Abstract
Background
Obesity and sarcopenia are both viewed as pathological body composition issues and indicators of compromised nutritional status. The aim of this study was to evaluate the effects of sarcopenic obesity on short- and long-term clinical outcomes.
Methods
The clinical data of patients who underwent radical gastrectomy between 2013 and 2019 were prospectively collected. Sarcopenia is defined by European Working Group on Sarcopenia in Older People (EWGSOP2) and obesity is defined by the body mass index (BMI). To identify the independent predictors of postoperative complications and overall survival, we performed univariate and multivariate logistic and Cox regression analyses.
Results
In a total of 508 cases, 372 (73.2%) cases were diagnosed with non-sarcopenia, 112 (22.0%) with non-obese sarcopenia, and 24 (4.7%) with sarcopenic obesity. In the multivariate logistic regression, both of non-obese sarcopenia (OR = 2.305, 95% CI = 1.444–3.679, P < 0.001) and sarcopenic obesity (OR = 2.589, 95% CI = 1.088–6.156, P = 0.031) were independent risk factors for postoperative complications. In the multivariate Cox analysis, only non-obese sarcopenia (HR = 2.614, 95% CI = 1.882–3.631, P < 0.001) was independent risk factor for overall survival.
Conclusions
Sarcopenic obesity serves as an independent risk factor for postoperative complications, but not for overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sarcopenia is a chronic condition affecting skeletal muscle throughout the body. It is characterized by a gradual decline in muscle mass and function, leading to adverse outcomes such as physical disability, reduced quality of life, and even death [1]. Recent research has demonstrated that gastric cancer patients with sarcopenia experience a significantly higher rate of postoperative complications and poorer oncological outcomes [2, 3].
Body mass index (BMI) is commonly utilized to evaluate nutritional status, yet they do not necessarily serve as accurate indicators of muscle mass [4]. The inexorable decline in skeletal muscle mass and function with advancing age is often accompanied by a concomitant increase in body fat. Sarcopenic obesity is a complex syndrome characterized by the concurrent presence of obesity and sarcopenia. Obesity can trigger muscle mass and function loss due to the detrimental effects of adipose tissue-dependent metabolic dysregulation. The intricate interplay between sarcopenia and obesity extends beyond their independent effects on health, as these conditions may also reinforce each other, creating a vicious cycle of muscle loss and fat gain. This reciprocal relationship is exacerbated by reduced physical activity, a common consequence of aging and disease. However, some studies suggest that overweight or obese cancer patients may have better outcomes compared to normal-weight patients [5, 6]. This phenomenon, known as the obesity paradox, appears to contradict the well-established link between obesity and poorer health outcomes.
The situation becomes even more complex in patients with sarcopenic obesity, a condition where both muscle loss and excess body fat are present, as the combined effects of these conditions may interact synergistically or antagonistically, impacting health outcomes in unpredictable ways. So far, the effect of sarcopenic obesity on clinical outcomes in cancer patients was unclear. This study aims to compare the differences in clinical characteristics between non-sarcopenia, non-obese sarcopenia, and sarcopenic obesity and their impact on short-term and long-term outcomes in patients with gastric cancer.
2 Methods
2.1 Patients
Patient data from those who underwent radical gastrectomy at the First Affiliated Hospital of Wenzhou Medical University between 2013 and 2019 was collected and analyzed. This study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University. The study included patients who met the following criteria: (1) histologically confirmed gastric adenocarcinoma and planned to undergo elective curative surgery for gastrointestinal cancer; (2) aged 18 years or older; (3) had abdominal computed tomography (CT) scan within 1 month before surgery; (4) signed informed consent and agreed to participate in the stud. Patients were excluded if they had: (1) preoperatively or during surgery confirmed cancer metastasis; (2) a diagnosis of double cancer or emergency surgery; (3) unavailable data on muscle quantity and quality due to unqualified CT images; (4) inability to undergo functional assessments due to physical or mental reasons.
