Abstract
Crop protection from algal grazers is a key area of concern, as grazing zooplankton and flagellates can decimate microalgae crops and impede economic viability of cultivation for biofuels and bioproducts. Inhibition of grazing by chemical and physical interference is one promising solution; however, there have been few empirical tests of this approach that use defense traits innate to algal crop species. Botryococcus braunii is of particular interest because a) it excretes high levels of hydrocarbons and exopolysaccharides and b) forms colonies and possesses chemical defenses. Here we conduct a controlled laboratory experiment to test whether B. braunii can mitigate losses to grazing by two distinct grazers, Daphnia magna and Poterioochromonas malhamensis, due to both chemical inhibition and physical interference linked to large/inedible colonies. We show that chemical and physical defenses interactively reduce the total effect of grazing, thus significantly increasing the biomass and growth rates of cultures of B. braunii and Nannochloropsis limnetica when either grazer is present. We also find that B. braunii medium enhances the growth of N. limnetica. Our study demonstrates how community engineering can identify synergies arising from algal co-cultivation (e.g., by using industrially relevant strains for crop protection). While our lab study serves as a proof-of-concept, future research should test this strategy at pilot scale; if successful, such ecological discoveries may help to reduce the costs of large-scale deployment of algal cultivation for sustainable foods, fuels, bioproducts (e.g., bioplastics), and carbon capture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protecting microalgal crops from losses to grazers, parasites, and pathogens is a key area of concern, as these organisms can cause rapid pond crashes that threaten the sustainability and economic viability of algae cultivation for bioproducts (Day et al. 2017; Lane 2022). In general, the most productive, fastest-growing microalgae (i.e., common targets for bioproducts) have small cell sizes which are optimal for maximizing resource uptake and thus growth rates, but are also highly susceptible to grazing by zooplankton including flagellates, rotifers, and cladocerans (Hillebrand et al. 2022). Specifically, taxa including Chlorella, Nannochloropsis, and Scenedesmus are promising due to their high productivity of lipids, proteins, and carbohydrates which can be used for a variety of products (e.g., animal feed, omega-3 fatty acid supplements, biofuels, bioplastics). However, they are also highly susceptible to grazing when grown in unprotected monocultures (Day et al. 2017; Ma et al. 2017; Thomas et al. 2017, 2019); this can create a trade-off between baseline productivity and grazing resistance capacity. There are several described strategies to overcome this trade-off between maximum productivity and increased risk of pond crash due to grazing or parasitism. Specifically, grazers and fungi can be controlled via external inputs like pesticides and fungicides (McBride et al. 2014; Van Ginkel et al. 2020; McGowen et al. 2023) or can be managed by applying pH, ammonia, or salinity changes that inhibit pests but not algae (Day et al. 2017; Ma et al. 2017; Thomas et al. 2017). However, using one of these solutions in isolation is unlikely to exhibit long-term efficacy against all aquatic grazers and parasites, because biological pest populations can respond adaptively, and their communities are dynamic. This calls for using complementary adaptive management strategies to stabilize large-scale production. Ideally, these interventions will not increase operational costs, energy input, or risk of pesticide release to the environment.
One approach with the potential to stabilize annual productivity is to use designed polycultures, such that different algae species or strains with complementary traits can overcome trade-offs faced by monocultures (Shurin et al. 2013; Kazamia et al. 2014; Nalley et al. 2014; Newby et al. 2016; Thomas et al. 2019), thus simultaneously maintaining baseline productivity and preventing pond crash. This can take a variety of forms, including, e.g., using chemically-defended strains (Bacellar Mendes and Vermelho 2013) or large-celled/colonial strains (Corcoran and Boeing 2012) to act as an “associational refuge” capable of protecting undefended but faster-growing strains (Shurin et al. 2013). Despite the promise of using chemically/physically defended strains for crop protection, we currently lack experimental evidence for the efficacy of this approach using industrially relevant strains.
One species of intensive focus in the algae industry is Botryococcus braunii due to its high production of extracellular hydrocarbons which can be non-destructively extracted for liquid biofuels (i.e., “algae milking”) (Moheimani et al. 2013; Griehl et al. 2015; Ennaceri et al. 2023) as well as its high excretion of exopolysaccharides in certain strains (Fernandes et al. 1989; Blifernez-Klassen et al. 2018). However, B. braunii may also have untapped potential in its role for protecting undefended algal crops. Shurin et al. (2013) indeed suggested this possibility for crop protection due to B. braunii’s ability to chemically inhibit other zooplankton and algae by secreting inhibitory levels of free fatty acids into the medium (Chiang et al. 2004), as well as its large colonies offering physical protection from grazing. Therefore, an important opportunity in designing robust algal communities is to test whether B. braunii can inhibit grazers without inhibiting undefended target algae (e.g., Nannochloropsis).
