Abstract
Using surfactants to extract oil, the anionic surfactant Karamay petroleum sulfonate (KPS), the zwitterionic surfactant octadecyl betaine (BS-18) and the nonionic surfactant coconut oil fatty acid diethanolamide (6501) were selected for adsorption experiments with minerals contained in the conglomerate reservoir with different mineral compositions to study the adsorption law of different types of surfactants. Zeolite and montmorillonite, which have the highest specific surface area and zeta potential among the minerals in the conglomerate reservoir, have the most obvious adsorption effect on surfactants, resulting in a large amount of adsorption of KPS and BS-18. The three types of surfactants were then used to conduct physical simulation oil recovery experiments with conglomerate core samples, and the results showed that 6501 had better overall performance, the best adsorption resistance, and a higher degree of recovery in oil recovery experiments, which provided a basis for the selection of surfactants in the process of chemical drive in conglomerate reservoirs.
Article Highlights
-
(1)
The complex mineral composition and physicochemical properties of conglomerate reservoirs in the Junggar Basin are analyzed.
-
(2)
The adsorption degree of different types of surfactants on rock minerals in conglomerate reservoirs was studied.
-
(3)
The formula for chemical oil recovery in conglomerate reservoirs can be optimized through analysis and research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the increasing demand for energy and the continuous development of oil and gas prediction and development technology, the study of special reservoirs such as conglomerates has received more and more attention. Conglomerate reservoirs are reservoirs dominated by coarse clastic rocks such as conglomerate, conglomerate sandstone, etc. China is dominated by terrestrial oil-producing reservoirs, and conglomerate reservoirs do not account for a high percentage in general, but they account for a higher percentage in some areas, such as in the Junggar Basin of Xinjiang, where the percentage of conglomerate reservoirs reaches nearly 50% (Zhu et al. 2015). The conglomerate reservoirs in the Junggar Basin have a wide variety of minerals, especially the authigenic clay minerals are widely distributed, which have an important influence on the storage performance of conglomerate reservoirs (Yu et al. 2023). The content and types of clay minerals reflect the mineral evolution process during reservoir deposition, which can reflect the physical characteristics of the reservoir to a certain extent (Li et al. 2023). In addition, Guo Hui and others found that zeolite cements are also widely developed in glutenite reservoirs in the Junggar Basin (Guo et al. 2022). The presence of large amounts of clay minerals with zeolite-like minerals is not conducive to reservoir development.
At present, in the process of extracting conglomerate reservoirs, most of the primary and secondary oil recovery can only collect one-third of the oil, and there is still a large amount of crude oil that is difficult to recover. With the continuous development of oil recovery technology, there are three oil recovery technologies such as chemical drive (Hammond et al. 2011; Murison et al. 2014; Zhao et al. 2014), but there are not many studies related to chemical drive in conglomerate reservoirs.
Chemical drive is a method of oil recovery in which chemical agents are added to the injected water to change the physical and chemical properties of the replacement fluid and the interface between the replacement fluid and the crude oil and rock minerals, thus facilitating the production of crude oil (Herawati et al. 2022; Wang 2001). Depending on the chemicals used, conventional chemical drives generally include alkali drives, polymer drives, surfactant/polymer binary composite drives and alkali/surfactant/polymer ternary composite drives. With the development of oil drive theory and the research and development of new oil drive agents, new types of chemical drives such as foam drive and nano-composite drive have also appeared (Muggeridge et al. 2013; Liao et al. 2017; Hosny et al. 2023; Wang et al. 2012). Among them, surfactants play an important role in tertiary oil recovery. Generally speaking, commonly used surfactants include cationic, anionic, zwitterionic and nonionic types.The adsorption of cationic surfactants on sandstone reservoirs is significant, while the adsorption of anionic surfactants on carbonate reservoirs is significant (Lee et al. 2012). Losses due to adsorption of anionic and cationic surfactants also increase at higher salinities, resulting in unsuitability of the surfactant (Belhaj et al. 2020). Researchers have been able to show that the main driving forces behind surfactant adsorption are the surfactant type, physical (lithology) and chemical properties of reservoir rocks (Bera et al. 2013). So different minerals will affect surfactants differently, and their adsorption on rock minerals have different patterns and characteristics.
Most surfactants are stable and suitable under general conditions, and their adsorption has both positive and negative aspects (Kumar and Mandal 2019). Appropriate adsorption of surfactants on mineral surfaces can alter important interfacial properties and thus improve recovery, while excessive adsorption can be costly and of limited effectiveness (Zhong et al. 2019). Surfactant oil recovery can weaken the tension between the oil–water interface, change the wettability of the rock, so that the water and oil phases in the rock pores can be driven out, so that the oil-driving efficiency and recovery rate can be further improved (Hirasaki et al. 2011; Baek et al. 2022). The main factor that makes surfactants susceptible to depletion in porous media such as conglomerate reservoirs is adsorption, which ultimately leads to reduced recovery. The adsorption of surfactants is mainly physical and chemical adsorption such as electrical adsorption, ion exchange adsorption, intermolecular gravitational adsorption, and formation of hydrogen bond adsorption. The most common form of surfactant consists of a hydrocarbon tail chain and a polar head, and its main mechanism of adsorption is the electrostatic attraction between the charged solid surface and the charged polar head group of the surfactant molecule (Zhang et al. 2015).
In this paper, the minerals contained in the conglomerate reservoir were first analyzed, and the physicochemical properties of the minerals in the conglomerate reservoir were investigated through the determination of specific surface and zeta potential. Then different types of surfactants were selected for adsorption experiments with conglomerate reservoir minerals to determine the degree of interaction between different types of surfactants and conglomerate reservoir minerals. Finally, oil recovery experiments were conducted on natural conglomerate cores saturated with oil using chemical formulations of different types of surfactants to relate recovery rates to dynamic adsorption experiments. The selection and use of surfactants determine the oil recovery efficiency of chemical drive, and the study of the adsorption law between surfactants and rock minerals in conglomerate oil reservoirs has theoretical significance and practical guidance for the development of conglomerate oil reservoirs.