All patients underwent routine perioperative examinations, and the following data were collected: (1) demographics: age, gender, and body mass index (BMI); (2) nutritional status: nutrition risk screening 2002 (NRS 2002) [7], serum albumin concentration (hypoalbuminemia defined as < 35 g/L), and serum hemoglobin concentration (anemia defined as < 120 g/L for males and < 110 g/L for females); (3) comorbidities: Charlson comorbidity index (CCI) [8] and abdominal surgery history; (4) skeletal muscle assessment: skeletal muscle mass index (SMI), skeletal muscle radiodensity (SMD), and handgrip strength; (5) tumor characteristics: tumor location and TNM stage; (6) operative details: laparoscopy-assisted surgery, operative time, combined resection, and type of resection; (7) clinical outcomes: Clavien-Dindo classification [9] for grade II and higher complications and overall survival status.
2.2 Diagnosis of sarcopenia
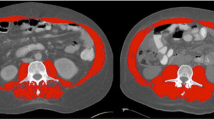
CT assessment serves as a gold standard method for detecting body composition and abnormal body composition phenotypes. The cross-sectional non-contrast CT image at the third lumbar vertebra (L3) level was selected, and a Hounsfield unit (HU) threshold of 29 to 150 was applied to differentiate muscle from other nearby tissues. Professional CT post-processing software (Advantage Workstation 4.5, General Electric Healthcare, USA) automatically calculated the muscle areas and mean SMD. The skeletal muscle mass index (SMI, cm2/m2) was obtained by dividing the muscle area by the square of height. Low muscle quantity, represented by low SMI, was defined as < 34.9 cm2/m2 for females and < 40.8 cm2/m2 for males [10]. Meanwhile, low muscle quality, represented by low SMD, was defined as < 28.6 HU for females and < 38.5 HU for males [11]. Handgrip strength, measured on the dominant hand using an electronic hand dynamometer (EH101; Camry, Guangdong Province, China), was used to represent muscle strength.
According to the European Working Group on Sarcopenia in Older People 2018 (EWGSOP2), sarcopenia was defined as low handgrip strength plus low muscle quantity or low handgrip strength plus low muscle quality [12]. Considering the racial differences, low handgrip strength was defined as < 18 kg for females and < 26 kg for males according to the Asian Working Group for Sarcopenia [13].
2.3 Follow-up
In the post-surgical care regimen, systematic follow-up assessments were scheduled every 3 months for the initial 24-month period, subsequently shifting to biannual health maintenance examinations. These comprehensive follow-ups comprised evaluations of the postoperative quality of life, meticulous physical examinations, extensive laboratory analyses, appropriate imaging studies, and, when clinically justified, endoscopic assessments to ensure comprehensive surveillance. To quantify treatment outcomes, overall survival (OS) was determined from the surgical date until death resulting from any cause, thereby encompassing the pivotal metrics of therapeutic efficacy and long-term patient outcomes.
2.4 Statistics
Categorical variables were presented as counts with percentages and analyzed using either the Pearson's Chi-square test or Fisher's exact test. Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR) and analyzed using either the student’s t test or the Mann–Whitney U test. Univariate logistic and Cox regression analyses were performed to identify potential prognostic factors. Variables with a P-value less than 0.10 were included in the subsequent multivariate analysis. All statistical analyses were performed using SPSS version 23 (IBM, Armonk, NY).
3 Results
3.1 Baseline characteristics
Initially, 601 patients were enrolled. After excluding patients with distant metastasis, those with unavailable radiographic data, individuals unable to undergo muscle function testing, and those who underwent emergency surgeries, a total of 508 patients with curable gastric cancer were included in this study (Fig. 1). Among them, 372 (73.2%) cases were diagnosed with non-sarcopenia, 112 (22.0%) with non-obese sarcopenia, and 24 (4.7%) with sarcopenic obesity. Patients' demographic and clinicopathologic characteristics are.
listed in Table 1. Compared to sarcopenia patient without obesity, patients with sarcopenic obesity had significantly higher BMI (21 kg/m2 vs. 26.4 kg/m2, P < 0.001) and SMI (39 cm2/m2 vs. 45 cm2/m2, P < 0.001), and lower SMD (33.5 HU vs. 29.5 HU, P < 0.001). Other baseline characteristics were comparable between these two groups.