In this study we tested whether B. braunii inhibits two common and important freshwater grazers and one algal species via both chemical and physical interference. We used Daphnia magna and Poterioochromonas malhamensis as our two taxonomically and functionally distinct grazer species, and Nannochloropsis limnetica as a small (< 4 µm diameter), fast-growing target alga for co-culture with B. braunii. Daphnia magna was chosen as a representative large-bodied (length < 1 mm as juvenile; 1–5 mm as adult), filter-feeding arthropod zooplankton grazer with a larger prey size, while the golden alga Poterioochromonas was chosen as a representative small-celled (< 10 µm diameter) flagellate with a smaller prey size that consumes cells via phagocytosis; both are common worldwide and are capable of strong top-down control of algal biomass (Day et al. 2017; Ma et al. 2023). We experimentally tested the following hypotheses: H1) B. braunii reduces algal biomass losses due to grazing via a combination of chemical and physical interference; and H2) B. braunii does not chemically inhibit N. limnetica growth.
Materials and methods
Algae and zooplankton cultures
The strain of Botryococcus braunii used in this experiment was isolated from a bloom in Eawag’s experimental ponds (Dübendorf, Switzerland, 47°24′18.2″ N 8°36′31.7″ E) in April 2023. According to partial 18S sequence matches it belongs to the A-clade and is closely related to previously described strains including, e.g., OIT-560, OIT-284, and CCAP 807/1. It also exhibits traits similar to some B-clade strains of B. braunii such as flotation, which is linked to high hydrocarbon content. Poterioochromonas malhamensis (CCAP 933/1C) and Nannochloropsis limnetica (SAG 18.99) were obtained from culture collections. Daphnia magna was obtained from a clonal culture maintained for several years at Eawag and was fed N. limnetica as a food source in the weeks leading up to the experiment. Prior to the experiment, all algae and grazer cultures were maintained at 20 °C in COMBO medium with double the concentration of all nutrients described in the original medium recipe (Kilham et al. 1998) to allow for higher algal biomass density (hereafter referred to as 2X COMBO).
Experimental design
For each of the three grazer treatments (grazer-free control, P. malhamensis, D. magna), there were four B. braunii treatments: 1) control (no B. braunii); 2) B. braunii culture filtrate only, representing only chemical interference; 3) B. braunii colonies only, representing primarily physical interference; and 4) B. braunii colonies and medium together, representing a combination of both chemical and physical interference of grazing. Treatment 2 (B. braunii filtrate only) was made by collecting 0.2-µm filtered medium (i.e., filtrate from Treatment 3) and individually adding 2X COMBO stock nutrients and vitamins to ensure nutrients were initially in excess. Treatment 3 (B. braunii colonies) was made by filtering B. braunii culture through a 0.2-µm PES Stericup filter and resuspending colonies in fresh 2X COMBO medium. Treatment 4 (colonies and medium) was created by adding 2X COMBO stock nutrients and vitamins directly to a dense culture of B. braunii. Specifically, for Treatments 3 and 4, B. braunii from stationary phase cultures was added at a constant density of 1498 ± 264 SD relative fluorescence units (RFU), equal to 1.1 × 104 colonies mL−1. It should be noted that Treatment 3 (colonies only) isolates the effects of physical interference only earlier in the experiment, while later it likely also represents chemical interference as B. braunii metabolites accumulate in the medium (i.e., previous work finds that B. braunii extracellular products are relatively low in the first week of growth and peak after around 15 days (Blifernez-Klassen et al. 2018)). Treatments 2 and 4 are meant to represent the full chemical fingerprint of B. braunii medium. This chemical fingerprint includes exuded metabolites of B. braunii that have been previously described, including free fatty acids, hydrocarbons, and exopolysaccharides (Chiang et al. 2004; Blifernez-Klassen et al. 2018; Ennaceri et al. 2023); it also includes changes in inorganic chemical parameters such as pH. While it was not feasible to measure pH in the experimental units, the pH of stationary phase B. braunii culture medium used in the experiment was near 9 (compared to pH 8 in the control COMBO medium). We thus cannot entirely rule out pH effects, but we assume that this difference in pH has minimal influence and that chemical effects are driven largely by exuded metabolites.