2 Experimental section
2.1 Materials and instruments
2.1.1 Materials
-
(1)
Surfactants: ① Anionic surfactant: karamay petroleum sulfonate, this is also the surfactant currently used in chemical oil recovery of conglomerate reservoirs in Xinjiang, abbreviated as KPS; ② Nonionic surfactant: coconut oil fatty acid diethanolamide, abbreviated as 6501; ③ Zwitterionic surfactant: octadecyl betaine, abbreviated as BS-18;
-
(2)
Polymer: Salt-resistant polymer, polyacrylamide (HPAM) as the main component, relative molecular weight 15 million, abbreviated as HPAM;
-
(3)
Chemical reagents: NaHCO3: analytically pure; NaCl: analytically pure;
-
(4)
Mineral and rock materials: ① Minerals: zeolite, montmorillonite, kaolinite, illite, chlorite, sodium feldspar, calcite; ② Cores: conglomerate core from Junggar Basin in XinJiang; ③ Chemically driven extractives from conglomerate reservoirs: Oil bearing mud and sand, it was extracted with petroleum ether, dried, pulverized, and sieved to a particle size of about 800 mesh before the experiment;
-
(5)
Experimental water: conglomerate reservoir formation water, NaHCO3 type, mineralization 10000mg/L, its parameters are shown in Table 1.
Table 1 Formation water parameters of conglomerate reservoir
2.1.2 Experimental instruments
High Resolution Field Emission Scanning Electron Microscope, FEIVerios460, FEI, USA; Ultraviolet–Visible Spectrophotometer, UV-2200, Shunyu Hengping, Shanghai; Interfacial Tension Meter, TX-500C, Zhongchen, Shanghai; Specific Surface Pore Sizing Analyzer, V-Sorb2800P, Beijing Guoyi Precision Measurement Technology Co. Zeta Potential Analyzer, ZetasizerNanoZS, Malvern, UK; X-ray Diffraction Analyzer, D8Quest, Bruker, Germany; Magnetic Stirring Kettle with Water Bath, Keheng, Shanghai; Multi-functional Experimental Device for Oil Recovery in Core, Haian Petroleum Research Instrument Co.
2.2 Methods
2.2.1 Analysis of mineral composition and content of conglomerate reservoirs
Both conglomerate reservoir blocks and extracts were ground into fine powdery samples, and the mineral compositions were quantitatively analyzed by X-ray diffraction analysis, according to the standard of the oil and gas industry of the People’s Republic of China, SY/T 5162-2018, “Analysis method for clay minerals and ordinary non-clay minerals in sedimentary rocks by the X-ray diffraction”.
2.2.2 Mineral specific surface determination in conglomerate reservoirs
The specific surface and pore size of the conglomerate samples and the minerals contained in the reservoir were determined by a nitrogen adsorption specific surface pore size analyzer using the BET method and the Langmuir model as the basic working principle. Block samples were used for the conglomerates instead of powder samples, which preserves the fixed structure of the samples as much as possible and is closer to the real specific surface of the conglomerate reservoirs.
2.2.3 Zeta potential measurement
The nature of the surface charge of rock minerals and surfactants was determined by means of a zeta potential meter using Doppler electrophoresis as the basic operating principle.
2.2.4 Surfactant interfacial tension determination
By adopting the rotating drop method and taking into account the actual situation at the production site, a concentration of 3000 ppm was selected as the measurement concentration of surfactant, and the surfactant solution prepared from the formation water of the conglomerate reservoir was loaded into a sample tube, to which a drop of crude oil extracted from the conglomerate reservoir was then added. According to the oil and gas industry standard of the People’s Republic of China, SYT5370-2018, “Test method for surface tension and interfacial tention”, under the action of centrifugal force, gravity and interfacial tension, the crude oil of the low-density phase forms an ellipsoidal or cylindrical droplet in the surfactant solution of the high-density phase, and the shape of which is determined by the rotational speed and interfacial tension. Using an interfacial tensiometer, the equilibrium interfacial tension was determined after the interfacial tension was stabilized by setting the temperature at 30 °C (to simulate the formation temperature) and rotating at an angular speed of 5000 r/s. The equilibrium interfacial tension was determined by using a tensiometer.
2.2.5 Wettability test
Natural core sand with particle size less than 0.25 mm and dehydrated crude oil shall be mixed evenly at a mass ratio of 7:1, and placed in an oven at 30 °C (target formation temperature) for thermal aging for more than 48 h. The aged oil-bearing core sand shall be adhered to quartz with Double-sided tape to form an oil-bearing natural core sand mold, and different types of surfactant solutions shall be prepared with injected water as the aqueous phase, measure the contact angle of surfactant solution on the surface of oil bearing natural core sand model using a fully automatic contact angle tester.
2.2.6 Adsorption experiments
-
(1)
Standard curve drawing
Surfactant solutions were prepared with stratum water, and each surfactant was configured with five different concentrations of 10 ppm, 30 ppm, 50 ppm, 100 ppm and 200 ppm. The wavelength profiles of the five different concentration solutions were measured by ultraviolet spectrophotometer, and the absorbance at the same peak (wavelength) was determined, and the standard curve was plotted by connecting the five points.
-
(2)
Static adsorption experiment
Dilute the surfactant solution prepared with formation water to a series of concentrations as the initial concentration, noted as C0; the conglomerate reservoir rock minerals and surfactant solution were added to a stoppered, mill-necked conical flask at a solid–liquid ratio of 1:9, shake it well, then cover the stopper and seal it well; place the conical flask in a constant-temperature water bath at 30 °C with a rotational speed of 60 r/min, and leave it there for 24 h (at this time, the adsorption has reached equilibrium) Remove; the adsorbed solution after shaking well poured into the centrifuge tube, centrifugal separation at 4000 r/min speed for 30 min; the upper clear liquid in the centrifuge tube was taken, shaken well and the concentration of surfactant in the clear liquid was determined. This concentration is the equilibrium concentration when the adsorption reaches equilibrium and is denoted as Ct (Ni et al. 2018); The static adsorption capacity was calculated according to the following formula (1):
In the equation, Γ-static adsorption capacity, denotes the number of milligrams of surfactant adsorbed per gram of mineral, mg/g; V-volume of surfactant solution, L;C0-initial concentration of surfactant in solution, mg/L; Ct-final concentration of surfactant solution after solution adsorption equilibrium, mg/L; G-mass of conglomerate cores, minerals, g.
