Abstract
Open algal cultivation platforms often suffer crop losses to herbivorous grazers that have potential to devastate biomass production within a few days. While a number of studies suggest synthetic chemicals as control agents for voracious algal grazers, environmental and safety concerns associated with the use of these chemicals encourage the exploration of alternative biological control agents. We hereby propose the application of a biosurfactant produced by Bacillus subtilis C9 (referred to as C9-biosurfactant) for controlling cladoceran grazers commonly found in algal cultivation systems. The results indicated that C9-biosurfactant completely eradicated Daphnia pulex and Moina macrocopa within 24 hours when concentrations were equal to or exceeded 6 mg/L. Moreover, supplying C9-biosurfactant into the cultures of selected algal species with and without cladoceran grazers indicated no adverse effect of C9-biosurfactant on the growth and lipid productivity of algal crops, while cladocerans were selectively controlled by C9-biosurfactant even under the presence of their prey. These results thus indicate that C9-biosurfactant could be an effective biocontrol agent for cladoceran grazers at industrial algal cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Anthropogenic climate change and population growth have resulted in a global-scale consensus to mitigate the usage of unsustainable energy sources, and concomitantly initiated active research and investment efforts to at least partially replace fossil fuels with renewable and carbon-neutral energy sources1. Microalgae, in particular, have been given considerable attention because of their relatively high growth rates compared to terrestrial biofuel crops and the possibilities of utilizing flue gases and excess nutrients in wastewater sources for their prolific growth2,3. Nonetheless, the production of microalgae-derived biofuels and bioproducts is still at the explorative stage, and a number of challenges have arisen from mass cultivation of algal biomass to downstream processes for producing target products2,3,4,5,6. Especially, achieving high and reliable biomass production at the industrial-scale poses a great and pressing challenge to make entire production chain cost-competitive7,8.
While the mass production of algal biomass is primarily achieved in outdoor non-sterile systems due to the constraints associated with the vast capital and operational costs of sterile closed systems, the foremost issues in outdoor cultivation of short-listed algal species for the production of biofuels and bioproducts are culture contamination and population crashes9,10,11. In particular, the grazing pressure of herbivorous zooplanktons can cause significant reductions in productivity and quality of harvested biomass, which in turn cause losses in overall profitability12. Cauchie et al., for example, measured a 99% reduction in algal chlorophyll-a due to Daphnia grazing over several days in an open pond system12,13. Similarly, a one-year-long operation of wastewater-fed open algal ponds experienced repeated outbreaks of cladoceran Daphnia, and it was subsequently confirmed in laboratory that this cladoceran grazer imposed 12.5–87.87% reductions in the dry cell weight (DCW) of common microalgal strains found in outdoor open ponds14. In addition, White and Ryan estimated algal crop losses to grazers at 20% ± 10% based on their industrial-scale operation of outdoor algal ponds15. It is therefore imperative to develop cost-effective strategies for controlling unpredictable blooms of algal grazers because loss of even a single algal reactor in an array can dramatically impact overall facility productivity and yield9,11,15,16.
Synthetic chemicals have thus been explored to mitigate algal crop losses from zooplankton grazers in algal production systems17,18,19,20,21. For example, the disinfectant hypochlorite was used to control the rotifer Brachionus calyciflorus in laboratory cultures of the microalga Chlorella kessleri19,21,22. In addition, Wang et al. outlined the lethal concentrations of three synthetic pesticides on B. calyciflorus and Daphnia pulex23. Synthetic compounds, however, may have negative impacts on non-target organisms and/or adjacent ecosystems24,25. Therefore, sustainable cost-effective strategies that control growth and proliferation of grazing zooplanktons in algal cultivation platforms without the use of synthetic chemicals are needed17,26,27,28,29,30.
In terrestrial agriculture, microbial secondary metabolites have been regarded as promissory alternatives to synthetic pesticides because of their diverse metabolites with high biological activities31,32. In particular, Bacillus spp. lead active research and development efforts because the strains of Bacillus are known to excrete peptides and lipopeptides, such as fungicine, iturin, bacillomicine, and others that have antifungal, antibacterial, and high surfactant activity33. Although a variety of biological control products based on Bacillus species are available for agronomical use to control agricultural pests, few studies explored the direct application of the secondary metabolites of Bacillus in the systems targeting to grow algal biomass33.
Previously, Kim et al. isolated Bacillus subtilis C9 that produces surfactin, a lipopeptide biosurfactant with antibacterial, antiviral, and anti-biofilm forming activities34,35. While B. subtilis and its biosurfactant were known to exhibit biocontrol activities against mosquito pupae and phytopathogenic fungi31,36,37,38, the purpose of this study was to explore the biocontrol activity of recovered biosurfactant from the culture of B. subtilis C9 (referred hereupon as C9-biosurfactant) on common cladoceran grazers in algal cultivation platforms14,39. Grazer-introduced cultures were first subjected to different concentrations of C9-biosurfactant to test whether cladoceran grazers were effectively controlled by C9-biosurfactant. Because the industrial applicability of any biocontrol compound must assure its non-toxicity to non-target species, we further explored the influence of supplemental C9-biosurfactant on the growth and/or lipid productivity of two microalgal strains with and without the presence of cladoceran grazers.
Results and Discussion
Recovery and identification of C9-biosurfactant
The average surface tension of cell-free supernatants from triplicate cultures of B. subtilis C9 after a 72-hour-long cultivation period was 29.8 ± 1.1 mN/m, and the average concentration of acid precipitate in the respective culture supernatant was 0.9 ± 0.2 g/L. 0.3 ± 0.1 g/L of C9-biosurfactant was then recovered from acid precipitates by dissolving C9-biosurfactant in demineralized water and freeze-drying the biosurfactant solution. Our subsequent mass spectrometry analysis of C9-biosurfactant exhibited the highest peak at m/z 1058.67, where the highest peak of HPLC-grade surfactin from B. subtilis was also observed (Fig. S1). While the molecular ion species of surfactin can be detected in either protonated or sodium adduct forms, Kim et al. also reported a high peak of C9-biosurfactant at m/z 1058, where the sodium adduct of surfactin is putatively present34,35,40. In addition, the CMD values of aqueous solutions of C9-biosurfactant and HPLC-grade surfactin at an identical concentration of 0.3 g/L were 50 and 60, indicating that the relative proportion of surfactin in C9-biosurfactant was roughly 83% (Figs S2 and S3). These results thus indicated the production of biosurfactant in the culture of B. subtilis C9 and further confirmed surfactin as the main surfactant compound of C9-biosurfactant34,35,40. Although C9-biosurfactant tested in this study was obtained from triplicated batch cultivation of B. subtilis C9, it should be also noted that the concentration of surfactin in the culture of B. subtilis C9 could substantially vary under different cultivation conditions, and direct quantification of surfactin in C9-biosurfactant will be required especially when determining the application doses of C9-biosurfactant in industrial algal cultivation41.