Aliquots from a stationary phase N. limnetica culture were added at equal densities (153 ± 15 SD RFU, equal to 3.1 × 105 cells mL−1) to each of the four B. braunii treatments. These mixtures were dispensed into sterile 24-well plates with a volume of 2 mL per well, randomized order in well plates, and 6 replicates per treatment (except for 1 rep. lost in the P. malhamensis treatment), in Infors incubators set to 20 °C, rotating at 100 rpm, with 12h:12h light:dark cycle and 104 ± 3.2 µmol photons m−2 s−1. Juvenile D. magna of similar age and size were used for the experiment, with one individual per well; 100 µL of stationary phase P. malhamensis culture was added to each well (i.e., a 5% v/v dilution yielding 4.5 × 104 cells mL−1). These grazer densities were chosen as pre-trials showed that they were sufficient to exert control on biomass. Growth was tracked using in vivo chlorophyll fluorescence (recorded in RFU) as a proxy for total algal biomass, with excitation/emission at 460/685 nm respectively, using a Biotek Cytation 5 plate reader. The maximum fluorescence value achieved over time was used as a metric for the highest total algal biomass achieved during the experiment for each experimental unit. Note that such fluorescence values capture chlorophyll present in N. limnetica as well as B. braunii and, to a lesser extent, P. malhamensis, which also yields a relatively weak chl-a fluorescence signal due to its lower chlorophyll content. As chlorophyll content can change with environmental conditions or cell health, fluorescence may not linearly relate to cell counts or biomass; however, we interpret fluorescence as indicative of total photosynthetically active biomass, which may be more directly related to the density of live and metabolically active algae. The experiment was conducted for a total of 18 days. We report algal growth for the first 3 days for D. magna treatments, as D. magna survival, which was recorded daily in experimental units via microscopy, was high during this period (96%) reflecting an effective grazing treatment and was sufficient to observe differences across treatments (Fig. S1). However, D. magna survival gradually declined after day 3 across all B. braunii treatments, thus reducing efficacy of the grazing treatment. We report 18 days of growth for the other grazing treatments. On day 18, Lugol-preserved samples were collected for estimating final cell densities. From these preserved samples, cell counts were obtained by imaging with a Leica DMi8 microscope; cells were then enumerated in ImageJ v1.52a. Final cell counts were obtained for the target algae species, N. limnetica, and for P. malhamensis. Growth rates were calculated as ln(RFUday3/RFUday0)/3 to capture the initial change in fluorescence during nutrient-replete exponential growth over the first 3 days (i.e., while nutrients are in excess in the first few days, we are more certain that effects are due to physical/chemical defenses and not due to differences in nutrient levels). Effects of grazers on growth rate and biomass were interpreted in relation to the respective B. braunii treatment with grazers absent, as this allows us to more directly quantify the net effect of grazing on total algal biomass. For example, the effect of Daphnia on N. limnetica biomass (as % change) when grown in B. braunii filtrate is:
where BD = biomass with Daphnia and in B. braunii filtrate and B0 = biomass with no grazer and in B. braunii filtrate.
2-way ANOVAs were used to test for the individual and interactive effects of chemical and physical interference on the growth rate and biomass of all treatments; detailed ANOVA results are in Table S1; Tukey HSD was used to test for differences between individual treatments. Statistics were performed in R version 4.2.2 (R Core Team 2020). All data and code are available on Zenodo (https://doi.org/10.5281/zenodo.13237033).