-
(3)
Dynamic adsorption experiments
Using the multifunctional core oil recovery experimental device (Fig. 1), the conglomerate core was loaded into the gripper, and each surfactant was loaded into the intermediate container, the thermostat system was turned on, and the constant temperature was simulated for about one hour at the simulated stratigraphic temperature (30 °C). The injection pump was turned on, and the amount of the injection was adjusted, and the water was injected firstly, until the injection pressure was stabilized; the surfactant is then injected and samples are continuously taken at the outflow end to detect changes in surfactant concentration until the surfactant concentration is equal to or close to the initial concentration injected; finally, water was injected again to drive out the surfactant until the concentration of surfactant in the effluent was equal to or close to 0. Based on the measured concentration of surfactant in the effluent sample and the volume of the sample, the dynamic adsorption amount of surfactant was calculated by using the principle of material balance (Chen et al. 2016).
2.2.7 Oil recovery experiments through physical simulation
The experiment simulated the actual conglomerate reservoir chemical drive production site, firstly, the polymer/surfactant binary composite drive mode was selected, and the natural conglomerate core was evacuated by vacuum pump for 4 h, and then the natural conglomerate core was saturated with crude oil under the condition of 30 °C for 20 h, and the oil-bearing saturation (SO) and pore volume (PV) were calculated.
Prepare the multifunctional core drive oil recovery device, simulate the actual formation pressure of 12 MPa, the actual formation temperature of 30 C, and prepare the simulated injection water with the same mineralization as the formation. Start the advection pump, set the flow rate to 0.3 ml/min, and start injecting water to drive the oil, injecting two core pore volumes (i.e., 2PV) of water, at which time the water content at the extraction end can basically reach over 90%; then change the polymer/surfactant binary drive, open the corresponding valve, turn off the valve corresponding to the water drive, and after injecting a predetermined volume (2PV), turn off the valve corresponding to the chemical drive, and finally carry out the subsequent water drive for 2PV. Record the amount of oil recovered at each stage with the injection pressure to calculate the degree of oil recovery.
3 Results and discussion
3.1 Influence of mineral components
3.1.1 Composition and content of minerals
Conglomerate reservoirs are different from sandstone reservoirs in that they are very non-homogeneous, not only in physical characteristics such as pore structure, but also in the extreme complexity of the mineral rocks of which they are basically composed. Natural conglomerate cores were used to analyze the mineral composition and ingredients.
Firstly, the surface of the conglomerate core sample was scanned by energy dispersive spectroscopy (EDS) to obtain the mineral element composition (Fig. 2). From the surface energy spectrum scanning elements of the conglomerate sample in Fig. 2, it can be inferred that the surface of the conglomerate reservoir contains a large amount of Si and Al elements in addition to conventional elements such as C, N, O, and S. The Al element content reaches 13%, and the Si element content reaches 25%. Therefore, it is inferred that the surface of the reservoir particles contains a large amount of water aluminum silicate products.
The surface minerals of the conglomerate reservoir were then characterized by scanning electron microscopy (SEM). It was observed that the surface composition of the conglomerate reservoir skeleton particles was complex, with the presence of a large number of clay minerals as well as zeolite minerals. The characteristic of kaolinite is most prominent in the sample, with page shaped kaolinite monomers and aggregates visible everywhere, as shown in Fig. 3a; Simultaneously, illite with bridging pseudo hexagonal crystals was observed, as shown in Fig. 3b; There are also coniferous and Flat noodles shaped chlorite secondary growth on the mineral surface, Fig. 3c, honeycomb and flame like illite and montmorillonite mixed layer filling the interior of the pores, Fig. 3d, and single crystal structure turbidite zeolites and spherical particle square zeolites, Fig. 3e, f.
Characterization of surface mineral morphology in conglomerate reservoir. a Kaolinite observed on the surface of the conglomerate reservoir (image resolution 10 um), b Illite observed on the surface of the conglomerate reservoir (image resolution 2 um), c Chlorite observed on the surface of the conglomerate reservoir (image resolution 5 um), d Illite Montmorillonite mixed layer observed on the surface of the conglomerate reservoir (image resolution 30 um), e Turbidite zeolite observed on the surface of the conglomerate reservoir (image resolution 5 um), f Square zeolite observed on the surface of the conglomerate reservoir (image resolution 5 um)
Finally, the mineral content was analyzed by X-ray diffraction (XRD), and the results of the mineral content analysis of the natural core samples from the conglomerate reservoir were considered to be before the conglomerate reservoir was exploited, and the results of the mineral content analysis of the chemically driven extracts from the conglomerate reservoir were considered to be after the conglomerate reservoir was exploited, and the results are shown in Fig. 4.
The natural conglomerate reservoir is dominated by feldspar and quartz, with 34.1% feldspar and 37.5% quartz; The cementing material is dominated by sulfate minerals and clay minerals, with contents of 10.8% and 10.5%, the content of carbonate minerals is relatively low at 2.6%, and the content of zeolite minerals is about 3.7%, mainly turbidite and square zeolite, with a very small amount of corundum present. The complexity and variability of mineral types in conglomerate reservoirs have many hidden effects on the recovery efficiency of late-stage surfactants, which include the fact that surfactants can adsorb different grades of minerals on each mineral, and losses can occur, thus affecting the recovery rate.
