering both kidneys. Dynamic acquisition over 60–80 s.
-
12.
Offset frequencies for individual metabolites must be specified a priori. It is important to note that the frequency might be different than the in vivo version of a given phantom metabolite and thus it is important to verify the correct frequency.
-
13.
Monitor the respiration continuously throughout the entire experiment and if necessary adapt the TR accordingly for the 13C examination.
-
14.
Example: 2D spectral-spatial for a 300 g rat at 3 T (20): TR = 1 s; TE = 10 ms; flip angle = 90° on [1-13C]lactate, flip angle = 15° on [1-13C]pyruvate;, flip angle = 90° on [13C]bicarbonate and flip angle = 90° on [1-13C]alanine; slice orientation = axial oblique; FOV = (80 × 80) mm; matrix size = 32 × 32; 5 mm × 5 mm real spatial resolution; 1 slice with 1–2 cm thickness covering both kidneys. Dynamic acquisition over 60–80 s.
-
15.
Be careful with the frequency shift between in vivo and ex vivo phantom conditions.
-
16.
B0 shimming is particularly important, since macroscopic magnetic field inhomogeneities shorten T2*, and potentially shift the peak positions relative to the main magnetic field. Shimming should be performed on a voxel enclosing only the kidney using either the default iterative shimming method or manual shimming.
-
17.
It is often possible to retain a similar B1 amplitude for similar sized animals. 13C Tx gain and Tx/Rx frequency is largely similar in rats and mice and thus the differences should be small between experiments (typically below 1 dB and 50 Hz).
-
18.
A good starting point is to use the same relative resolution as for rats. For this, reduce the FOV to the mouse body width and keep the matrix size the same.
References
Blantz RC, Deng A, Miracle CM, Thomson SC (2007) Regulation of kidney function and metabolism: a question of supply and demand. Trans Am Clin Climatol Assoc 118:23–43
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 100(18):10158–10163. https://doi.org/10.1073/pnas.1733835100
Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH, Gallagher FA, Keshari KR, Kjaer A, Laustsen C, Mankoff DA, Merritt ME, Nelson SJ, Pauly JM, Lee P, Ronen S, Tyler DJ, Rajan SS, Spielman DM, Wald L, Zhang X, Malloy CR, Rizi R (2018) Hyperpolarized (13)C MRI: path to clinical translation in oncology. Neoplasia 21(1):1–16. https://doi.org/10.1016/j.neo.2018.09.006
Laustsen C (2016) Hyperpolarized renal magnetic resonance imaging: potential and pitfalls. Front Physiol 7:72. https://doi.org/10.3389/fphys.2016.00072
Qi H, Mariager CO, Lindhardt J, Nielsen PM, Stodkilde-Jorgensen H, Laustsen C (2018) Effects of anesthesia on renal function and metabolism in rats assessed by hyperpolarized MRI. Magn Reson Med 80(5):2073–2080. https://doi.org/10.1002/mrm.27165
Ardenkjaer-Larsen JH, Bowen S, Petersen JR, Rybalko O, Vinding MS, Ullisch M, Nielsen NC (2018) Cryogen-free dissolution dynamic nuclear polarization polarizer operating at 3.35 T, 6.70 T, and 10.1 T. Magn Reson Med. https://doi.org/10.1002/mrm.27537
Comment A, van den Brandt B, Uffmann K, Kurdzesau F, Jannin S, Konter JA, Hautle P, Wenckebach WT, Gruetter R, van der Klink JJ (2007) Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn Reson B Magn Reson Eng 31B(4):255–269. https://doi.org/10.1002/cmr.b.20099
