Abstract
The timing of DNA replication in mammals is crucial for minimizing errors and influenced by genome usage and chromatin states. Replication timing in the newly formed mammalian embryo remains poorly understood. Here, we have investigated replication timing in mouse zygotes and 2-cell embryos, revealing that zygotes lack a conventional replication timing program, which then emerges in 2-cell embryos. This program differs from embryonic stem cells and generally correlates with transcription and genome compartmentalization of both parental genomes. However, consistent and systematic differences existed between the replication timing of the two parental genomes, including considerably later replication of maternal pericentromeric regions compared to paternal counterparts. Moreover, maternal chromatin modified by Polycomb Repressive Complexes in the oocyte, undergoes early replication, despite belonging to the typically late-replicating B-compartment of the genome. This atypical and asynchronous replication of the two parental genomes may advance our understanding of replication stress in early human embryos and trigger strategies to reduce errors and aneuploidies.
Similar content being viewed by others
Introduction
DNA replication is a fundamental process ensuring accurate transfer of genetic information when cells divide. Its precise orchestration follows a well-defined temporal order, known as the replication timing program1,2. Replication timing has emerged as a key cellular fingerprint that is associated with various genomic features, such as gene density, gene transcription, histone modifications, DNA methylation, and 3D genome organization3,4,5,6,7,8,9,10. Furthermore, it exhibits a remarkable level of synchrony across single cells and their homologous chromosomes11,12. Significant advancements in our comprehension of replication timing have been facilitated by a range of sequencing technologies collectively referred to as Repli-seq11,12,13. More recently, the advent of single-cell sequencing approaches have revolutionized the investigation of replication timing in individual cells11,12,14. Single-cell Repli-seq examines copy number variations of S phase cells and normalizes them against G1 reference cells.
Despite this significant progress, our understanding of replication timing heavily relies on established replication programs derived from in vitro cell lines. However, little is known about how de novo replication programs are established after fertilization in the developing embryo15,16. Early studies based on incorporation of fluorescent nucleotide analogs and microscopy in mouse embryos have shown that early embryonic replication is structured and asynchronous between the maternal and paternal genomes17,18.
Human and mouse oocytes and embryos are characterized by several transformative changes in their epigenetic landscapes19,20,21,22,23, and a permissive transcriptional environment that allows for unusually high retrotransposon activity and the emergence of alternative retrotransposon-initiated transcripts24,25,26,27. As the paternal genome is unpacked from the fertilizing sperm, its protamines are replaced by histones, and both the maternal and paternal genomes must exit the transcriptionally silenced states that existed in the gametes to initiate embryonic genome activation28, while undergoing major morphological changes29. Collectively, these factors could place the preimplantation genome under considerable exertion, and might contribute to the reported instances of replication stress during the first cellular divisions in mouse and human embryos30,31. Consequently, exceptional factors might influence and govern the replication and its timing at the early stages of mammalian embryo development.
In this study, we investigate replication timing patterns in the mouse zygote and 2-cell stage embryo by developing single-embryo Repli-seq. Our data reveal an apparently uniform replication timing in the zygote, and the emergence of a replication timing program in 2-cell embryos. In 2-cell embryos, replication timing is generally associated with transcription, Lamina Associated Domains (LADs), genome compartmentalization, and inherited histone modifications. However, the two parental genomes display consistent and systematic differences. In particular, we find a specific association between late replication of the pericentromeric regions in the maternal genome, and early replication of chromatin marked by H3K27me3 in the oocyte. Our data characterizes a unique asynchronous DNA replication timing in the early embryo, which is intricately linked to the changing 3D chromatin organization, properties inherited from the oocyte, and those established de novo in the early embryo.
Results
Single-embryo Repli-seq reveals atypical replication timing in zygotes and 2-cell embryos
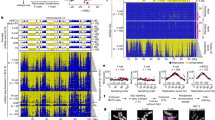
To better understand the mechanisms underlying the establishment of replication timing during early mammalian embryogenesis, we developed a single-embryo Repli-seq technique to study replication timing in the zygote and 2-cell stage of mouse embryos (Fig. 1a). The reliability of Repli-Seq relies on the precise selection of cells in S phase, therefore, we first employed EdU labeling at specific time points following fertilization. This allowed us to determine the exact timing of the zygote’s entry into and exit from the first S phase (Fig. 1a–c). For the 2-cell embryos, we additionally used Nocodazole to arrest in vitro fertilized mouse zygotes in metaphase for 4 h, aligning their cell cycles and facilitating precise selection of G1 or S phase cells based on EdU incorporation (Fig. 1a–c).