The mineral composition of the conglomerate reservoirs has not changed essentially after chemical recovery. Feldspar and quartz, as the backbone minerals of the reservoir, are still most present. The proportion of carbonate minerals and clay minerals has become slightly smaller, the proportion of sulfate minerals and corundum has slightly increased, and the obvious change is the zeolite minerals, whose content has increased from 3.7 to 8.1%.
Clay minerals, a group of minerals that are relatively active in nature in conglomerate reservoirs, were analyzed for their relative content (Fig. 5). Kaolinite has the highest relative content of 36.9%, followed by a mixture of illite and montmorillonite at 30.5%, illite at 29.2%, and chlorite at the lowest relative content of 3.4%. It can be seen that there are large amounts of illite and montmorillonite in the clay minerals of conglomerate reservoirs, and both of them are transformed into mixed-layer minerals in large quantities during the diagenetic evolution, it is referred to as I-M mixed layer.
The clay mineral content in the total content after chemical drive did not change much, but the relative content changed significantly, the content of ilmenite mixed layer in the extracted material turned out to be the largest, increasing from 30.5 to 44%, illite content was the second largest, kaolinite content became significantly smaller, and the content of chlorite remained the smallest. In terms of mineral sensitivity, montmorillonite is a water-sensitive mineral, kaolinite is a quick-sensitive mineral, and illite and chlorite are in the middle.
The high percentage of zeolite and I-M mixed layer after chemical oil recovery indicates that these two minerals are very easy to hydrate, strong adsorption, a large number of adsorption of formation water and chemical agents, along with the injection of the formation of chemical together with the agent migration and be taken out of the formation. Therefore, in the face of conglomerate reservoirs with high content of clay minerals and zeolite minerals, it is especially important to choose suitable surfactants.
3.1.2 Physico-chemical properties of minerals
Specific surface measurements were performed on conglomerate reservoir core samples and contained minerals (Table 2). It can be seen that the specific surface of clay minerals is significantly larger than that of skeletal and carbonate minerals. As for the clay minerals, kaolinite has the smallest specific surface, 15.7 m2/g, illite has 20.62 m2/g, chlorite has 23.76 m2/g, montmorillonite has the largest specific surface area, 40.21 m2/g, and zeolite has a large specific surface, 31.58 m2/g. The specific surface of the actual core of the conglomerate is larger than that of the skeletal minerals quartz and feldspar, which also indicates that the constituent minerals of the conglomerate reservoir are more complex, with a large number of minerals with large specific surfaces present.
A larger specific surface means a stronger adsorption, and the strong specific surface free energy readily interacts physicochemically with chemical agents. Previous research also found that the clay minerals, montmorillonite adsorption is the strongest, it is a 2:1 type clay minerals, up and down the adjacent levels are O surface, the gravitational force between the crystal layer to intermolecular force is dominated by the interlayer gravitational force is weak, water molecules are easy to enter the crystal layer, montmorillonite exists lattice substitution, the number of cations that can be exchanged is many (Derjaguin and Landau et al. 1993), due to the lattice substitution produces more negative charge, around it, will inevitably be adsorbed equal amount of cations, hydrated cations to the clay to bring a thicker hydration film, which results in the expansion of montmorillonite. Montmorillonite and zeolite have strong adsorption, ion exchange, however, the two are not “one mother and one sibling”, zeolite is silica-aluminate minerals, montmorillonite is clay minerals, zeolite molecular sieve structure has a stronger adsorption, while montmorillonite will be swollen in contact with water. It has been analyzed by XRD that conglomerate reservoirs contain large amounts of I-M mixed layer minerals, which, in general, are more susceptible to swelling and dispersion in contact with water than single clay minerals.
Different types of conglomerate reservoir samples with different types of surfactants were selected to determine the nature of the surface charge of rock minerals and chemicals, and the results are shown in Table 3. The zeta potential distribution of conglomerate reservoirs and minerals ranges from 0 to − 20 mV, with a wide span of potentials, mostly belonging to unstable systems. The larger the specific surface area of the clay minerals, the more charged they are, such as montmorillonite and zeolite. Skeleton mineral feldspar and carbonate mineral calcite, the electrification is very small.KPS surfactant belongs to anionic surfactant, Zeta potential is around − 40 mV, the higher the concentration, the better the stability, the comprehensive cost considerations, the general oilfield production using concentration of 3000 ppm.Nonionic surfactant 6501 is less electrically charged, and zwitterionic surfactant BS-18 is charged with Minimum.
DLVO theory (the theory that describes colloidal stability) suggests that the stability of a colloidal system is the net structure of the double electric layer mutual repulsion and van der Waals mutual attraction between particles as they approach each other. It is in the form of a colloidal solution that clay minerals exist in subsurface reservoirs. The energy barrier between the particles as they approach each other comes from the mutual repulsion force, and when the particles have enough energy to overcome this barrier, the mutual suction force will cause the particles to approach further and stick together irreversibly (Elimelech and O’Melia 1990a, b; Zhao 2010). The presence of these physicochemically active minerals in conglomerate reservoirs has a great impact on surfactant injection, where zeolites and I-M mixtures have a huge specific surface with very strong chargeability, they adsorb a lot of formation water and surfactant, causing the formulation system of chemical oil recovery to be altered in the reservoir and reducing the energy efficiency of the surfactant.
3.2 Effect of interfacial tension
Surfactant solutions injected into oil formations can reduce the interfacial tension between oil and water, oil and rock, and change the wettability of the rock to improve recovery. Reducing the oil–water interface to ultra-low interfacial tension (10−3 mN/m) is one of the main criteria for surfactant systems used in tertiary oil recovery. It is widely recognized that only by reducing the oil–water interfacial tension to the ultra-low interfacial tension region, the residual crude oil in the reservoir void can be deformed and flowed (Wang et al. 1995). The three surfactants were formulated into an aqueous solution using simulated formation water, and the concentration of surfactants was consistent with that used at the production site, which was 3000 ppm, and the results of interfacial tension are shown in Fig. 6.