Results
Botryococcus braunii inhibits grazing by Daphnia magna through both chemical and physical interference
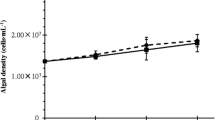
In the control treatment with no B. braunii present, D. magna rapidly reduced N. limnetica biomass (Fig. 1A) over the first three days, with a mean 62% decrease in max. chl-a fluorescence (Fig. 2A; 74.4% loss if one outlier is excluded). Conversely, the presence of B. braunii filtrate inhibited grazing by D. magna, allowing N. limnetica density to increase despite the presence of the grazers, though max. chl-a fluorescence was still significantly reduced (mean 29% loss compared to grazer-free controls). The treatments applying B. braunii colonies and colonies + medium provided even further protection from D. magna grazing, with total algal biomass (i.e., B. braunii and N. limnetica combined) losses of only 4.6% and 10.1%, respectively, compared to grazer-free controls). The 95% CIs overlapping zero indicate no significant declines in biomass (Fig. 2A) or growth rate (Fig. 2C) when B. braunii colonies are present. ANOVA revealed significant individual effects of adding B. braunii medium as well as B. braunii colonies. Moreover, there was a significant interactive effect between the two, in terms of both algae biomass loss (p < 0.02) and growth rate change (p < 0.001); i.e., the effect of adding B. braunii medium is reduced when B. braunii colonies are present, and vice versa (see Table S1 for ANOVA tables). At the final time point, N. limnetica cell densities were highly variable across replicates, but on average were relatively low in the B. braunii medium treatment (7.8 × 105 ± 4.5 × 105 SD cells mL−1). When B. braunii cells and medium were present, N. limnetica densities were 2.0 × 106 ± 2.1 × 106 cells mL−1 (Fig. 3); these cell densities are on average 46% lower than in the corresponding grazer-free control (3.8 × 106 ± 1.0 × 106 cells mL−1). While this indicates N. limnetica were still grazed upon, it also confirms that B. braunii prevents the rapid depletion of N. limnetica biomass that we observed in the absence of B. braunii (see control in Fig. 1A), and that the remaining biomass is comprised of a mixture of N. limnetica and B. braunii.
Chlorophyll-a fluorescence (as a proxy for total algal biomass, log-scale) over time in the four Botryococcus braunii treatments, with (A) D. magna as grazer, (B) Poterioochromonas malhamensis as grazer, and (C) no grazer added. Both grazers caused strong reductions in biomass (circles in A, B) compared to the grazer-free controls (C) when Nannochloropsis limnetica was grown alone in fresh medium; however, loss of total algal biomass was mitigated with the addition of B. braunii’s filtered culture medium, B. braunii colonies alone, and the combination of B. braunii medium and colonies. Points show mean and SD of replicates (n = 6); lines represent loess fits. Note that fluorescence captures biomass of all photosynthetic organisms when present in a given treatment: N. limnetica (present in all treatments), B. braunii (present in half of treatments) and, to a lesser degree, the mixotrophic flagellate P. malhamensis (only in treatments shown in Fig. 1B)
Net effects of grazing on biomass and growth rate under different B. braunii treatments. Shown are grazing effects on (A, B) maximum chl-a fluorescence (as a proxy for total algal biomass) and (C, D) initial growth rates from day 0–3 caused by the different grazers (D. magna in A, C; P. malhamensis in B, D). Biomass loss calculations show the % change in maximum fluorescence (i.e., highest fluorescence value over time) in each experimental unit relative to the mean grazer-free control in the corresponding B. braunii treatment; growth rate changes show the difference in growth rate in each experimental unit compared to the mean grazer-free control. Points are data for individual replicates; black bars show mean and 95% CI per treatment
Cell densities of N. limnetica at the end of the experiment (day 18). Data show counts for individual replicates alongside mean and SD of cell densities. Data are only shown for experimental units with live Daphnia; missing Daphnia treatments are those in which Daphnia died prior to day 18. Cell counts were performed on a randomly chosen subset of 3 of 6 replicates
Botryococcus braunii inhibits grazing by Poterioochromonas malhamensis through both chemical and physical interference
In the control treatment with no B. braunii present, P. malhamensis significantly reduced N. limnetica biomass (mean 67% decrease in max. chl-a fluorescence, Fig. 1B, 2B) and growth rates (Fig. 2D) compared to the grazer-free controls. However, the presence of B. braunii medium mitigated grazing losses by P. malhamensis, as max. chl-a fluorescence of N. limnetica only decreased by a mean of 1.6% compared to grazer-free control (i.e., no significant biomass loss). In contrast to treatments with D. magna as grazer, the B. braunii colonies (43% decrease) and colonies + medium (27% decrease) treatments allowed for greater grazing losses to P. malhamensis than B. braunii medium alone over the full 18-day incubation (Fig. 2B). However, their initial (day 0–3) growth rates did not differ from those of the grazer-free controls (Fig. 2D). ANOVA again showed a significant interaction between effects of B. braunii medium and B. braunii colonies (p < 10–5 for both biomass and growth rate; Table S1). Final cell densities of N. limnetica were higher in B. braunii medium (5.0 × 106 ± 2.4 × 106 cells mL−1) when compared to colonies + medium (2.7 × 106 ± 1.0 × 106 cells mL−1), colonies only (1.0 × 106 ± 5.0 × 105 cells mL−1), or the treatment without B. braunii (1.3 × 106 ± 4.0 × 105 cells mL−1) (Fig. 3). Final counts of P. malhamensis showed that B. braunii medium reduced the density of P. malhamensis by 56% compared to the control, while treatments containing B. braunii colonies tended to cause increases in P. malhamensis cell density (Fig. 4A). P. malhamensis final density, however, was not correlated with N. limnetica final density (Fig. 4B; Spearman ρ = -0.3, p = 0.4).