C9-biosurfactant acts as an effective control agent for cladoceran Daphnia and Moina
While a diverse array of physical, biological, and chemical measures were recently explored to successfully control blooms of algal grazers22, our results indicated that C9-biosurfactant can also be implemented to eradicate cladoceran grazers in industrial algal cultivation platforms. The results showed 6 mg/L of C9-biosurfactant was enough to cause complete mortality of both Daphnia pulex and Moina macrocopa within 12 hours (Fig. 1). Our subsequent test with HPLC-grade surfactin from Bacillus subtilis also indicated the complete mortality of cladocerans within 12 hours at concentrations equal to or above 6 mg/L, confirming that the main surfactant compound of C9-biosurfactant resulted in the mortality of algal grazers (Figs S2 and S5).
The results further suggested that the cladoceran-control activity of C9-biosurfactant is dependent on the concentration of C9-biosurfactant. In particular, the data obtained 1 hour after the application of C9-biosurfactant exhibited substantial increases in the percent mortalities of both cladocerans with increasing concentrations of C9-biosurfactant (Fig. 1). While Vollenbroich et al. similarly reported that the antiviral activity of surfactin substantially increased with increasing surfactin concentration42, dose-dependent mortality response of cladocerans may provide important information that can be exploited to achieve successful cladoceran-control in a shorter period of time. Nonetheless, complete eradication of cladoceran grazers within 1 day following the application of C9-biosurfactant at all tested concentrations indicates C9-biosurfactant can act as an effective biocontrol agent for cladoceran algal grazers in industrial algal cultivation systems, especially because invading cladoceran grazers often require at least 1–2 days to substantially consume dominant microalgal species22,39,43.
Although this study was focused on demonstrating the biocontrol activity of C9-biosurfactant on cladoceran grazers, microzooplanktons, including rotifers and ciliated protists, are also known to detrimentally contaminate industrial algal cultivation platforms. Indeed, order-of-magnitude reductions in microalgal biomass have been well acknowledged for ciliate-dominated zooplankton in commercial Spirulina (Arthrospira) cultivation sites and for rotifer-dominated zooplankton in High Rate Algal Ponds (HRAPs)22,29,39,43,44. Such devastatingly high level of algal crop losses will impede making algae-based products a commercial reality, thus exploring the effectiveness of C9-biosurfactant in controlling other frequent algal grazers will be vitally important to further support the implementation of C9-biosurfactant at industrial algal cultivation.
Indeed, previous studies have reported that surfactants generally exhibit biocontrol activities against different organisms38,45,46. Geetha et al., for example, noted the mosquito pupicidal activity exhibited by the biosurfactant of Bacillus subtilis, and Manonmani et al. further confirmed that the culture supernatant of Bacillus subtilis was found to kill both larval and pupal stage of mosquito species of Anopheles stephensi, Culex quinquefasciatus, and Aedes aegyti, while a significantly lesser amount of biosurfactant was required to kill the mosquito pupae38,45. In addition, Lechuga et al. explored the toxicity of different surfactants on the luminescent bacterium Vibrio fischeri, and reported that V. fischeri was more sensitive to the toxic effects of the surfactants than was cladoceran Daphnia46. Nonetheless, it is evident that the cladoceran-control activity of C9-biosurfactant bears substantial industrial potential for managing blooms of cladoceran grazers in algal cultivation platforms29,47, especially given that large-bodied cladocerans are typically thought to control microalgal production much more effectively than other small-bodied herbivorous grazers because their algal ingestion is less influenced by the size of resident algal strains29,47.
Although the exact mode of action of allelopathic C9-biosurfactant on cladoceran grazers requires further exploration, it was speculated that a reduction in the surface tension of the water caused by C9-biosurfactant was likely to require more energetic costs during swimming that may have substantially contributed to the observed cladoceran mortality36,37. Vollenbroich et al. reported the disintegration of mycoplasma membranes after treating mycoplasma-contaminated mammalian cells with surfactin, and concluded that the disintegration of bacterial membrane was due to a physicochemical interaction of the membrane-active surfactant with the outer part of the lipid membrane bilayer, whereas the low cytotoxicity of surfactin for mammalian cells was observed42,48. Although the cladoceran-control activity of C9-biosurfactant is likely to depend on the life cycle and nutritional status of cladocerans49,50, a physicochemical interaction of surfactin with the carapace of cladocerans may also be an important contributor to the observed cladoceran mortality36,37,42,48,51.
C9-biosurfactant does not negatively influence the biomass and lipid productivity of algal crops
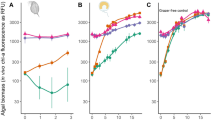
Any potential biocontrol agents in agriculture should be tested for their toxicity to crop species prior to warranting their application in agricultural fields33. Likewise, the application of C9-biosurfactant in algal cultivation systems cannot be justified without evaluating the influence of C9-biosurfactant on the growth and productivity of selected algal crops. The results of our flasks-scale experiments, however, clearly indicated no growth inhibiting effect of C9-biosurfactant on Chlorella sp. HS2 and Scenedesmus deserticola JD052 (Fig. 2 and Table 1).
The specific growth rates of each algal species were not statistically different under varying concentrations of C9-biosurfactant (p-values > 0.05), except the culture of Chlorella sp. HS2 amended with 60 mg/L of C9-biosurfactant, which had a lower specific growth rate than that of the control without C9-biosurfactant (p-value < 0.05) (Table 1). The well-plate experiments similarly indicated that the supplemental surfactin did not negatively influence the growth of Chlorella sp. HS2 and Scenedesmus deserticola JD052, except in the case of adding 60 mg/L of surfactin into the culture of Chlorella sp. HS2 where the final cell density was only about half that of the control with no supplemental surfactin (Fig. S7). These results indicated no growth inhibiting effect of C9-biosurfactant and surfactin on the selected Chlorella and Scenedesmus strains at the concentrations below 60 mg/L, which were enough to cause complete cladoceran mortality (Fig. 1).
The DCW and lipid content of Chlorella sp. HS2 and Scenedesmus deserticola JD052 substantially increased under 6 and 20 mg/L treatments of C9-biosurfactant (Fig. 3). While no substantial increase in final DCW was measured under 60 mg/L of C9-biosurfactant for either algal strain, increasing the concentration of C9-biosurfactant up to 60 mg/L continuously enhanced the lipid content of Scenedesmus deserticola JD052 (Fig. 3). In contrast, the lipid content of Chlorella sp. HS2 was highest in the cultures amended with 6 mg/L of C9-biosurfactant, and the Chlorella cultures amended with 60 mg/L of C9-biosurfactant exhibited no considerable difference in the lipid content compared to that of the control with no supplemental C9-biosurfactant (Fig. 3). The subsequent FAME analysis further indicated minor changes (i.e., 1–5%) in the proportion of each type of fatty acid under varying concentrations of C9-biosurfactant: oleic (C18:1n9c) and α-linolenic (C18:3n3) acids exhibited substantial shifts in the cultures of Scenedesmus deserticola JD052 and the additive C9-biosurfactant substantially influenced the proportions of palmitic (C16:0) and linoleic (C18:2n6c) acids in the cultures of Chlorella sp. HS2 (Tables 2 and 3).