BS-18 has the lowest interfacial tension of 10−3 mN/m. Although its interfacial tension is the lowest, its solubility deteriorates due to the increase of hydrophobic groups as a result of the long carbon chain. In the solubilization of ionic micelles, the solubilization is mainly governed by the hydrocarbon chain length of the solubilizer molecules because the polar substances do not enter the interior of the micelles, but only solubilize on the surface of the micelles (Gong et al. 2019). On the other hand, the water solubility of 6501 was good, and the interfacial tension of 6501 was also ultra-low, with the interfacial tension in the range of 10−2 mN/m and 10−3 mN/m. The interfacial tension of KPS was the highest among the three surfactants, with the interfacial tension of 10−2 mN/m.
3.3 Surface wettability reversal
The experiment simulated the actual oil reservoir surface sample making, the surface is oily, the contact angle of conglomerate reservoir formation water is more than 100°, 0.3% concentration KPS can change the wettability of the rock surface, the contact angle is about 60°, the contact angle of 0.3% concentration betaine is about 31°, and the 0.3% concentration 6501 changes the wettability of the rock surface with a better effect, and the contact angle is about 30% (Fig. 7). And choosing a point on the sample thin section, dropping 6501 first, and then dropping simulated formation water at the same point, it was found that the contact angle was reduced to a very low level (Fig. 8), so that the surface was changed from oleophilic to hydrophilic. The contact angle on the surface of the oil-bearing natural core sand model was reduced, and the wettability was obviously reversed, so that the oil film on the surface of the oil-bearing natural core sand model could be stripped off and the residual oil could be initiated, which is very important for improving the crude oil recovery (Sun et al. 2015).
KPS was compounded with 6501 in a 1:1 ratio and the contact angle decreased to 46.8° (Fig. 9). The combination of anionic surfactants and nonionic surfactants can also have positive effects, and many complex oil reservoirs can improve their performance through the combination of surfactants.
In addition to alkanes, most crude oils contain small amounts of surface-active polar components, and divalent cations combined with acidic components in the oil can control the wettability of the oil–water-rock system. Mugele et al. (2015) removed divalent ions from the water, and observed that the contact angle between the water and the rock surface was reduced by about 10°, which could improve crude oil recovery by several percentage points (Herminghaus 2012). In this paper, the ionic surfactant was replaced with a nonionic surfactant, which again reduces the number of ions in the water that can be exchanged with the rock system, and controls the wettability by controlling the adsorption of ions to the solid–liquid interface, which from the results does change the wettability of the surface.
3.4 Adsorption results
3.4.1 Establishment of standard curve
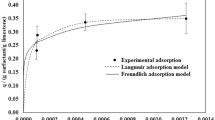
Standard curves were fitted with UV spectrophotometry for the three surfactants and the fit was good as shown in Table 4.
3.4.2 Static adsorption
The experimental results showed that the order of magnitude of adsorption of the anionic surfactant KPS on the rock minerals of the conglomerate reservoir was as follows: montmorillonite/zeolite > illite > chlorite > kaolinite > conglomerate core > calcite > feldspar (Fig. 10). KPS adsorption on montmorillonite and zeolite reached more than 40 mg/g, and the adsorption equilibrium concentration was also the highest, and the adsorption equilibrium was reached at 5000 ppm. Adsorption was lowest on the reservoir backbone mineral feldspar at about 6 mg/g, adsorption on calcite ~ 7 mg/g, with an adsorption equilibrium of 2000 ppm concentration on both. The amount of KPS adsorbed on the conglomerate core was about 9 mg/g, and the equilibrium concentration of adsorption was about 3500–4000 ppm. Combining the results of specific surface and zeta potential analysis, montmorillonite and zeolite have the largest specific surface area and both of them have high zeta potentials, which can be seen that they are active in physicochemical properties, so they cause a large amount of surfactant adsorption, while feldspars and calcite are on the contrary, so their adsorption amount is lower.
According to the same method, BS-18 and 6501 were used to do static adsorption experiments, and the results showed that the adsorption amount of BS-18 on rock minerals was comparable to that of KPS (Fig. 11), and the adsorption amount on zeolite and montmorillonite was up to 40 mg/g. The adsorption on clay minerals was still larger than that on conglomerate cores, and the adsorption amount on conglomerate cores was 5 mg/g.
The adsorption amount of 6501 was much lower than that of KPS and BS-18 (Fig. 12). 6501 adsorbed only 11–13 mg/g on zeolite and montmorillonite, and the concentration of the adsorption equilibrium was relatively low, with adsorption saturated at 3,000 ppm, and the adsorption amount was less than 3 mg/g on the conglomerate core.
It can be seen that adsorption of anionic surfactants is also inevitable in the face of conglomerate reservoirs with complex mineral compositions, and even though electrical adsorption with negatively charged mineral rocks is reduced, multilayer adsorption still occurs. Del Hoyo et al. (2008) found that the adsorption of anionic surfactants in the interlayer space of montmorillonite, kaolinite, illite, etc. did not change the X-ray maps, suggesting adsorption on the surfaces or in the structural channels of these minerals. While all clay minerals adsorbed nonionic surfactants increased in stability. Surfactants interact with hydroalumino-silicates through functional groups of organic compounds, variable cations of clay minerals formed by ion–dipole or hydrogen bonding, and on the other hand, rearrangement of adsorbed surfactant molecules has been observed. So the interaction of the surfactant with the mineral produces adsorption while altering both.
3.4.3 Dynamic adsorption
The experiments were conducted according to the dynamic adsorption experimental conditions in Sect. 1.2.5 with a surfactant concentration of 3000 ppm. Injecting surfactant into a conglomerate core and driving it out with water, and the following results were obtained (Figs. 13, 14 and 15).
KPS surfactant dynamic adsorption capacity: 0.255 mg/g.
BS-18 surfactant dynamic adsorption capacity: 0.261 mg/g.
6501 surfactant dynamic adsorption capacity: 0.046 mg/g.