Xu T, Mayer D, Gu M, Yen YF, Josan S, Tropp J, Pfefferbaum A, Hurd R, Spielman D (2011) Quantification of in vivo metabolic kinetics of hyperpolarized pyruvate in rat kidneys using dynamic 13C MRSI. NMR Biomed 24(8):997–1005. https://doi.org/10.1002/nbm.1719
Yen YF, Kohler SJ, Chen AP, Tropp J, Bok R, Wolber J, Albers MJ, Gram KA, Zierhut ML, Park I, Zhang V, Hu S, Nelson SJ, Vigneron DB, Kurhanewicz J, Dirven HA, Hurd RE (2009) Imaging considerations for in vivo 13C metabolic mapping using hyperpolarized 13C-pyruvate. Magn Reson Med 62(1):1–10. https://doi.org/10.1002/mrm.21987
Bertelsen LB, Nielsen PM, Qi H, Norlinger TS, Zhang X, Stodkilde-Jorgensen H, Laustsen C (2016) Diabetes induced renal urea transport alterations assessed with 3D hyperpolarized 13 C,15 N-Urea. Magn Reson Med. https://doi.org/10.1002/mrm.26256
Laustsen C, Østergaard JA, Lauritzen MH, Nørregaard R, Bowen S, Søgaard LV, Flyvbjerg A, Pedersen M, Ardenkjær-Larsen JH (2013) Assessment of early diabetic renal changes with hyperpolarized [1-13C]pyruvate. Diabetes Metab Res Rev 29(2):125–129. https://doi.org/10.1002/dmrr.2370
Laustsen C, Hansen ES, Kjaergaard U, Bertelsen LB, Ringgaard S, Stodkilde-Jorgensen H (2015) Acute porcine renal metabolic effect of endogastric soft drink administration assessed with hyperpolarized [1-13c]pyruvate. Magn Reson Med. https://doi.org/10.1002/mrm.25692
Ohliger MA, von Morze C, Marco-Rius I, Gordon J, Larson PEZ, Bok R, Chen HY, Kurhanewicz J, Vigneron D (2017) Combining hyperpolarized (13) C MRI with a liver-specific gadolinium contrast agent for selective assessment of hepatocyte metabolism. Magn Reson Med 77(6):2356–2363. https://doi.org/10.1002/mrm.26296
Cunningham CH, Chen AP, Albers MJ, Kurhanewicz J, Hurd RE, Yen YF, Pauly JM, Nelson SJ, Vigneron DB (2007) Double spin-echo sequence for rapid spectroscopic imaging of hyperpolarized 13C. J Magn Reson 187(2):357–362. https://doi.org/10.1016/j.jmr.2007.05.014
Leupold J, Månsson S, Stefan Petersson J, Hennig J, Wieben O (2009) Fast multiecho balanced SSFP metabolite mapping of 1H and hyperpolarized 13C compounds. Magn Reson Mater Phys 22(4):251–256
Niles DJ, Gordon JW, Huang G, Reese S, Adamson EB, Djamali A, Fain SB (2018) Evaluation of renal metabolic response to partial ureteral obstruction with hyperpolarized (13) C MRI. NMR Biomed 31(1). https://doi.org/10.1002/nbm.3846
Wiesinger F, Weidl E, Menzel MI, Janich MA, Khegai O, Glaser SJ, Haase A, Schwaiger M, Schulte RF (2012) IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn Reson Med 68(1):8–16. https://doi.org/10.1002/mrm.23212
Qi H, Nielsen PM, Schroeder M, Bertelsen LB, Palm F, Laustsen C (2018) Acute renal metabolic effect of metformin assessed with hyperpolarised MRI in rats. Diabetologia 61(2):445–454. https://doi.org/10.1007/s00125-017-4445-6
Josan S, Yen Y-F, Hurd R, Pfefferbaum A, Spielman D, Mayer D (2011) Application of double spin echo spiral chemical shift imaging to rapid metabolic mapping of hyperpolarized [1-13C]-pyruvate. J Magn Reson 209(2):332–336. https://doi.org/10.1016/j.jmr.2011.01.010
Gordon JW, Niles DJ, Adamson EB, Johnson KM, Fain SB (2016) Application of flow sensitive gradients for improved measures of metabolism using hyperpolarized (13) c MRI. Magn Reson Med 75(3):1242–1248. https://doi.org/10.1002/mrm.25584