Dry cell weight (g/L) and lipid content (% DW) of harvested biomass of Chlorella sp. HS2 (a) and Scenedesmus deserticola JD052 (b) following the harvest of 250-mL flask cultures grown under varying concentrations of C9-biosurfactant in batch cultivation mode. Error bars represent the standard deviation from the mean for triplicate independent measurements.
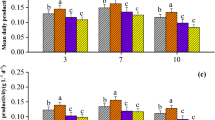
The lipid productivities of Chlorella and Scenedesmus were significantly enhanced with the supplemental C9-biosurfactant (p-values < 0.05) in most cases (but see Chlorella cultures amended with 60 mg/L of C9-biosurfactant – Fig. 4). The highest lipid productivities of Chlorella sp. HS2 and Scenedesmus deserticola JD052 were 0.178 and 0.128 g/L/day at C9-biosurfactant concentrations of 6 and 20 mg/L, respectively. Such increments corresponded to 35 and 51% of increases in the lipid productivities compared to the control cultures of each algal strain. While the amount of triacylglycerols (TAG) in harvested algal biomass would be a direct indicator for understanding the influence of supplemental biosurfactant on the bioenergy potential of selected algal strains, Griffiths and Harrison emphasized that lipid productivity itself could be a key characteristic for choosing algal species for biodiesel production52. These results thus suggest that the additive C9-biosurfactant does not compromise the growth or lipid productivity of the selected algal crops, and further indicate the possibility of utilizing C9-biosurfactant to promote the bioenergy potential of harvested biomass at industrial algal cultivation.
Lipid productivity of the cultures of Chlorella sp. HS2 (a) and Scenedesmus deserticola JD052 (b). Lipid productivity was calculated by multiplying lipid content (%) with corresponding biomass productivity after harvesting flask cultures treated with varying concentrations of C9-biosurfactant at the end of either 6 or 7 day-long batch cultivation period. Error bars indicate 95% confidence intervals for the mean.
The increased lipid productivities of Chlorella sp. HS2 and Scenedesmus deserticola JD052 may be resulted from increased nutrient uptake into cell bodies with the additive C9-biosurfactant. Taoka et al., for example, reported ca. 4% increase in the total lipid content of Thraustochytrium aureum with the addition of 1% Tween 80, a non-ionic surfactant, and speculated that the addition of surfactant likely interfered with the permeability of cell membranes and enhanced the nutritional uptakes into the cell body53. The authors reported significant increases in the proportions of both saturated and monounsaturated fatty acids, while the percentage of unsaturated fatty acids decreased with the addition of Tween 8053. Our results, however, indicated the opposite trend: the proportions of saturated and mono-unsaturated fatty acids were generally declined with increasing concentration of C9-biosurfactant, whereas the proportion of unsaturated fatty acids tended to increase with increasing C9-biosurfactant concentration mainly due to substantial increase in the proportion of polyunsaturated fatty acids (Tables 2 and 3). The increase in the relative proportion of unsaturated fatty acids has been acknowledged as a compensating response to the decreased thylakoid membrane fluidity under low temperature conditions for a green alga, Tetraselmis sp., and an increase in the level of polyunsaturated fatty acids particularly was suggested as a shift in the structure of membrane-forming lipids to spatially increase membrane fluidity that potentially enables more efficient exchange of metabolites54,55,56. Although few studies addressed the influence of biosurfactant on algal membrane fluidity, more careful evaluation on the mechanisms involved in possible shifts in membrane fluidity will be necessary to clearly understand algal response to the additive biosurfactant.
It should be, however, noted that algal response to biosurfactant is closely dependent on the type of biosurfactant and the algal strains that are being cultivated46,57. Rieß and Grimme, for instance, reported that diethyleneglycol monohexadecylether (C16E2), a nonionic surfactant, was shown to act as an instantaneous inhibitor of cell growth and reproduction of Chlorella fusca, whereas a cationic surfactant cetyltrimethylammonium chloride (CTAC) was shown to induce only minor acute effects on photosynthesis and respiration57. A recent study further tested the toxicity of alkylglucosides on the freshwater alga, Selenastrum capricornutum and reported that these non-ionic surfactants are relatively innocuous to Selenastrum after a 72-hour algal growth inhibition test, while substantial toxicity of these surfactants were confirmed with cladoceran Daphnia magna46. In addition, Ahn et al. confirmed that the additive surfactin slightly inhibited the growth of Chlorella vulgaris and a Scenedesmus species, suggesting the possibility that two algal strains tested in this study had intrinsically greater tolerance against high surface activity of surfactin than other algal species58. These authors, however, also noted that the growth of Navicula sp. seemed to be stimulated with the additive surfactin58. Therefore, the influence of C9-biosurfactant on a wide variety of algal crop species should be systematically evaluated throughout a long-term operation to successfully achieve the industrial application of C9-biosurfactant as an effective biocontrol agent.
Applying C9-biosurfactant in industrial algal cultivation platforms
While the results presented in Fig. 1 were based on the cases in which algal cells were not provided throughout the experimental period, it is true that prey availability is not limiting for the growth of algal grazers in industrial algal cultivation platforms59. It is thus vitally important to evaluate the effectiveness of C9-biosurfactant in controlling cladoceran grazers in the presence of algal prey because the nutritional status of cladocerans can substantially influence the cladoceran-control activity of C9-biosurfactant49,50.
Although Fig. 1 indicated the complete eradication of cladocerans within 12 hours at the C9-biosurfactant concentration of 6 mg/L, the results clearly showed that more than 12 hours were required to completely eradicate cladoceran grazers with 6 mg/L of C9-biosurfactant under the presence of algal prey (Figs 5b and 6b). In particular, the respective percent mortalities of Daphnia pulex were 45 and 60% in the cultures of Chlorella sp. HS2 and Scenedesmus deserticola JD052, and 40 and 70% of the respective percent mortalities of Moina macrocopa were observed in the cultures of Chlorella sp. HS2 and Scenedesmus deserticola JD052 after treating with 6 mg/L of C9-biosurfactant for 12 hours (Figs 5b and 6b). The delayed mortality response of cladoceran grazers at the C9-biosurfactant concentration of 6 mg/L was reflected in lower algal cell densities than those of the tube cultures amended with higher C9-biosurfactant concentrations (Figs 5a and 6a), and no algal growth limiting effect of C9-biosurfactant was observed at the C9-biosurfactant concentrations above 6 mg/L (Figs 5a and 6a). These results indicate that longer exposure of C9-biosurfactant to cladoceran grazers is likely to be necessary to achieve complete eradication in the presence of algal prey, and reaffirm that the additive C9-biosurfactant does not substantially limit the growth of two selected algal strains.