It can be seen that the amount of dynamic adsorption is smaller than the amount of static adsorption, this is because the dynamic adsorption is simulated the oil recovery process for the experiment, the surfactant passes through the core and does not reach the adsorption saturation on the rock minerals, but the dynamic adsorption is more in line with the real state of the surfactant in the oil recovery process. From the results of the dynamic adsorption amount, the dynamic adsorption amount of 6501 was smaller than that of KPS and BS-18, with BS-18 having the largest adsorption amount.
3.5 Effect on recovery rate
3.5.1 Relationship between adsorption and recovery rate
The current chemical drive formulation used in oil recovery sites in conglomerate reservoirs is a binary blend solution of 3000 ppm concentration of KPS and 1800 ppm concentration of HPAM. So according to this formulation, dynamic adsorption experiments were carried out on conglomerate natural cores first, and then oil recovery experiments were carried out by physical simulation, and the experimental data were recorded to obtain Table 5, in which a relationship can be established between the amount of adsorption of the chemical agent and the total degree of recovery.
Based on the specific surfaces of the four conglomerate natural cores, it can be seen that the minerals contained in the four cores are different, and rock minerals with larger specific surfaces will have stronger adsorption capacity. The experimentally obtained adsorption amount was used to establish a relationship with the degree of oil recovery (Fig. 16).
Finally, the correlation between the adsorption amount of poly and surface agent and the oil recovery efficiency was obtained to be very good, with a negative correlation, indicating that the smaller the adsorption amount is, the higher the degree of oil recovery is.
Relationship equation between oil recovery efficiency and surfactant adsorption:
Relationship equation between oil recovery efficiency and polymer adsorption:
3.5.2 Recovery of different types of surfactants
Simulating the actual production situation of oil fields, chemical drive adopts a polymer/surface binary drive mode. In order to better compare the effects in parallel, HPAM is uniformly used for polymers, and different types of surfactants are used. Three chemical drive formulas are formulated, which are: (1) 3000 ppm KPS + 1800 ppm HPAM; (2) 3000 ppm 6501 + 1800 ppm HPAM; and (3) 3000 ppm BS-18 + 1800 ppm HPAM. Natural cores of conglomerate with the same physical properties were selected, and the injection pressure simulated the formation pressure of 12 MPa, and three sets of oil recovery experiments were done through physical simulation, and then compared in parallel.
In this case, the final degree of recovery after oil recovery with the formulation with KPS surfactant was 86.89%, and the chemical recovery partially increased the recovery by 17% points (Fig. 17). Subsequent water drives after the KPS drive improved recovery by 14% points.
The final degree of recovery after drive oil recovery with the formulation of BS-18 surfactant was 90.85%, and the chemical drive oil recovery improved the recovery by 23% points (Fig. 18). Subsequent water drives after BS-18 drives improved recovery by 15% points.
The final degree of recovery after drive oil recovery with the formulation of BS-18 surfactant was 91.45%, and the chemical drive oil recovery improved the recovery by 26% points (Fig. 19). Subsequent water drives after BS-18 drives improved recovery by 12% points.
According to the experimental results, the total oil recovery degree of 6501 is slightly higher than that of BS-18, and both of them reach more than 90%. KPS has the lowest level of oil recovery. However, comparing the results of chemical recovery (when injecting the second to fourth PV numbers), the 6501 binary system was the most effective in improving recovery. 6501 has the smallest adsorption on conglomerate reservoirs and minerals, and the small adsorption will make the surfactant more efficient in driving oil. The BS-18 binary system is also more effective in improving recovery, which will be slightly inferior to the effect of 6501 and superior to the KPS binary system, but the oil on the mineral surface of the rock driven by BS-18 becomes easier to be stripped off, so the effect of its subsequent water drive in improving recovery is the best.
The –SO3− group and hydrocarbon group of the anionic surfactant KPS have strong adsorption with the surface of clay minerals, which is mainly due to the electrical attraction of metal active centers (Al3+, Fe3+, Fe2+, Ca2+, Mg2+, etc.) on the surface of clay to the surfactant ions, the electrical attraction between the Stern layer of the loaded negatively charged clay surface and the –SO3− of surfactant, the dispersive and induced forces and hydrogen bonding between the surface of the clay minerals and the surfactant ions, as well as multilayered adsorption resulting from the colloidization of surfactants that have already adsorbed on the clay minerals. BS-18 also belongs to ionic surfactants, but because the structure of zwitterionic surfactants contains both anionic hydrophilic groups and cationic hydrophilic groups, so zwitterionic surfactants to change the wettability of the surface of the rock and mineral is better, and has a super-low interfacial tension, which makes up for the shortcomings of the larger adsorption amount (Hou 2016). The nonionic surfactant 6501 does not exist in solution in an ionic state, so it has high stability, is not easily affected by the presence of strong electrolytes, and is not easily affected by acids and bases, can be used in combination with ionic surfactants, has good compatibility, has good solubility in various solvents, and does not adsorb strongly on solid surfaces, thus increasing the degree of recovery to a high degree, and can be used as a preferred surfactant in conglomerate reservoirs with complex mineral compositions.
4 Conclusions
-
(1)
The mineral composition of conglomerate reservoir is diversified, containing a large number of minerals with active physicochemical properties, which are easy to interact with surfactants. In the conglomerate reservoir, there are I-M mixed layer minerals formed by montmorillonite and illite transforming and mixing with each other, as well as zeolite minerals with molecular sieve structure, which have the largest specific surface and the strongest electrically charged properties, which cause a large number of adsorption of surfactants, resulting in the loss and change of the chemical oil recovery formula system in the subsurface, which has a certain impact on the oil recovery efficiency;
-
(2)
The three surfactants we selected, KPS, BS-18, and 6501, all have good compatibility with the oil–water system of conglomerate reservoirs. However, in terms of reducing oil–water interfacial tension and changing surface wettability, BS-18 and 6501 are superior to KPS currently used in chemical oil recovery of conglomerate reservoirs in Xinjiang.