A Cell densities of P. malhamensis at the end of the experiment (day 18). Data show counts for individual replicates alongside mean and SD of cell densities. Cell counts were performed on a randomly chosen subset of 3 of 6 replicates. (B) P. malhamensis densities plotted against N. limnetica densities. Each point represents cell densities in one experimental unit
Botryococcus braunii medium enhances growth of Nannochloropsis limnetica
Compared to N. limnetica grown alone in fresh medium (max. fluorescence of 4678 RFU ± 384 SD and growth rate of 0.50 ± 0.01 day−1), N. limnetica grown in the used medium of B. braunii had 13% higher max. fluorescence (5279 RFU ± 461 SD, p = 0.13, Tukey HSD) and 8% higher growth rates (0.54 ± 0.01 day−1 p = 0.007, Tukey HSD), indicating an overall enhancement of growth due to B. braunii medium (Fig. 1C, Fig. 5). Max. fluorescence was highest in the treatment containing B. braunii colonies and medium (5780 ± 718 SD, 24% higher than N. limnetica grown alone). ANOVA showed a significant interaction between effects of B. braunii medium and B. braunii colonies for growth rate (p < 0.001) and significant independent effects of B. braunii medium and B. braunii colonies for max. fluorescence (p < 0.03, Table S1). Growth rates were slower when N. limnetica was added to stationary phase B. braunii; however, the fact that biomass increased at all over time indicates the potential for synergistic interactions and overyielding in co-culture compared to monoculture. Moreover, final cell counts show that N. limnetica cell densities in the presence of B. braunii colonies and/or medium are at least as high as those when N. limnetica is grown alone (Fig. 3). In summary, B. braunii does not inhibit, and may even facilitate N. limnetica growth.
Discussion
The results of our study provide support for our hypotheses that (H1) B. braunii inhibits both grazers via a combination of chemical and physical factors, and (H2) B. braunii does not chemically inhibit Nannochloropsis growth. Our results, although they are limited to the laboratory microcosm scale, provide a precedent for engineering algal communities with multiple synergies. Specifically, we propose that B. braunii could simultaneously function as a crop protection agent for fast-growing algae such as Nannochloropsis species that can be harvested for higher-value products, while also producing extracellular hydrocarbons (Ennaceri et al. 2023) and exopolysaccharides (Wang et al. 2022).
Potential generalities, and context-dependencies, of using defended algae strains for crop protection
While we use Nannochloropsis as a focal ‘fast-grower’ we imagine that this principle could also apply to other small undefended taxa, e.g., Synechocystis that can be used for sustainable PHA bioplastic production (Koch et al. 2020), Haematococcus for antioxidants (Shah et al. 2016), or a variety of other small strains of interest for feed or fuel. Although in this study we use freshwater organisms, the general principle should apply equally well to brackish or saltwater cultivation. The fact that biomass loss was not completely prevented by B. braunii also suggests that this approach should be used in conjunction with complementary approaches to modify the biology and chemistry of pond systems for crop protection to more comprehensively manage complex pond communities (McBride et al. 2014; Day et al. 2017; Ma et al. 2017; Thomas et al. 2017; Fisher et al. 2019; Van Ginkel et al. 2020; McGowen et al. 2023).