The culture densities of Chlorella sp. HS2 under the grazing pressure of Daphnia pulex (top) and Moina macrocopa (bottom) with varying concentrations of C9-biosurfactant throughout a 72-hour-long cultivation period (a) and the corresponding percent mortalities of Daphnia pulex (top) and Moina macrocopa (bottom) (b). Note that no mortalities of both cladocerans were observed in the cultures received 0 mg/L of C9-biosurfactant throughout the experimental period. Error bars represent the standard error of the mean.
The culture densities of Scenedesmus deserticola JD052 under the grazing pressure of Daphnia pulex (top) and Moina macrocopa (bottom) with varying concentrations of C9-biosurfactant throughout a 96-hour-long cultivation period (a) and the corresponding percent mortalities of Daphnia pulex (top) and Moina macrocopa (bottom) (b). Note that no mortalities of both cladocerans were observed in the cultures received 0 mg/L of C9-biosurfactant throughout the experimental period. Error bars represent standard error of the mean.
Although the complete eradication of cladoceran grazers was observed within 12 hours upon the application of C9-biosurfactant at the concentrations of 20 and 60 mg/L, the percent mortalities of cladoceran grazers were greater in the cultures of Scenedesmus after 12 hours under 6 mg/L of C9-biosurfactant than in the cultures of Chlorella. Such differences in cladoceran mortality may be the results of different ingestion rate of algal grazers by the size of algal prey14. Cho et al., for example, tested the ingestion rate of Daphnia sp. with different algal strains and reported that the large-sized microalga, Pediastrum sp. was most resistant to the grazing pressure of Daphnia, while relatively smaller unicellular algal species such as Parachlorella sp. were effectively grazed by the cladoceran grazer14. Cell size and long axis length of microalgae were particularly hypothesized as master traits that inherently control other ecological traits related to the trends in competition and facilitation, especially because size confers a defense against grazing30,60,61,62. Indeed, the cell density of Chlorella sp. HS2 was more drastically decreased than that of Scenedesmus deserticola JD052 under the presence of cladoceran grazers without supplemental C9-biosurfactant (Figs 5a and 6a). It is therefore likely that small-sized unicellular Chlorella sp. HS2 was more effectively ingested by cladoceran grazers than colonial Scenedesmus deserticola JD052, and potential differences in algal ingestion efficiency seemed to contribute to the lower percent mortalities of cladoceran grazers in the culture of Chlorella sp. HS2 under 6 mg/L of C9-biosurfactant. These results thus reemphasize potential differences in resistance against grazing between different algal species, and further suggest the importance of carefully selecting algal crop species to effectively achieve crop protection against grazing consumers because the extent of crop loss to algal grazers may significantly vary with the choice of algal crop species30.
Although the biosurfactants of Bacillus subtilis are known to withstand a wide range of pH values, exposure to long hours of sunlight, UV radiation, as well as moist heat up to 121 °C36,45, continuous or semi-continuous operation of algal reactors may necessitate greater inputs of C9-biosurfactant to successfully control algal grazers due to typically greater biomass-specific grazing rates than biomass-specific growth rates in continuous cultures59,63. In addition, the exposure regime of biocontrol agent (i.e., continuous vs. pulse exposure) was known to play a critical role in determining the effectiveness of selected control agents. While the complete eradication of cladoceran grazers may not be desirable because of the potential ecological imbalances and the establishment of possibly unmanageable alien-grazers27,30, future research will need to test the cladoceran-control activity of C9-biosurfactant under varying operational conditions with high-frequency monitoring of ecological communities to assure the industrial applicability of C9-biosurfactant64. Furthermore, the environmental impact of the possible release of biosurfactant to waterbodies should be evaluated across different trophic levels to ensure safe and reliable application of C9-biosurfactant, although biosurfactants are generally considered to be less toxic and more biodegradable than synthetic surfactants34.
It has to be also noted that the yield of biosurfactant from the culture of B. subtilis C9 can be substantially enhanced by optimizing growth medium or selecting cost-effective growth substrate35,41,65. Kim et al., for example, carefully optimized both macro- and micro- nutrients of the growth medium of B. subtilis C9 and achieved the production of C9-biosurfactant at the concentration of 7.0 g/L35. Gudina et al. further reported 1.31 g/L of average biosurfactant production from B. subtilis #573 grown in corn steep liquor (CSL) with the low surface tension values between 29.7 and 31.0 mN/m of cell-free supernatants without dilution41. Moreover, Fox and Bala tested waste potato substrates as a low-cost carbon source for surfactant production by B. subtilis ATCC 2133, and observed a significant drop of surface tension with the recovered biosurfactant65. High yield of biosurfactant from B. subtilis with agro-waste particularly indicates the possibility of achieving the production of C9-biosurfactant in a cost-effective manner41; further investigation into producing C9-biosurfactant with waste nutrient sources could substantially contribute to the industrial application of C9-biosurfactant as an effective biocontrol agent.
Although there is unlikely to be a one-size-fits-all solution for managing unpredictable blooms of algal grazers at industrial algal biomass production, the results of this study clearly supported the potential of C9-biosurfactant as an effective biocontrol agent for cladoceran algal grazers. The additive C9-biosurfactant, however, did not inhibit the growth and lipid productivity of Chlorella sp. HS2 and Scenedesmus deserticola JD052, supporting the non-toxicity of C9-biosurfactant to the selected algal crops. While managing algal pests in industrial algal cultivation platforms is likely to require the harmonious utilization of carefully screened control strategies15, combining the C9-biosurfactant with other effective biocontrol measures will be needed to successfully reduce or completely eliminate algal grazers. Additionally, we urge more careful investigations into optimizing the application strategy of C9-biosurfactant under varying outdoor cultivation conditions at the scales more relevant to industrial algal cultivation.
Methods
Strain selection
Bacterial strain
Kim et al. previously isolated Bacillus subtilis C9 that produces an effective biosurfactant by the method of oil-film collapsing assay34,35,66. The biosurfactant of B. subtilis C9 was determined to be a lipopeptide consisting of a C14–15 fatty acid tail linked to a peptide moiety consisting of 7 amino acid residues that was identical to the peptide moiety of surfactin34,35,67. While the lipopeptides produced by Bacillus strains are considered as the main compounds involved in the biocontrol activities of Bacillus on phytopathogens31,68, surfactin is particularly known for its remarkable surfactant properties and interactions with artificial and biomembrane systems (e.g., bacterial protoplasts or enveloped viruses) that were postulated to give rise to its antibiotic activities34,35,42,48.