-
(3)
Although KPS is an anionic surfactant, it still adsorbs a lot with the negatively charged conglomerate reservoir because of multilayer adsorption. BS-18 is also an ionic surfactant, and its degree of adsorption is basically comparable to that of KPS. Whereas nonionic surfactant 6501 does not ionize in water and it does not contain ion-exchangeable anions and cations. When it encounters clay minerals, it forms hydrogen bonds with the polar group on the surface of the mineral crystal layer, and then adsorbs on the surface of the clay particles. Hydrogen bonding is not a chemical bond, but a special kind of intermolecular force. So facing conglomerate reservoir minerals, the adsorption of nonionic surfactants will be less than that of ionic surfactants;
-
(4)
The larger the adsorption amount of the surfactant, i.e., the lower the degree of its action in the oil recovery process in conglomerate reservoirs, so it will cause a decrease in oil recovery efficiency. The adsorption amount of KPS is large, so its oil recovery efficiency is the lowest, the adsorption amount of BS-18 is about the same as that of KPS, but the oil recovery efficiency will be higher than that of KPS, because BS-18 has hydrophilic and oleophilic amphiphilic properties, and it can change the wettability of the rock surface and reduce the interfacial tension between oil and water, which can make up for some of the loss generated by the adsorption. 6501 has the lowest adsorption amount and has been verified to have the highest oil recovery efficiency, so the nonionic surfactant 6501 can be used as the main surfactant for chemical oil recovery in conglomerate reservoirs.
Data availability
No datasets were generated or analysed during the current study.
References
Baek KH, Liu MY, Francisco J, Argüelles V, Gayan A, Abeykoon OR (2022) The effect of surfa-ctant partition coefficient and interfacial tension on oil displacement in low-tension polymer floo-ding. J Petrol Sci Eng 214:110487. https://doi.org/10.1016/j.petrol.2022.110487
Belhaj AF, Elraies KA, Mahmood SM, Zulkifli NN, Akbari S, Hussien OSE (2020) The effect of surfa-ctant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. J Petrol Explor Prod Technol 10:125–137. https://doi.org/10.1007/s13202-019-0685-y
Bera A, Kumar T, Ojha K, Mandal A (2013) Adsorption of surfactants on sand surface in enhanc-ed oil recovery: isotherms, kinetics and thermodynamic studies. Appl Surf Sci 284:87–99. https://doi.org/10.1016/j.apsusc.2013.07.029
China Petroleum and Natural Gas Industry Standard SY/T 5162-2018 (2018) X-ray diffraction analys-is method for Clay mineral and common non Clay mineral in Sedimentary rock. China Petroleum Industry Press. (in Chinese)
China Petroleum and Natural Gas Industry Standard SY/T 5370-2018 (2018) Surface and Interfacial Tension Measurement Method. China Petroleum Industry Press. (in Chinese)
Chen QS, Zhang EY, Liu WD, Shi YM, Xu K, Hou JW (2016) Analysis of ASP compound flooding adsorption behavior on conglomerate reservoirs in Xinjiang. Sci Tech Engrg 16(22):53–59
DelHoyo C, Dorado C, Rodríguez-Cruz MS, Sánchez-Martín MJ (2008) Physico-chemical study of selected surfactant-clay mineral systems. J Therm Anal Calorim 94:227–234. https://doi.org/10.1007/s10973-007-8934-6
Derjaguin B, Landau L (1993) Theory of the stability of strongly charged lyophobic sols and of the ad-hesion of strongly charged particles in solutions of electrolytes. Prog Surf Sci 14:733–762. https://doi.org/10.1016/0079-6816(93)90013-l
Elimelech M, O’Melia CR (1990a) Effect of particle size on collision effificiency in the deposition of Brownian particles with electrostatic energy barriers. Langmuir 6:1153–1163
Elimelech M, O’Melia CR (1990b) Kinetics of deposition of colloidal particles in porous media. Environ Sci Technol 24(10):1528–1536. https://doi.org/10.1021/es00080a012
Gong LY, Liao GZ, Chen QS, Luan HX, Feng YJ (2019) Swollen surfactant micelles: properties and applications. Acta Phys -Chim Sin 35(8):816–828. https://doi.org/10.3866/pku.whxb201810060
Guo H, Ji BQ, Yang S, Wang R, Zhang SC, Li JS, Zhang SY, Zou NN, Shi JA (2022) Formation and petroleum geological significance of zeolite cements in Permian glutenite reservoirs in Huanmahu Sag, the Junggar Basin. Acta Pet Sin 43(3):341–354. https://doi.org/10.7623/syxb202203002
Hammond PS, Unsal E (2011) Spontaneous imbibition of surfactant solution into an oil-wet capillary: wettability restoration by surfactant contaminant complexation. Langmuir 27:4412. https://doi.org/10.1021/la1048503
Herawati I, Permadi P, Rochliadi A, Marhaendrajana T (2022) Adsorption of anionic surfactant on sandstone reservoir containing clay minerals and its effect on wettability alteration. Energy Rep 8:11554–11568. https://doi.org/10.1016/j.egyr.2022.08.268
Herminghaus S (2012) Universal phase diagram for wetting on mesoscale roughness. Phys Rev Lett 10(9):236102. https://doi.org/10.1103/physrevlett.109.236102
Hirasaki GJ, Miller CA, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16(04):889–907. https://doi.org/10.2118/115386-pa
Hosny R, Zahran A, Abotaleb A, Ramzi M, Mubarak MF, Zayed MA, Shahawy AE, Hussein MF (2023) Nanotechnology impact on chemical-enhanced oil recovery: a review and bibliometric analysis of recent developments. ACS Omega 8(49):46325–46345. https://doi.org/10.1021/acsomega.3c06206
Hou BF (2016) Research on the modification of rock surface wettability by surfactants and its enhanced oil recovery. Ph. D. Dissertation, China University of Petroleum (East China)