Shang H, Sukumar S, von Morze C, Bok RA, Marco-Rius I, Kerr A, Reed GD, Milshteyn E, Ohliger MA, Kurhanewicz J, Larson PEZ, Pauly JM, Vigneron DB (2017) Spectrally selective three-dimensional dynamic balanced steady-state free precession for hyperpolarized C-13 metabolic imaging with spectrally selective radiofrequency pulses. Magn Reson Med 78(3):963–975. https://doi.org/10.1002/mrm.26480
Schulte RF, Sperl JI, Weidl E, Menzel MI, Janich MA, Khegai O, Durst M, Ardenkjaer-Larsen JH, Glaser SJ, Haase A, Schwaiger M, Wiesinger F (2013) Saturation-recovery metabolic-exchange rate imaging with hyperpolarized [1-13C] pyruvate using spectral-spatial excitation. Magn Reson Med 69(5):1209–1216. https://doi.org/10.1002/mrm.24353
Lau AZ, Chen AP, Hurd RE, Cunningham CH (2011) Spectral-spatial excitation for rapid imaging of DNP compounds. NMR Biomed 24(8):988–996. https://doi.org/10.1002/nbm.1743
Eichhorn TR, Takado Y, Salameh N, Capozzi A, Cheng T, Hyacinthe JN, Mishkovsky M, Roussel C, Comment A (2013) Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. Proc Natl Acad Sci U S A 110(45):18064–18069. https://doi.org/10.1073/pnas.1314928110
von Morze C, Bok RA, Sands JM, Kurhanewicz J, Vigneron DB (2012) Monitoring urea transport in rat kidney in vivo using hyperpolarized 13C magnetic resonance imaging. Am J Physiol Ren Physiol. https://doi.org/10.1152/ajprenal.00640.2011
Qi H, Norlinger TS, Nielsen PM, Bertelsen LB, Mikkelsen E, Xu Y, Stodkilde Jorgensen H, Laustsen C (2016) Early diabetic kidney maintains the corticomedullary urea and sodium gradient. Physiol Rep 4(5). https://doi.org/10.14814/phy2.12714
Nielsen PM, Szocska Hansen ES, Norlinger TS, Norregaard R, Bonde Bertelsen L, Stodkilde Jorgensen H, Laustsen C (2016) Renal ischemia and reperfusion assessment with three-dimensional hyperpolarized (13) C,(15) N2-urea. Magn Reson Med 76(5):1524–1530. https://doi.org/10.1002/mrm.26377
Johansson E, Olsson LE, Månsson S, Petersson JS, Golman K, Ståhlberg F, Wirestam R (2004) Perfusion assessment with bolus differentiation: a technique applicable to hyperpolarized tracers. Magn Reson Med 52(5):1043–1051. https://doi.org/10.1002/mrm.20247
von Morze C, Larson PE, Hu S, Keshari K, Wilson DM, Ardenkjaer-Larsen JH, Goga A, Bok R, Kurhanewicz J, Vigneron DB (2011) Imaging of blood flow using hyperpolarized [(13)C]urea in preclinical cancer models. J Magn Reson Imaging 33(3):692–697. https://doi.org/10.1002/jmri.22484
Svensson J, Mansson S, Johansson E, Petersson JS, Olsson LE (2003) Hyperpolarized 13C MR angiography using trueFISP. Magn Reson Med 50(2):256–262. https://doi.org/10.1002/mrm.10530
Durst M, Koellisch U, Gringeri C, Janich MA, Rancan G, Frank A, Wiesinger F, Menzel MI, Haase A, Schulte RF (2014) Bolus tracking for improved metabolic imaging of hyperpolarised compounds. J Magn Reson (San Diego, CA: 1997) 243:40–46. https://doi.org/10.1016/j.jmr.2014.02.011
Tang S, Jiang W, Chen HY, Bok R, Vigneron DB, Larson PE (2015) A 2DRF pulse sequence for bolus tracking in hyperpolarized 13C imaging. Magn Reson Med 74(2):506–512. https://doi.org/10.1002/mrm.25427
Schmidt R, Laustsen C, Dumez J-N, Kettunen MI, Serrao EM, Marco-Rius I, Brindle KM, Ardenkjaer-Larsen JH, Frydman L (2014) In vivo single-shot 13C spectroscopic imaging of hyperpolarized metabolites by spatiotemporal encoding. J Magn Reson 240:8–15. https://doi.org/10.1016/j.jmr.2013.12.013