Cladoceran algal grazers
While the zooplankton communities of algal cultivation systems are likely to substantially vary across different sites and seasons39, Daphnia pulex and Moina macrocopa were selected as model cladoceran grazers based on their blooms during our year-long operation of wastewater-fed open algal ponds14. Laboratory clones of Daphnia pulex and Moina macrocopa were obtained from a local supplier (Biozoa Biological Supply Co. Ltd, Korea) and Gyeongsang National University (Tongyeong, Korea), respectively. Cladoceran cultures were then maintained at 25 °C in 500-mL beakers at the light intensity of 150 µmol·m−2·s−1 and 14: 10 L/D photoperiod in COMBO medium69. Each zooplankton culture was fed with either Chlorella sp. HS2 or Scenedesmus deserticola JD052 at the algal cell density of 1 × 106 cells/mL. Cladoceran density at each culture was maintained above 300 individuals/L and each beaker was gently shaken twice a day to ensure that algal cells stayed in suspension.
Microalgal strains
While our year-long operation of wastewater-fed open ponds indicated the year-round presence of Chlorella and Scenedesmus at greater dominance levels than most other algal colonizers14,70, Chlorella sp. HS2 and Scenedesmus deserticola JD052 were previously isolated from local wastewater sources. The bacteria-free seed culture of each of these algal strains was subsequently obtained by treating xenic algal cultures in BG-11 medium with ultrasonication and micropicking71. Because the species of Chlorella and Scenedesmus are particularly recognized for their industrial potential for the production of biofuels and bioproducts7,8, we selected these isolated strains to evaluate the influence of C9-biosurfactant on the growth and productivity of algal crops. Prior to the inoculation, samples withdrawn from bacteria-free seed cultures of two algal strains were streaked onto five different types of agar plates that contained organic carbon sources (i.e., YM, R2A, LB, TSA, and BG-11+ 100 ppm of glucose) to assure the absence of culturable bacteria8,70,71.
Production and recovery of biosurfactant from Bacillus subtilis C9
The stock culture of B. subtilis C9 was stored at −80 °C in Luria-Bertani (LB) medium supplemented with 20% (v/v) of glycerol. Upon streaking the stock culture on LB agar plates, an inoculum was prepared by inoculating a single colony of B. subtilis C9 into 10-mL of LB broth in 125-mL Erlenmeyer flask that was subsequently cultivated at 37 °C for 12 hours. The 12-hour-old inoculum was used to seed 200-mL of LB broth medium supplemented with 5 g/L of glucose in each of triplicated 1-L culture flasks; the initial optical density of each culture following inoculation was 0.05 at 600 nm and three culture flasks were incubated at 37 °C for 72 hours on an orbital shaker at 170 rpm. The composition of LB medium was (g/L): NaCl 10.0; tryptone 10.0; yeast extract 5.041,72.
The recovery of C9-biosurfactant was performed as described in previous studies34,35,41. Briefly, the cultures were first centrifuged at 7500 rpm for 20 minutes to remove bacterial cells after a 72-hour-long cultivation period. Upon confirming the production of biosurfactant by measuring the surface tension of culture broth with a ring tension meter at 25 °C (K10ST; Kruss, Hambrug, Germany), the cell-free supernatants were adjusted to pH 2 with 6 M HCl and were subsequently incubated overnight at 4 °C to promote the precipitation of biosurfactant34,35,41. The precipitates were then collected by centrifugation at 10000 rpm for 20 minutes at 4 °C. Afterwards, the biosurfactant of B. subtilis C9 was dissolved in a minimal amount of demineralized water and biosurfactant solution was subsequently lyophilized after adjusting pH to 7 using 1 M NaOH41. The freeze-dried products were weighed to calculate the yield of biosurfactant prior to storing at −20 °C. A known volume of culture medium was used to dissolve the recovered biosurfactant of B. subtilis C9 to explore its biocontrol activity against cladoceran algal grazers and its influence on the growth and productivity of selected algal strains under different concentration gradients. Although direct quantification of surfactin in C9-biosurfactant was not performed in this study, the relative proportion of surfactin in C9-biosurfactant was estimated by comparing critical micelle dilution (CMD) of aqueous solutions of C9-biosurfactant and HPLC-grade surfactin at an identical concentration of 0.3 g/L. CMD was the dilution necessary to reach the point where the surface tension starts to dramatically increase, and was known to be proportional to the amount of surfactin present in the original sample34,73.
Experimental design
Assessment of the biocontrol activity of C9-biosurfactant against cladoceran algal grazers
Chlorella-fed adult cladocerans were first gently harvested by filtering the liquid with a 500 µm mesh filter and carefully rinsed with clean COMBO medium to remove any debris. Subsequently, each of 10-mL glass tubes received 10 individuals of either Daphnia pulex or Moina macrocopa. The average adult body sizes of Daphnia pulex and Moina macrocopa were 1.8 ± 0.3 mm and 1.2 ± 0.2 mm, respectively. Each glass tube was then tested at C9-biosurfactant concentrations of 0, 6, 20, and 60 mg/L in plain COMBO medium. Alive and dead individuals were counted thrice after 1, 12, and 24 hr by considering the cladocerans settled onto the bottom of glass tube without visual movement after gentle shaking as dead. In addition to the recovered C9-biosurfactant, HPLC-grade surfactin from B. subtilis (Sigma-Aldrich, St. Louis, MO, USA) was tested under identical experimental conditions at four concentrations (0, 6, 20, and 60 mg/L) in plain COMBO medium to confirm whether the main surfactant compound of C9-biosurfactant is primarily contributing to its cladoceran control activity34,35. All experiments were performed in a temperature-controlled room at 25 ± 1 °C under the light intensity of 150 µmol·m−2·s−1, and the pH level of each tube culture ranged between 7.0 and 8.0 throughout the experimental period, ensuring no significant influence of ammonia toxicity on the mortality of algal grazers74.