Kumar A, Mandal A (2019) Critical investigation of zwitterionic surfactant for enhanced oil recovery from both sandstone and carbonate reservoirs: adsorption, wettability alteration and imbibition studies. Chem Eng Sci 209:115222. https://doi.org/10.1016/j.ces.2019.115222
Lee DH, Chang HW, Cody RD (2004) Synergism effect of mixed surfactant solutions in remediation of soil contaminated with PCE. Geo Sci 8:319–323. https://doi.org/10.1007/bf02910251
Li BW, Sun LH, Liu XG, Feng C, Zhang ZR, Huo X (2023) Effects of clay mineral content and types on pore-throat structure and interface properties of the conglomerate reservoir: a case study of baikouquan formation in the Junggar Basin. Minerals 13(1):9. https://doi.org/10.3390/min13010009
Liao GZ, Wang Q, Wang HZ, Liu WD, Wang ZM (2017) Chemical flooding development status and prospect. Acta Pet Sin 38(2):196–207. https://doi.org/10.7623/syxb201702007
Mugele F, Bera B, Cavalli A, Siretanu I, Maestro A, Duits M, Cohen-Stuart M, Ende D, Stocker I, Collo SI (2015) Ion adsorption-induced wetting transition in oil-water-mineral systems. Sci Rep 5:10519. https://doi.org/10.1038/srep10519
Murison J, Semin B, Baret JC, Herminghaus S, Schröter M, Brinkmann M (2014) Wetting heterogeneities in porous media control flow dissipation. Phys Rev Appl 2(3):034002. https://doi.org/10.1103/physrevapplied.2.034002
Muggeridge A, Cockin A, Webb K, Frampton H, Collins I, Moulds T, Salino P (2013) Recovery rates, enhanced oil recovery and technological limits. Philos T R Soc A 372(2006):20120320. https://doi.org/10.1098/rsta.2012.0320
Ni XM, Li ZH, Wang YB (2018) Adsorption characteristics of anionic surfactant sodium dodecylbenzene sulfonate on the surface of montmorillonite minerals. Front Chem 6:390. https://doi.org/10.3389/fchem.2018.00390
Sun JF, Dai Y, Chen QS, Wu YQ, Zhao WQ, Li RH, Luan HX (2015) The effect of reversal of rock surface wettability on enhanced oil recovery by binary composite flooding. Daily Chem Ind 45(9):500–508 ((in Chinese))
Wang D, Butler R, Zhang J, Seright R (2012) Wettability survey in bakken shale with surfactant-formulation imbibition. SPE Reserv Eval Eng 15:695–705. https://doi.org/10.2118/153853-pa
Wang DM (2001) Development of daqing oil field in the new century: collected papers of academician Wang demin’s report. Petroleum Industry Press: Beijing, 404–420. (in Chinese)
Wang YF, Zhang CG, Hou WG (1995) Surfactant and its applications in oil fields. Petroleum Industry Press: Beijing, 2. (in Chinese)
Yu ZC, Wang ZZ, Adenutsi CD (2023) Genesis of authigenic clay minerals and their impacts on reservoir quality in tight conglomerate reservoirs of the Triassic Baikouquan formation in the Mahu Sag, Junggar Basin, Western China. Mar Petrol Geol. 148:106041. https://doi.org/10.1016/j.marpetgeo.2022.106041
Zhang L (2015) Study on Characteristics of adsorption and retention of amphiphilic polymers and surfactants on rocks and minerals. M. A. Dissertation, China University of Petroleum (East China)
Zhao FL (2010) Oilfield chemistry. China University of Petroleum Press, Beijing, pp 11–13 (in Chinese)
Zhao HN, Cheng XH, Zhang OD, Huang JB, Liu CJ, Zhao B (2014) Mixed cationic and anionic surfactant systems achieve ultra-low interfacial tension in the karamay oil field. Acta Phys Chim Sin 30(4):693–698. https://doi.org/10.3866/pku.whxb201402121
Zhong X, Pu H, Zhou Y, Zhao JX (2019) Comparative study on the static adsorption behavior of zwitterionic surfactants on minerals in middle bakken formation. Energy Fuels 33:1007–1015. https://doi.org/10.1021/acs.energyfuels.8b04013
Zhu SQ, Qian GB, Liu SS, Wang YJ, Xu CF (2015) Secondary development of conglomerate reservoir in Karamay. Petroleum Industry Press, Beijing, pp 5–20 (in Chinese)
Acknowledgements
Firstly, the author would like to express gratitude to Shaanxi Key Laboratory of Chemical Additives for Industry for providing us with the experimental instruments and site, which enabled our research to be completed smoothly. Secondly, we would like to thank the Petroleum and Natural Gas Research Center of the School of Earth and Space at Peking University for their assistance in our experiment. Lastly, we would like to express our special thanks to the editors and reviewers for their valuable comments.
Funding
This work was supported by the National Natural Science Foundation of China [No.51904180] and the Xinjiang Conglomerate Reservoir Laboratory Development Project [2020D04045].
Author information
Authors and Affiliations
Contributions
X. Y.: Conceptualization, Methodology, Writing-Original Draft, Investigation, Data Curation. Y. T. and Y. S.: Conceptualization, Supervision, Writing-Review and Editing. X. W. and R. L.: Resources, Data Curation. H. Z. and S.Z.: Investigation, Data analysis, image mapping and revision. B. L.: Resources, Methodology. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to publish
The Author confrms: that the work described has not been published before (except in the form of an abstract or as part of a published lecture, review, or thesis); that it is not under consideration for publication elsewhere; that its publication has been approved by all co-authors, if any; that its publication has been approved (tacitly or explicitly) by the responsible authorities at the institution where the work is carried out.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, X., Tian, Y., Shi, Y. et al. Effect of adsorption of different types of surfactants on conglomerate reservoir minerals on chemical oil recovery efficiency. Geomech. Geophys. Geo-energ. Geo-resour. 10, 150 (2024). https://doi.org/10.1007/s40948-024-00868-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40948-024-00868-5