Our results provide additional evidence that species-specific chemical inhibition can be used to inhibit grazers without inhibiting algal productivity. A previous meta-analysis showed the chemical compounds in recycled cultivation medium on average cause inhibitory effects on algal productivity (Loftus and Johnson 2017); therefore, finding algae species that are chemically compatible (as in our study) is of utmost importance, both for the purpose of recycling culture water and for the purpose of creating a selective chemical environment that favors low grazer densities. There is also potential to take advantage of dissolved metabolites produced by algae and bacteria that stimulate algal productivity, for example plant growth hormones like indole acetic acid (Brisson et al. 2021; Negi et al. 2024). Indeed, our results suggest a positive effect of used B. braunii medium on N. limnetica, which warrants further study. Nannochloropsis gaditana growth, for example, increases when grown mixotrophically with added glucose and glycerol (Menegol et al. 2019); therefore, a possible explanation is that in our study N. limnetica metabolized exopolysaccharides excreted by B. braunii, thus enhancing its growth. However, an increase in dissolved organic carbon from B. braunii exudates could also cause indirect effects, such as enhancing the growth of bacteria or even mixotrophs like P. malhamensis. We did in fact note higher P. malhamensis density when B. braunii colonies and medium were present (Fig. 4A), suggesting the possibility that these mixotrophs benefited from more exudates and/or free-living bacteria in such B. braunii treatments. However, we observed no clear effects of P. malhamensis density on N. limnetica cell density (Fig. 4B); it is thus possible that P. malhamensis switched to smaller bacterial prey or osmotrophy instead of ingesting N. limnetica. The influence exerted by B. braunii’s diverse microbiome (Blifernez-Klassen et al. 2021) on grazers and pathogens in algal systems also warrants in-depth investigation. In summary, the net effect of B. braunii in our experiment is likely a combination of inhibition and stimulation of our study organisms, both direct and indirect. Future research, especially over longer time periods and at larger scales, is needed to understand the complex dynamics that may arise even from a relatively simple system such this.
Although this microcosm study provides a clear proof of concept for a community engineering strategy using B. braunii to inhibit grazers and stabilize culture biomass, we emphasize that many factors will determine whether it is as effective at commercial scale as in our lab study. For example, we assess only one genotype of each organism; other distinct genotypes of each organism studied here are likely to have differences in key traits (e.g., chemical exudate profiles or colony size across strains/clades of B. braunii, differential tolerance to allelopathic inhibition in target algae such as Nannochloropsis), which could either enhance or diminish the net protective effect of B. braunii. While free fatty acid excretion has been linked to chemical inhibition by B. braunii (Chiang et al. 2004; Thomas et al. 2023), there is likely much more to uncover regarding the mechanisms of inhibition caused by the complex suite of bioactive metabolites excreted by different B. braunii strains. Different species or genotypes of grazers (or combinations of grazers), and other types of pests like viral, fungal, or bacterial pathogens may (or may not) be inhibited in a similar manner as the two grazers used here. It should also be noted that our B. braunii medium treatments represented a single pulse disturbance in terms of chemical inhibition and thus could have temporary effects; therefore, impacts of chemical inhibition may differ if applied in a continuous or semi-continuous manner. While we used high densities of B. braunii, reflecting its maximum biomass at carrying capacity, using a lower relative density of B. braunii could alter results by changing the total strength of interactions with both Nannochloropsis and with grazers. Additionally, effects of other abiotic factors (e.g., temperature, salinity, pH/alkalinity, CO2 addition), which are outside the scope of this study, could significantly alter the effects of chemical inhibition and thus be optimized in tandem with the crop protection strategy described in our study.
Future perspectives on the microalgae “eco-refinery” concept
The biorefinery concept describes an approach in which commercial facilities process a variety of cellular components, usually from an algal monoculture, into distinct bio-based products in order to maximize the economic and environmental benefits of algae farming (Laurens et al. 2017; Khoo et al. 2019). Complementary to the biorefinery approach, the “eco-refinery” framework described in this study additionally allows the separation of different algae species, grown together in the pond ecosystem, into different fractions prior to downstream conversion pathways. Because the size of B. braunii colonies (up to 200 µm in diameter) is significantly greater than cell size of strains of industrial interest (e.g., Nannochloropsis or Picochlorum, which are < 5 µm), it is feasible to selectively harvest the large B. braunii colonies (e.g., with a 20-µm filtration apparatus), perform non-destructive extraction of hydrocarbons in the extracellular matrix (Moheimani et al. 2013; Griehl et al. 2015), and return live colonies to ponds. The under 20-µm fraction, containing primarily the target small algae, could then be dewatered and processed using current biorefinery methods. The relative cell densities of each species in a pond could also be controlled by dynamically changing the fraction of each that is harvested and removed from/returned to the system. It is the opinion of the authors, therefore, that the eco-refinery approach has the potential improve bioprocess design while also preventing pond crashes due to pests. We again stress, however, that these methods must be tested and validated at larger scales. We therefore suggest that an expanding branch of research should evaluate potential benefits of integrating ecologically-based crop protection measures with novel bioprocess engineering pathways to enhance the reliability and efficiency of algal bioproduct generation.