Influence of C9-biosurfactant on the growth of selected algal crops
The influence of C9-biosurfactant on the growth of Chlorella sp. HS2 and Scenedesmus deserticola JD052 was tested to determine whether C9-biosurfactant negatively influenced the growth and productivity of selected algal strains. Prior to inoculation, triplicated 250-mL Erlenmyer flasks were subjected to four different concentrations of C9-biosurfactant (0, 6, 20, and 60 mg/L) in plain BG-11 medium. Either Chlorella sp. HS2 or Scenedesmus deserticola JD052 was then inoculated into each flask culture at the cell density of 1 × 106 cells/mL with the final working volume of 100-mL. Each flask culture was supplemented with 5% CO2 through an autoclavable aeration tube with an inner diameter of 1 mm at 0.1 volume gas per volume culture per minute (vvm), and all cultures were shaken at 100 rpm on an orbital shaker under continuous light at the intensity of 150 µmol·m−2·s−1. Flask cultures were harvested two days after stationary growth phase was reached, and the harvested biomass was subsequently lyophilized to explore the influence of C9-biosurfactant on the biomass and lipid productivity of two algal strains. In addition, Chlorella sp. HS2 and Scenedesmus deserticola JD52 were cultured in 24-well plates without supplemental CO2 at four different concentrations of HPLC-grade surfactin from B. subtilis (0, 6, 20, and 60 mg/L) in plain BG-11 medium to confirm whether the main surfactant compound of C9-biosurfactant negatively influenced the growth of selected algal species under continuous light at the intensity of 150 µmol·m−2·s−1. The final working volume for each of quintuplicated wells was 1.5 mL and the well plates were shaken at 100 rpm on an orbital shaker after covering with PARAFILM® to minimize evaporative loss. Both flask and well plate experiments were performed in a temperature-controlled room at 25 ± 1 °C.
Exploring the biocontrol activity of C9-biosurfactant in grazer-introduced algal cultures
Because blooms of cladoceran grazers in algal cultivation systems will occur under the presence of algal crops, the grazer-control activity of C9-biosurfactant was further evaluated by monitoring the percent mortality of cladoceran grazer in algal cultures at different concentrations of C9-biosurfactant. Each of 10-mL glass tubes was first inoculated with either Chlorella sp. HS2 or Scenedesmus deserticola JD052 at the cell density of 1 × 106 cells/mL, and subsequently received 10 individuals of either Daphnia pulex or Moina macrocopa that were carefully rinsed with clean COMBO medium after being fed for at least 48 hours with the identical algal strain of the culture they were added. Each of triplicated tube cultures was then subjected to four different concentrations of C9-biosurfactant (0, 6, 20, and 60 mg/L) in plain COMBO medium at 25 ± 1 °C under the light intensity of 150 µmol·m−2·s−1. Both alive and dead cladocerans were counted every 12 hours, and algal cell density in each culture was monitored daily throughout a 72-hour-long experimental period. In addition, the growth of each algal strain without the presence of cladoceran grazers was monitored for 72 hours in triplicated tube cultures in plain COMBO medium under identical light and temperature conditions. Because dissolved CO2 and agitation may negatively influence the survival of cladocerans by limiting the exchange of CO2 and exerting shear stress, all tube cultures were only gently shaken twice a day without supplemental CO2 to ensure that algal cells stayed in suspension27,43.
Analytical methods
Identification of C9-biosurfactant by mass spectrometry
While previous studies indicated a lipopeptide surfactin as the main surfactant compound of C9-biosufactant, the recovered C9-biosurfactant and HPLC-grade surfactin from Bacillus subtilis (Sigma-Aldrich, St. Louis, MO, USA) were submitted to mass spectrometry analysis after dissolving each compound into methanol at the concentration of 1 mg/L34,35. High-resolution mass spectra were collected using a Bruker micrOTOF-Q II mass spectrometer (Bruker Daltonics, Bremen, Germany) in ESI positive ion mode over the mass range of m/z 50–2000 under the following optimized settings: end plate offset, −500 V; capillary, 4800 V; nebulizer gas, 0.4 bar; dry gas, 4.0 liters/min; dry gas temperature, 180 °C. The obtained spectra of C9-biosurfactant and HPLC-grade surfactin were subsequently compared to assure the presence of surfactin in the recovered C9-biosurfactant.
Algal growth measurement
Culture growth was measured by either counting cell numbers (i.e., microalgal cell density) under a Nikon FX-A microscope (Optical Analysis Corp., Nashua, Japan) with a hemocytometer (C-chip, NanoEnTek, USA) or measuring optical density at 680 nm using a microplate reader (BioTek, Bad Friedrichshall, Germany). The dry cell weight (DCW) was also determined by filtration onto pre-weighed GF/C filters (Whatman, UK). After rinsing with distilled water, the filters were dried at 105 °C overnight. The filters were then reweighed after drying and the DCW was calculated from the difference between the filter weight with and without algal biomass. In addition, the specific growth rate of each flask culture was calculated during exponential growth phase as:
where µ was the specific growth rate, and C1 and C2 were culture densities at time 1 (t1) and time 2 (t2), respectively.
Lipid content and fatty acid methyl ester (FAME) composition of harvested algal biomass
Each of 250-mL flask cultures was harvested at the end of batch cultivation period by removing supernatant after centrifugation at 4000 rpm for 10 min, and the harvested biomass was subsequently lyophilized and stored in the freezer at −70 °C until further analysis. The lipid content of harvested biomass was analyzed by extracting total lipids from freeze-dried biomass with chloroform-methanol (2:1 (v/v)) following a slightly modified version of Bligh and Dyer’s method7,75,76. FAME composition of lyophilized biomass was analyzed using a gas chromatograph (Shimadzu GC-2010, Japan). Each of 50 mg samples was first placed into capped test tubes, saponified with 1 mL of a saturated KOH-CH3OH solution at 75 °C for 10 min, and submitted to methanolysis with 5% HCl in methanol at 75 °C for another 10 min8,77. Fatty acids containing phase was subsequently separated by adding 2 mL of distilled water and recovered8,77. The components were identified by comparing their retention times and peak areas with those of FAME Mix, C8-C24 (18918-1AMP, Supelco, Sigma-Aldrich, St. Louis, MO, USA)8,76,77.
Algal gazer mortality
The % mortality of cladoceran grazer in each tube culture was calculated as:
where n0 was the total number of cladoceran grazer at time 0, and nt was the number of alive individuals at each time point. The individuals settled onto the bottom of tube culture without visual movement after gentle shaking were considered as dead, and no newborn cladocerans were observed across all experimental treatments.
Statistical analysis
The results were presented as either mean values or mean values ± standard deviation from at least triplicated independent measurements unless denoted otherwise. The statistical significance of differences in specific growth rate and lipid productivity between treatments was evaluated by using 95% confidence intervals for the mean.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Change history
20 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Day, J. G., Slocombe, S. P. & Stanley, M. S. Overcoming biological constraints to enable the exploitation of microalgae for biofuels. Bioresour. Technol. 109, 245–251 (2012).
Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2007).
Hu, Q. et al. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 54, 621–639 (2008).
Chen, C.-Y., Yeh, K.-L., Aisyah, R., Lee, D.-J. & Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 102, 71–81 (2011).
Lammers, P. J. et al. Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 22, 166–186 (2017).