3.2 Short-term outcomes
The results of short-term outcomes are listed in Table 2. The incidence of surgical complications was 14% in the non-sarcopenic group, 17.9% in the sarcopenic non-obese group, and 20.8% in the sarcopenic obese group. For medical complications, the incidence was 7% in the non-sarcopenic group, 16.1% in the sarcopenic non-obese group, and 16.7% in the sarcopenic obese group. According to the Clavien-Dindo classification, postoperative complications of grade II or higher occurred in 118 (23.2%) patients. In the univariate logistic analysis, age ≥ 65 (OR = 1.581, 95% CI = 1.039–2.406, P = 0.032), hypoproteinemia (OR = 1.604, 95% CI = 0.999–2.576, P = 0.051), sarcopenia (sarcopenia without obesity vs. non-sarcopenia, OR = 2.305, 95% CI = 1.444–3.679, P < 0.001; sarcopenia with obesity vs. non-sarcopenia, OR = 2.589, 95% CI = 1.088–6.156, P = 0.031), laparoscopic surgery (OR = 0.667, 95% CI = 0.428–1.041, P = 0.075) were potential influence factors for postoperative complication. Subsequently, these factors were incorporated into the multivariate logistic regression. Finally, only sarcopenia, including non-obese sarcopenia and sarcopenic obesity (sarcopenia without obesity vs. non-sarcopenia, OR = 2.305, 95% CI = 1.444–3.679, P < 0.001; sarcopenia with obesity vs. non-sarcopenia, OR = 2.589, 95% CI = 1.088–6.156, P = 0.031) were independent risk factors for postoperative complications. In the subgroup analysis, sarcopenia, regardless of the presence of obesity, demonstrated a potential adverse impact on the postoperative complication across different populations (Fig. 2) (Table 3).
3.3 Long-term outcomes
During the median follow-up time of 46.3 months, 163 (32.1%) patients died. Among them, 96 (25.8%) cases died in the non-sarcopenia group, 57 (50.9%) in the non-obese sarcopenia group, and 10 (41.7%) in the sarcopenic obesity group. Kaplan–Meier curves demonstrate that sarcopenic patients without obesity have the poorest survival rates compared to non-sarcopenic patients and sarcopenic patients with obesity (Fig. 3). In the univariate Cox analysis, age ≥ 65 (HR = 1.650, 95% CI = 1.202–2.264, P = 0.002), male (HR = 1.525, 95% CI = 1.040–2.234, P = 0.031), low BMI (≤ 18.5/18.5–25, HR = 1.945, 95% CI = 1.153–3.281, P = 0.013; > 25/18.5–25, HR = 0.889, 95% CI = 0.596–1.328, P = 0.567), NRS 2002 score ≥ 3 (HR = 1.609, 95% CI = 1.176–2.202, P = 0.003), hypoproteinemia (HR = 1.704, 95% CI = 1.219–2.382, P = 0.002), anemia (HR = 1.882, 95% CI = 1.379–2.568, P < 0.001), non-obese sarcopenia (sarcopenia without obesity vs. non-sarcopenia, HR = 2.614, 95% CI = 1.882–3.631, P < 0.001; sarcopenia with obesity vs. non-sarcopenia, HR = 1.694, 95% CI = 0.883–3.251, P = 0.113), tumor size ≥ 5 cm (HR = 2.510, 95% CI = 1.838–3.426, P < 0.001), TNM stage (II/I, HR = 3.743, 95% CI = 2.122–6.603, P < 0.001; III/I, HR = 8.385, 95% CI = 5.019–14.008, P < 0.001), total gastrectomy (HR = 2.127, 95% CI = 1.564–2.892, P < 0.001), laparoscopic surgery (HR = 0.648, 95% CI = 0.459–0.913, P = 0.013), combined organ resection (HR = 1.939, 95% CI = 1.214–3.097, P = 0.006), and postoperative complication (HR = 1.452, 95% CI = 1.035–2.038, P = 0.031) were potential influence factors for overall survival. These factors were incorporated into the multivariate Cox regression. Finally, only non-obese sarcopenia (HR = 2.875, 95% CI = 2.060–4.014, P < 0.001), tumor size ≥ 5 cm (HR = 1.501, 95% CI = 1.081–2.085, P = 0.015), TNM stage (II/I, HR = 2.940, 95% CI = 1.643–5.262, P < 0.001; III/I, HR = 6.886, 95% CI = 4.032–11.760, P < 0.001), and total gastrectomy (HR = 1.417, 95% CI = 1.030–1.949, P = 0.032). In the subgroup analysis, it appears that obesity may mitigate the adverse impact of sarcopenia on overall survival (Fig. 4).