Conclusions
Our study provides a proof of concept that B. braunii, an alga that excretes extracellular hydrocarbons and polysaccharides, can also protect small, fast-growing algae from grazing pressure, thus providing early evidence for a novel mechanism of protecting algal crop productivity in designed polycultures at mass scale for a suite of complementary renewable bioproducts. Future work is needed to test and validate this novel strategy at pilot and commercial scales.
Data availability
All data and code used are publicly available on Zenodo (https://doi.org/10.5281/zenodo.13237033).
References
Bacellar Mendes LB, Vermelho AB (2013) Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol Biofuels 6:152
Blifernez-Klassen O, Chaudhari S, Klassen V, Wördenweber R, Steffens T, Cholewa D, Niehaus K, Kalinowski J, Kruse O (2018) Metabolic survey of Botryococcus braunii: Impact of the physiological state on product formation. PLoS ONE 13:0198976
Blifernez-Klassen O, Klassen V, Wibberg D, Cebeci E, Henke C, Rückert C, Chaudhari S, Rupp O, Blom J, Winkler A, Al-Dilaimi A, Goesmann A, Sczyrba A, Kalinowski J, Bräutigam A, Kruse O (2021) Phytoplankton consortia as a blueprint for mutually beneficial eukaryote-bacteria ecosystems based on the biocoenosis of Botryococcus consortia. Sci Rep 11:1726
Brisson V, Mayali X, Bowen B, Golini A, Thelen M, Stuart RK, Northen TR (2021) Identification of effector metabolites using exometabolite profiling of diverse microalgae. mSystems 6:e0083521
Chiang I-Z, Huang W-Y, Wu J-T (2004) Allelochemicals of Botryococcus braunii (Chlorophyceae). J Phycol 40:474–480
Corcoran AA, Boeing WJ (2012) Biodiversity increases the productivity and stability of phytoplankton communities. PLoS ONE 7:e49397
Day JG, Gong Y, Hu Q (2017) Microzooplanktonic grazers – A potentially devastating threat to the commercial success of microalgal mass culture. Algal Res 27:356–365
Ennaceri H, Nwoba EG, Ogbonna CN, Bahri PA, Moheimani NR (2023) Progress of non-destructive hydrocarbon extraction technology of Botryococcus braunii. Algal Res 2:103156
Fernandes HL, Tomé MM, Lupi FM, Fialho AM, Sá-Correia I, Novais JM (1989) Biosynthesis of high concentrations of an exopolysaccharide during the cultivation of the microalga Botryococcus braunii. Biotechnol Lett 11:433–436
Fisher CL, Ward CS, Lane PD, Kimbrel JA, Sale KL, Stuart RK, Mayali X, Lane TW (2019) Bacterial communities protect the alga Microchloropsis salina from grazing by the rotifer Brachionus plicatilis. Algal Res 40:101500
Griehl C, Kleinert C, Griehl C, Bieler S (2015) Design of a continuous milking bioreactor for non-destructive hydrocarbon extraction from Botryococcus braunii. J Appl Phycol 27:1833–1843
Hillebrand H, Acevedo-Trejos E, Moorthi SD, Ryabov A, Striebel M, Thomas PK, Schneider M (2022) Cell size as driver and sentinel of phytoplankton community structure and functioning. Funct Ecol 36:276–293
Kazamia E, Riseley AS, Howe CJ, Smith AG (2014) An engineered community approach for industrial cultivation of microalgae. Ind Biotechnol 10:184–190
Khoo CG, Dasan YK, Lam MK, Lee KT (2019) Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour Technol 292:121964
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Koch M, Bruckmoser J, Scholl J, Hauf W, Rieger B, Forchhammer K (2020) Maximizing PHB content in Synechocystis sp. PCC 6803: a new metabolic engineering strategy based on the regulator PirC. Microb Cell Fact 19:231
Lane TW (2022) Barriers to microalgal mass cultivation. Curr Opin Biotechnol 73:323–328
Laurens LML, Markham J, Templeton DW, Christensen ED, Van Wychen S, Vadelius EW, Chen-Glasser M, Dong T, Davis R, Pienkos PT (2017) Development of algae biorefinery concepts for biofuels and bioproducts; a perspective on process-compatible products and their impact on cost-reduction. Energy Environ Sci 10:1716–1738
Loftus SE, Johnson ZI (2017) Cross-study analysis of factors affecting algae cultivation in recycled medium for biofuel production. Algal Res 24:154–166
Ma M, Wei C, Huang W, He Y, Gong Y, Hu Q (2023) A systematic review of the predatory contaminant Poterioochromonas in microalgal culture. J Appl Phycol 35:1103–1114