Pate, R., Klise, G. & Wu, B. Resource demand implications for US algae biofuels production scale-up. Appl. Energy 88, 3377–3388 (2011).
Yun, J.-H. et al. Hybrid operation of photobioreactor and wastewater-fed open raceway ponds enhances the dominance of target algal species and algal biomass production. Algal Res. 29, 319–329 (2018).
Kakarla, R. et al. Application of high-salinity stress for enhancing the lipid productivity of Chlorella sorokiniana HS1 in a two-phase process. J. Microbiol. 56, 56–64 (2018).
Greenwell, H., Laurens, L., Shields, R., Lovitt, R. & Flynn, K. Placing microalgae on the biofuels priority list: a review of the technological challenges. J. R. Soc. Interface 7, 703–726 (2010).
McBride, R. C. et al. Contamination management in low cost open algae ponds for biofuels production. Ind. Biotechnol. 10, 221–227 (2014).
Peng, L., Lan, C. Q., Zhang, Z., Sarch, C. & Laporte, M. Control of protozoa contamination and lipid accumulation in Neochloris oleoabundansculture: effects of pH and dissolved inorganic carbon. Bioresour. Technol. 197, 143–151 (2015).
Day, J. G., Gong, Y. & Hu, Q. Microzooplanktonic grazers–a potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 27, 356–365 (2017).
Cauchie, H., Hoffmann, L., Jaspar-Versali, M., Salvia, M. & Thomé, J. Daphnia magna Straus living in an aerated sewage lagoon as a source of chitin: ecological aspects. Belg. J. Zool (1995).
Cho, D.-H. et al. Microalgal diversity fosters stable biomass productivity in open ponds treating wastewater. Sci. Rep. 7, 1979 (2017).
White, R. L. & Ryan, R. A. Long-term cultivation of algae in open-raceway ponds: lessons from the field. Ind. Biotechnol. 11, 213–220 (2015).
Richardson, J. W. et al. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 4, 96–104 (2014).
Beyter, D. et al. Diversity, productivity, and stability of an industrial microbial ecosystem. Appl. Environ. Microbiol. 82, 2494–2505 (2016).
Lincoln, E., Hall, T. & Koopman, B. Zooplankton control in mass algal cultures. Aquaculture 32, 331–337 (1983).
Van Ginkel, S. W. et al. The prevention of saltwater algal pond contamination using the electron transport chain disruptor, rotenone. Algal Res. 18, 209–212 (2016).
Zmora, O. R. A. Microalgae for aquaculture: microalgae production for aquaculture in Handbook of Microalgal Culture: Biotechnology and Applied Phycology (eds Richmond, A. & Hu, Q.) 365–379 (Blackwell Publishing, 2013).
Park, S. et al. The selective use of hypochlorite to prevent pond crashes for algae-biofuel production. Water Environ. Res. 88, 70–78 (2016).
Montemezzani, V., Duggan, I. C., Hogg, I. D. & Craggs, R. J. Screening of potential zooplankton control technologies for wastewater treatment High Rate Algal Ponds. Algal Res. 22, 1–13 (2017).
Wang, H., Zhang, W., Chen, L., Wang, J. & Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 128, 745–750 (2013).
Pimentel, D. & Edwards, C. A. Pesticides and ecosystems. BioScience 32, 595–600 (1982).
Fox, J. E., Gulledge, J., Engelhaupt, E., Burow, M. E. & McLachlan, J. A. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. USA 104, 10282–10287 (2007).
Kazamia, E., Aldridge, D. C. & Smith, A. G. Synthetic ecology–a way forward for sustainable algal biofuel production? J. Biotechnol. 162, 163–169 (2012).
Montemezzani, V., Duggan, I. C., Hogg, I. D. & Craggs, R. J. A review of potential methods for zooplankton control in wastewater treatment High Rate Algal Ponds and algal production raceways. Algal Res. 11, 211–226 (2015).
Shurin, J. B. et al. Industrial‐strength ecology: trade‐offs and opportunities in algal biofuel production. Ecol. Lett. 16, 1393–1404 (2013).
Smith, V. H. & Crews, T. Applying ecological principles of crop cultivation in large-scale algal biomass production. Algal Res. 4, 23–34 (2014).
Yun, J.-H., Smith, V. H., La, H.-J. & Chang, Y. K. Towards managing food-web structure and algal crop diversity in industrial-scale algal biomass production. Curr. Biotechnol. 5, 118–129 (2016).
Deravel, J. et al. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl. Microbiol. Biotechnol. 98, 6255–6264 (2014).
Thakore, Y. The biopesticide market for global agricultural use. Ind. Biotechnol. 2, 194–208 (2006).
Correa, O. S. & Soria, M. A. Potential of Bacilli for biocontrol and its exploration in sustainable agriculture in Plant Growth and Health Promoting Bacteria (ed. Maheshwari, D.) 197–209 (Springer, 2010).
Kim, H.-S. et al. A lipopeptide biosurfactant produced by Bacillus subtilis C9 selected through the oil film-collapsing assay. J. Microbiol. Biotechnol. 7, 180–188 (1997).
Kim, H.-S. et al. Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J. Ferment. Bioengineer. 84, 41–46 (1997).
Geetha, I. & Manonmani, A. Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis ssp. subtilis (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. Lett. Appl. Microbiol. 51, 406–412 (2010).
Geetha, I., Manonmani, A. & Paily, K. Identification and characterization of a mosquito pupicidal metabolite of a Bacillus subtilis subsp. subtilis strain. Appl. Microbiol. Biotechnol. 86, 1737–1744 (2010).
Geetha, I., Prabakaran, G., Paily, K., Manonmani, A. & Balaraman, K. Characterisation of three mosquitocidal Bacillus strains isolated from mangrove forest. Biol. Control 42, 34–40 (2007).
Montemezzani, V., Duggan, I. C., Hogg, I. D. & Craggs, R. J. Zooplankton community influence on seasonal performance and microalgal dominance in wastewater treatment High Rate Algal Ponds. Algal Res. 17, 168–184 (2016).
Song, Y., Talaty, N., Datsenko, K., Wanner, B. L. & Cooks, R. G. In vivo recognition of Bacillus subtilis by desorption electrospray ionization mass spectrometry (DESI-MS). Analyst 134, 838–841 (2009).
Gudiña, E. J., Fernandes, E. C., Rodrigues, A. I., Teixeira, J. A. & Rodrigues, L. R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 6 (2015).
Vollenbroich, D., Özel, M., Vater, J., Kamp, R. M. & Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 25, 289–297 (1997).
Montemezzani, V., Duggan, I. C., Hogg, I. D. & Craggs, R. J. Control of zooplankton populations in a wastewater treatment High Rate Algal Pond using overnight CO2 asphyxiation. Algal Res. 26, 250–264 (2017).