Vinding MS, Laustsen C, Maximov II, Sogaard LV, Ardenkjaer-Larsen JH, Nielsen NC (2013) Dynamic nuclear polarization and optimal control spatial-selective 13C MRI and MRS. J Magn Reson (San Diego, CA: 1997) 227:57–61. https://doi.org/10.1016/j.jmr.2012.12.002
Varma G, Wang X, Vinogradov E, Bhatt RS, Sukhatme VP, Seth P, Lenkinski RE, Alsop DC, Grant AK (2016) Selective spectroscopic imaging of hyperpolarized pyruvate and its metabolites using a single-echo variable phase advance method in balanced SSFP. Magn Reson Med 76(4):1102–1115. https://doi.org/10.1002/mrm.26004
Mansson S, Petersson JS, Scheffler K (2012) Fast metabolite mapping in the pig heart after injection of hyperpolarized 13C-pyruvate with low-flip angle balanced steady-state free precession imaging. Magn Reson Med 68(6):1894–1899. https://doi.org/10.1002/mrm.24183
Reed GD, von Morze C, Bok R, Koelsch BL, Van Criekinge M, Smith KJ, Hong S, PEZ L, Kurhanewicz J, Vigneron DB (2014) High resolution 13C MRI with hyperpolarized urea: in vivo T2 mapping and 15N labeling effects. IEEE Trans Med Imaging 33(2):362–371. https://doi.org/10.1109/TMI.2013.2285120
Reed GD, von Morze C, Verkman AS, Koelsch BL, Chaumeil MM, Lustig M, Ronen SM, Sands JM, Larson PEZ, Wang ZJ, Ardenkjær Larsen JH, Kurhanewicz J, Vigneron DB (2015) Imaging renal urea handling in rats at millimeter resolution using hyperpolarized magnetic resonance relaxometry. ArXiv 1511:200
Laustsen C, Stokholm Norlinger T, Christoffer Hansen D, Qi H, Mose Nielsen P, Bonde Bertelsen L, Henrik Ardenkjaer-Larsen J, Stodkilde Jorgensen H (2015) Hyperpolarized C urea relaxation mechanism reveals renal changes in diabetic nephropathy. Magn Reson Med. https://doi.org/10.1002/mrm.26036
Qi H, Mariager CO, Nielsen PM, Schroeder M, Lindhardt J, Norregaard R, Klein JD, Sands JM, Laustsen C (2019) Glucagon infusion alters the hyperpolarized (13) C-urea renal hemodynamic signature. NMR Biomed 32(1):e4028. https://doi.org/10.1002/nbm.4028
Schulte RF, Sacolick L, Deppe MH, Janich MA, Schwaiger M, Wild JM, Wiesinger F (2011) Transmit gain calibration for nonproton MR using the Bloch-Siegert shift. NMR Biomed 24(9):1068–1072. https://doi.org/10.1002/nbm.1657
Zhao L, Mulkern R, Tseng CH, Williamson D, Patz S, Kraft R, Walsworth RL, Jolesz FA, Albert MS (1996) Gradient-echo imaging considerations for hyperpolarized 129Xe MR. J Magn Reson B 113:179–183
Acknowledgments
This chapter is based upon work from COST Action PARENCHIMA, supported by European Cooperation in Science and Technology (COST). COST (www.cost.eu) is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to enrich their ideas by sharing them with their peers. This boosts their research, career, and innovation.
PARENCHIMA (renalmri.org) is a community-driven Action in the COST program of the European Union, which unites more than 200 experts in renal MRI from 30 countries with the aim to improve the reproducibility and standardization of renal MRI biomarkers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Laustsen, C., von Morze, C., Reed, G.D. (2021). Hyperpolarized Carbon (13C) MRI of the Kidney: Experimental Protocol. In: Pohlmann, A., Niendorf, T. (eds) Preclinical MRI of the Kidney. Methods in Molecular Biology, vol 2216. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0978-1_29
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0978-1_29
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0977-4
Online ISBN: 978-1-0716-0978-1
eBook Packages: Springer Protocols