Ma M, Yuan D, Yue H, Park M, Gong Y, Hu Q (2017) Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2-mediated low culture pH. Algal Res 26:436–444
McBride RC, Lopez S, Meenach C, Burnett M, Lee PA, Nohilly F, Behnke C (2014) Contamination management in low cost open algae ponds for biofuels production. Ind Biotechnol 10:221–227
McGowen J, Knoshaug EP, Laurens LML, Forrester J (2023) Outdoor annual algae productivity improvements at the pre-pilot scale through crop rotation and pond operational management strategies. Algal Res 70:102995
Menegol T, Romero-Villegas GI, López-Rodríguez M, Navarro-López E, López-Rosales L, Chisti Y, Cerón-García MC, Molina-Grima E (2019) Mixotrophic production of polyunsaturated fatty acids and carotenoids by the microalga Nannochloropsis gaditana. J Appl Phycol 31:2823–2832
Moheimani NR, Cord-Ruwisch R, Raes E, Borowitzka MA (2013) Non-destructive oil extraction from Botryococcus braunii (Chlorophyta). J Appl Phycol 25:1653–1661
Nalley JO, Stockenreiter M, Litchman E (2014) Community ecology of algal biofuels: complementarity and trait-based approaches. Ind Biotechnol 10:191–201
Negi S, Daughton B, Carr CK, Klein B, Davis R, Banerjee S, Dale T (2024) Effect of plant growth-promoting molecules on improving biomass productivity in DISCOVR production strains. Algal Res 77:103364
Newby DT, Mathews TJ, Pate RC, Huesemann MH, Lane TW, Wahlen BD, Mandal S, Engler RK, Feris KP, Shurin JB (2016) Assessing the potential of polyculture to accelerate algal biofuel production. Algal Res 19:264–277
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Shah MMR, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front Plant Sci 7:531
Shurin JB, Abbott RL, Deal MS, Kwan GT, Litchman E, McBride RC, Mandal S, Smith VH (2013) Industrial-strength ecology: trade-offs and opportunities in algal biofuel production. Ecol Lett 16:1393–1404
Thomas PK, Dunn GP, Coats ER, Newby DT, Feris KP (2019) Algal diversity and traits predict biomass yield and grazing resistance in wastewater cultivation. J Appl Phycol 31:2323–2334
Thomas PK, Dunn GP, Passero M, Feris KP (2017) Free ammonia offers algal crop protection from predators in dairy wastewater and ammonium-rich media. Bioresour Technol 243:724–730
Thomas PK, Hietala DC, Cardinale BJ (2023) Tolerance to allelopathic inhibition by free fatty acids in five biofuel candidate microalgae strains. Bioresour Technol Reports 21:101321
Van Ginkel SW, El-Sayed WMM, Johnston R, Narode A, Lee HJ, Bhargava A, Snell T, Chen Y (2020) Prevention of algaculture contamination using pesticides for biofuel production. Algal Res 50:101975
Wang W-N, Li T, Li Y, Zhang Y, Wu H-L, Xiang W-Z, Li A-F (2022) Exopolysaccharides from the energy microalga strain Botryococcus braunii: Purification, characterization, and antioxidant activity. Foods 11:110
Acknowledgements
We thank Silvana Käser for maintaining Daphnia cultures and for assistance with the B. braunii isolation, Marta Reyes for maintaining phytoplankton cultures, Raphaël Bossart for 18S sequencing of B. braunii, and Eawag teaching staff for facilitating our collaboration. This manuscript is dedicated to the late Ginger Scales, an omnivorous grazer ca 4.8 ×105 µm in length who exhibited size-selective feeding on kibble and carrots. This work was funded by a seed grant from Eawag.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL. This research was funded by a seed grant from Eawag.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design the study; PKT, FA, and MF conducted the experiment; PKT performed statistical analyses, data visualizations, and wrote the draft manuscript; FA and AN reviewed and edited the draft manuscript; all authors approve its final version for submission.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, P.K., Arn, F.J., Freiermuth, M. et al. Botryococcus braunii reduces algal grazing losses to Daphnia and Poterioochromonas through both chemical and physical interference. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03330-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03330-x