4 Discussion
The global aging population coincides with the obesity pandemic. Both obesity and sarcopenia are considered pathological body composition phenotypes and impaired nutritional status conditions. However, the impact of obesity, as measured by BMI, on individuals with chronic diseases remains controversial. In certain populations, overweight and obesity have been associated with increased survival. The mechanisms underlying these paradoxical associations remain largely unknown, but several potential explanations have been proposed. One possibility is that BMI is an imprecise measure of body fat, as it does not differentiate between fat and lean body mass. Lean body mass has been negatively associated with increased mortality. Additionally, a number of studies have demonstrated an association between sarcopenia and increased adverse outcomes in cancer patients. However, the association of sarcopenic obesity with cancer is uncertain. In our study, we demonstrated that sarcopenia, characterized by the loss of muscle mass and function, plays a crucial role in predicting postoperative complications irrespective of obesity status. However, in term of overall survival, the impact of sarcopenia varies depending on the presence of obesity. Non-obese individuals with sarcopenia exhibit a higher risk of mortality compared to their obese counterparts with sarcopenia.
In this study, the prevalence of sarcopenia was 26.7%, and the prevalence of sarcopenic obesity was 4.7%. The prevalence of sarcopenic obesity in this study was lower than that reported in some previous studies [14, 15], which could be attributed to several reasons. Firstly, some studies did not employ strict diagnostic criteria for sarcopenia. For instance, Liu et al. diagnosed sarcopenia solely based on grip strength, thereby inflating the proportion of sarcopenic individuals, and correspondingly increasing the proportion of sarcopenic obesity cases. Secondly, obese individuals often have higher absolute muscle mass. Since sarcopenia defines low muscle mass standardized to height squared, the muscle mass index in obese patients is often greater. As demonstrated in the baseline characteristics of this study, using cutoff values for muscle mass index derived from the general population could lead to an underestimation of muscle mass in obese individuals. The prevalence of sarcopenic obesity reported by Şahin et al. in gastric cancer patients was 4.9%, which is similar to the findings of our study [16].
While sarcopenia and obesity are increasingly recognized as significant health issues individually, their combined impact remains poorly understood. Ozkok et al. found that sarcopenic obesity, characterized by the coexistence of sarcopenia and obesity, was associated with better physical performance and lower frailty compared to sarcopenia alone [17]. Consistently to our study, Liu et al. found in a 3-year prospective cohort study that sarcopenia, but not sarcopenic obesity, was a predictor of mortality in older men [14]. Contrary to our study, Prado et al. demonstrated that sarcopenic obesity was associated with poorer functional status compared to obese patients without sarcopenia. However, they did not compare the overall survival time differences between non-obese sarcopenia and sarcopenic obesity. In this study, both non-obese and obese sarcopenia were identified as independent risk factors for postoperative complications. However, only non-obese sarcopenia emerged as an independent risk factor for overall survival, whereas sarcopenic obesity did not. The different effects of sarcopenic obesity on postoperative complication and overall survival may be attributed to the significant metabolic disturbances and inflammation associated with obesity, which may lead to an insufficient response and recovery to surgical stress. However, in the long term, the detrimental effects of obesity might not manifest because patients died early due to cancer recurrence or other cancer-related factors. On the other hand, obesity may confer a certain physiological resilience, allowing patients to endure the effects of cancer cachexia for a longer duration compared to non-obese counterparts, potentially extending their survival. Besides, peritumoral adipose tissue inflammation in obese patients may also count for prolonged survival [6].