a Schematic of the procedure for obtaining zygotes and 2-cell embryos undergoing DNA replication. Upper panel, S phase zygotes were selected at defined times after fertilization. Lower panel, Nocodazole was utilized to synchronize the release of 2-cell embryos into the second cell cycle. b Microscopy of EdU pulse labeling of 2-cell embryos at indicated time points after release from Nocodazole synchronization. Scale bar = 5 µM. c Quantitation of EdU-incorporation in zygotes and 2-cell embryos at indicated time points after fertilization. Coloring illustrates the time points defined as Early, Mid, and Late S-phase. The number of independent embryos collected at each hourly stage: 3.5 (3), 4.5 (6), 5.5 (8), 6.5 (12), 7.5 (4), 8.5 (8), 9.5 (9), 10.5 (10), 11.5 (4), 12.5 (5), 19.5 (4), 20.5 (6), 21.5 (4), 22.5 (4), 23.5 (6), 24.5 (6), 25.5 (4), 26.5 (5), 27.5 (4), 28.5 (6), 29.5 (6), and 30.5 (7). The embryos were collected from 3 independent experiments. d Graphs showing normalized Repli-seq read densities of three S-phase 2-cell embryos at chromosome 6. Values are log2-fold differences relative to the G1-phase signal. e, f The heatmap (e) and graph (f) display normalized Repli-seq read densities in S- and G1-phase zygotes, 2-cell embryos, and ESCs on chromosome 6. The heatmap shows individual embryos, while the graph represents mean values. The values are presented as log2-fold differences relative to the G1-phase signal for each cell type. g Beeswarm plots of the mean absolute genome-wide deviation in each Repli-seq sample. The background deviation from G1 controls for each sample is indicated with gray boxes and wide bars showing the interquartile range and median, respectively. Black bars indicate the 25, 50, and 75 percentiles of the S-phase populations. P-values obtained by two-sided Mann-Whitney U-tests Benjamini–Hochberg corrected for multiple testing. h, i Heatmaps of individual binarized replication status values (h) and mean binarized replication status of S-Phase zygotes, 2-cell embryos and ESCs at chromosome 6 (i). The vertical order and left side bar diagram (h) reflect the Replication Score. j Tile plot showing relationships between S-phase progression and ranked Replication Score from Repli-seq data in mouse zygotes and 2-cell embryos. r- and p-values were obtained using Spearman’s rank correlation tests Benjamini-Hochberg corrected for multiple testing. Source data are provided as a Source Data file.
With accurate definition of the cell cycle times, we obtained a cohort comprising of 26 zygotes and 44 2-cell stage embryos through in vitro fertilization using C57BL/6N oocytes fertilized with CAST/EiJ sperm. These embryos were processed using a modified version of previously published sc-Repliseq pipelines (methods)11,12. To validate the reliability of our approach, we also generated replication timing profiles from individual mouse embryonic stem cells (ESCs). These replication timing profiles demonstrated a strong agreement with the previously published sc-Repli-seq profiles obtained from ESCs (Supplementary Fig. 1a-e, Supplementary Data 1)11. Based on our previous EdU determination, six, four, seven, and nine zygotes were assigned as G1, early S, mid S, and late S phase, respectively. For 2-cell embryos, seven, eleven, thirteen, and thirteen embryos were assigned to the G1, early S, mid S, and late S phase, respectively (Supplementary Data 2).
Our analysis of replication timing in individual 2-cell embryos revealed consistent replication timing profiles across individual samples (Fig. 1d). However, we observed marked changes in the overall level of difference between S phase cells and G1 cells during embryonic growth. Specifically, the zygote data exhibited a notably smaller overall deviation (Fig. 1e, f), whereas Repli-Seq profiles generated from ESCs showed a strong and localized deviation between their S and G1 cells, which was not attributed to read depth (Fig. 1e–g and Supplementary Fig. 1f, g, and Supplementary Data 3). 2-cell embryos displayed intermediate levels of S/G1 deviation, suggesting that the changes might coincide with the embryonic genome activation and the emergence of compartmentalization in the embryo genome9,10,32. While asynchronous replication between the two parental genomes in the zygote is a potential cause of the low S/G1 deviation, this could also indicate a uniform replication program that initiates from a high abundance of replication origins that are randomly distributed throughout the genome.