Yuan, D. et al. Biodiversity and distribution of microzooplankton in Spirulina (Arthrospira) platensis mass cultures throughout China. Algal Res. 30, 38–49 (2018).
Manonmani, A. M., Geetha, I. & Bhuvaneswari, S. Enhanced production of mosquitocidal cyclic lipopeptide from Bacillus subtilis subsp. subtilis. Indian J. Med. Res. 134, 476 (2011).
Lechuga, M., Fernández-Serrano, M., Jurado, E., Núñez-Olea, J. & Ríos, F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 125, 1–8 (2016).
Steiner, C. F. Context-dependent effects of Daphnia pulex on pond ecosystem function: observational and experimental evidence. Oecologia 131, 549–558 (2002).
Vollenbroich, D., Pauli, G., Ozel, M. & Vater, J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl. Environ. Microbiol. 63, 44–49 (1997).
Holm, N. P. & Shapiro, J. An examination of lipid reserves and the nutritional status of Daphnia pulex fed Aphanizomenon flos‐aquae. Limnol. Oceanogr. 29, 1137–1140 (1984).
Sterner, R. W., Hagemeier, D. D., Smith, W. L. & Smith, R. F. Phytoplankton nutrient limitation and food quality for. Daphnia. Limnol. Oceanogr. 38, 857–871 (1993).
Buchoux, S. et al. Surfactin-triggered small vesicle formation of negatively charged membranes: a novel membrane-lysis mechanism. Biophys. J. 95, 3840–3849 (2008).
Griffiths, M. J. & Harrison, S. T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21, 493–507 (2009).
Taoka, Y. et al. Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. J. Biosci. Bioeng. 111, 420–424 (2011).
Boelen, P., van Dijk, R., Damsté, J. S. S., Rijpstra, W. I. C. & Buma, A. G. On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 3, 26 (2013).
Shin, H. et al. Genome-wide transcriptome analysis revealed organelle specific responses to temperature variations in algae. Sci. Rep. 6, 37770 (2016).
Suzuki, I., Los, D. A., Kanesaki, Y., Mikami, K. & Murata, N. The pathway for perception and transduction of low‐temperature signals in Synechocystis. EMBO J. 19, 1327–1334 (2000).
Rieβ, M. & Grimme, L. Studies on surfactant toxicity to the freshwater alga Chlorella fusca: a common mode of action? Sci. Total Environ. 134, 551–558 (1993).
Ahn, C.-Y. et al. Selective control of cyanobacteria by surfactin-containing culture broth of Bacillus subtilis C1. Biotechnol. Lett. 25, 1137–1142 (2003).
Flynn, K. J., Kenny, P. & Mitra, A. Minimising losses to predation during microalgae cultivation. J. Appl. Phycol. 1–12 (2017).
Edwards, K. F., Thomas, M. K., Klausmeier, C. A. & Litchman, E. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnol. Oceanogr. 57, 554–566 (2012).
Litchman, E. & Klausmeier, C. A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39, 615–639 (2008).
Narwani, A. et al. Common ancestry is a poor predictor of competitive traits in freshwater green algae. PLoS One 10, e0137085 (2015).
Hansen, P. J., BjØrnsen, P. K. & Hansen, B. W. Zooplankton grazing and growth: scaling within the 2–2,000‐µm body size range. Limnol. Oceanogr. 45, 1891–1891 (2000).
Duquesne, S., Reynaldi, S. & Liess, M. Effects of the organophosphate paraoxon‐methyl on survival and reproduction of Daphnia magna: importance of exposure duration and recovery. Environ. Toxicol. Chem. 25, 1196–1199 (2006).
Fox, S. L. & Bala, G. A. Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresour. Technol. 75, 235–240 (2000).
Jain, D., Collins-Thompson, D., Lee, H. & Trevors, J. A drop-collapsing test for screening surfactant-producing microorganisms. J. Microbiol. Methods 13, 271–279 (1991).
Ullrich, C., Kluge, B., Palacz, Z. & Vater, J. Cell-free biosynthesis of surfactin, a cyclic lipopeptide produced by Bacillus subtilis. Biochemistry 30, 6503–6508 (1991).
Ongena, M. & Jacques, P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125 (2008).
Kilham, S. S., Kreeger, D. A., Lynn, S. G., Goulden, C. E. & Herrera, L. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159 (1998).
Cho, D.-H. et al. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 191, 481–487 (2015).
Cho, D. H. et al. Novel approach for the development of axenic microalgal cultures from environmental samples. J. Phycol. 49, 802–810 (2013).
Pathak, K. V. & Keharia, H. Identification of surfactins and iturins produced by potent fungal antagonist, Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) tree using mass spectrometry. 3 Biotech. 4, 283–295 (2014).
Cooper, D., Macdonald, C., Duff, S. & Kosaric, N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl. Environ. Microbiol. 42, 408–412 (1981).
Thomas, P. K., Dunn, G. P., Passero, M. & Feris, K. P. Free ammonia offers algal crop protection from predators in dairy wastewater and ammonium-rich media. Bioresour. Technol. 243, 724–730 (2017).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37, 911–917 (1959).
Lee, J.-Y., Yoo, C., Jun, S.-Y., Ahn, C.-Y. & Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 101, S75–S77 (2010).
Kang, Z. et al. A cost analysis of microalgal biomass and biodiesel production in open raceways treating municipal wastewater and under optimum light wavelength. J. Microbiol. Biotechnol. 25, 109–118 (2015).
Acknowledgements
The authors thank Dr. Seok-Joong Kang at the Department of Marine Biology and Aquaculture at Gyeongsang National University for kindly providing the lab clones of Moina macrocopa, and JY thanks Ted Harris of the Kansas Biological Survey for extremely thoughtful comments on the manuscript. This work was supported by the Advanced Biomass R&D Center (ABC) of the Global Frontier Program funded by the Ministry of Science and ICT of the Republic of Korea (ABC-2016922286), and a grant from KRIBB Research Initiative Program (www.kribb.re.kr).
Author information
Authors and Affiliations
Contributions
J.Y., D.C., and H.K. conceived and designed the experiment; J.Y. acquired data; J.Y., D.C., B.L., H.K., and Y.G.C. analyzed data; J.Y., H.K., and Y.G.C. wrote and revised the manuscript; all authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yun, JH., Cho, DH., Lee, B. et al. Application of biosurfactant from Bacillus subtilis C9 for controlling cladoceran grazers in algal cultivation systems. Sci Rep 8, 5365 (2018). https://doi.org/10.1038/s41598-018-23535-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23535-8
- Springer Nature Limited
This article is cited by
-
Biotechnologies for bulk production of microalgal biomass: from mass cultivation to dried biomass acquisition
Biotechnology for Biofuels and Bioproducts (2023)