Our study had some potential limitations. First, it was a single-center study, despite the large sample size. The findings should be validated by a multicenter, prospective analysis. Second, the observational design of our study does not allow us to draw firm conclusions about the causal relationship between sarcopenia and adverse clinical outcomes. However, data were prospectively collected to minimize recall bias. Third, the cut-off values for low SMI and low SMD were derived from our previous studies due to the lack of a unified standard [10, 11]. Nevertheless, we believe that using cut-off values from Chinese-specific large-sample studies can yield more accurate results, considering racial differences. Fourth, because we are in the surgical department where the majority of patients undergoing surgery present with curable gastric cancer, we did not record the data likes ascites, which may limit the generalization of this study's findings.
In conclusion, our study demonstrated that sarcopenia, irrespective of obesity status, is a significant independent risk factor for postoperative complications. Nevertheless, non-obese sarcopenia, but not sarcopenic obesity, is an independent predictor of overall survival. These finding suggests the presence of an obesity paradox in terms of long-term survival among sarcopenia patients.
Availability of data and materials
Data described in the article will be made available upon request pending application and approval.
References
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–46.
Zhang FM, Zhang XZ, Zhu GL, Lv LQ, Yan XL, Wu WX, Wang SL, Chen XL, Zhuang CL, Yu Z. Impact of sarcopenia on clinical outcomes of patients with stage I gastric cancer after radical gastrectomy: a prospective cohort study. Eur J Surg Oncol. 2022;48:541–7.
Zhang FM, Chen XL, Wu Q, Dong WX, Dong QT, Shen X, Shi HP, Yu Z, Zhuang CL. Development and validation of nomograms for the prediction of low muscle mass and radiodensity in gastric cancer patients. Am J Clin Nutr. 2021;113:348–58.
Després JP. BMI versus obesity subtypes in the era of precision medicine. Lancet Diabetes Endocrinol. 2023;11:382–4.
Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–8.
Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, Ostrovnaya I, Petruzella S, Reising A, Patel P, Mano R, Coleman J, Russo P, Liu CH, Dannenberg AJ, Chan TA, Motzer R, Voss MH, Hakimi AA. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21:283–93.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36.
Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95: e3164.
Zhuang CL, Shen X, Huang YY, Zhang FM, Chen XY, Ma LL, Chen XL, Yu Z, Wang SL. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: a propensity score-matched analysis from a large-scale cohort. Surgery. 2019;166:297–304.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300-7.e2.
Liu LK, Chen LY, Yeh KP, Lin MH, Hwang AC, Peng LN, Chen LK. Sarcopenia, but not sarcopenic obesity, predicts mortality for older old men: a 3-year prospective cohort study. J Clin Gerontol Geriatr. 2014;5:42–6.
Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–7.
Şahin MEH, Akbaş F, Yardimci AH, Şahin E. The effect of sarcopenia and sarcopenic obesity on survival in gastric cancer. BMC Cancer. 2023;23:911.
Ozkok S, Aydin CO, Sacar DE, Catikkas NM, Erdogan T, Bozkurt ME, Kilic C, Karan MA, Bahat G. Sarcopenic obesity versus sarcopenia alone with the use of probable sarcopenia definition for sarcopenia: associations with frailty and physical performance. Clin Nutr. 2022;41:2509–16.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 82171565, 82371586), the Program for Research-oriented Physician of Shanghai Tenth People's Hospital (2023YJXYSB003), and the Shanghai Tenth People's Hospital Clinical Research Youth Cultivation Program (YNCR2C014).
Author information
Authors and Affiliations
Contributions
The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, HF., Zhang, XZ., Zhang, FM. et al. Impacts of sarcopenia with or without obesity on patients with gastric cancer. Holist Integ Oncol 3, 44 (2024). https://doi.org/10.1007/s44178-024-00109-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00109-5