Notably, when we assessed the replication status of each embryo by methodology previously validated in ESCs11 (Fig. 1h, i), we found a close alignment between the assessment made by EdU incorporation (Fig. 1b, c) and the calculated Replication Score for each 2-cell embryos, but not for zygotes (Fig. 1j and Supplementary Data 4). To address the possibility that the low S/G1 deviation in zygotes being attributed to selecting zygotes outside of S phase due to unsynchronized fertilization by IVF, we also performed Repli-seq on zygotes using an adjusted methodology. Specifically, we shortened the incubation time of the oocyte with sperm from 3 h to 1 h and selected embryos for Repli-seq at timepoints following pronuclear formation. Zygotes treated in this manner corroborated the findings from the earlier experiments (Supplementary Fig. 1h–j). Given the limited and insignificant differences between G1 and S phase zygotes (Fig. 1f, g and Supplementary Fig. 1f), we decided to focus on the significant deviations seen in the 2-cell stage in our subsequent analyses of genome-wide replication timing.
Consistent differences in local replication timing between 2-cell embryos and ESCs
Although replication timing between our 2-cell embryos and ESCs ordered by the fraction of the genome scored as replicated11 showed many similarities throughout the genome, we also observed notable local differences between the two cell types (Fig. 2a). These distinct differences were consistent across individual samples of the two cell types, with some displaying markedly earlier or later replication in the 2-cell embryos compared to ESCs (Fig. 2a). To assess these differences in an unbiased manner and on a global scale, we performed k-means clustering of the genome-wide Repli-seq enrichment of S-phase 2-cell stage embryos and ESCs. This revealed globally organized replication timing patterns recurring within individual cells, with systematic and persistent differences between 2-cell embryos and ESCs (Fig. 2b, c and Supplementary Data 5). In ESCs, both clusters 2 and 3 replicated late, but in 2-cell embryos their replication timing differed (Fig. 2c, d). Cluster 3 replicated comparably early in 2-cell embryos and exhibited high transcriptional induction at the 2-cell stage, while the comparably early replicating ESC cluster 9 was associated with high transcription induction at 4 and 8-cell stage26 (Fig. 2e, f and Supplementary Fig. 2a). Indeed, previous studies have consistently demonstrated that replication timing is associated with local transcriptional potential and chromatin organization4,33,34,35.
a Heatmaps and graphs showing normalized Repli-seq read densities (upper), binarized replication status (middle) and mean binarized replication status (bottom) 2-cell embryos and ESCs at five genomic loci. Orange and green highlighted regions indicate loci with differences between these cell types. b Genome-wide heatmaps of k-means clustered signal enrichment and variation in Repli-seq samples from S- and G1-phase 2-cell embryos and ESCs. c Heatmaps present genome-wide binarized replication status in individual 2-cell embryo and ESC Repli-seq samples ordered as in (b). Rightmost heatmap shows differences in the mean binarized replication status between 2-cell embryos and ESCs. d Scatter plots showing the relationship between mean normalized S-phase Repli-seq read densities in ESCs and 2-cell embryos for all 40 kbp bins (top) or three selected clusters (bottom) (r-value: Pearson correlation coefficient). e Composite panel showing mean genome-wide transcript density (graph, left) and log2-fold differences relative to the mean (heatmap, right) from single oocyte or embryo RNA-seq quantified in 40 kbp bins ordered as in (b). f Bar diagrams showing the correlation between mean replication status from 2-cell embryos or ESCs and transcript density from the indicated stages. The signal was analyzed in 40 kbp bins and correlation was measured using Spearman rank correlation tests. g Heatmaps showing genome-wide histone marks, histone variant, and chromatin accessibility signal from oocytes. Signal was quantified in 40 kbp bins ordered as in (b) and FPKM-normalized. Leftmost heatmap, mean replication status (RS) differences as in c. h Genome-wide correlation of histone marks, histone variant, and chromatin accessibility data in oocytes (lowermost blue rhombs) relative to mean replication status from 2-cell embryos, ESCs, and the difference between these two. The signal was analyzed in 40 kbp bins and correlation measured using Spearman rank correlation tests. i Genome-browser tracks of mean binarized replication status (top), RNA-seq (middle), histone mark and chromatin accessibility (bottom). Example loci with relatively early 2-cell replication (green highlight). Source data are provided as a Source Data file.
In light of these observations, we sought to investigate the potential influence of maternally inherited epigenetic landscape on the observed differences in replication timing, in addition to transcriptional induction. To accomplish this, we examined the enrichment of epigenetic features using published ChIP-seq, Cut&Run, and ATAC-seq data from Metaphase II (MII) and fully grown GV oocytes (FGO)19,20,23,26,36,37,38,39 (Fig. 2g and Supplementary Fig. 2b, c and Supplementary Data 6). Interestingly, we identified a pronounced global association between replication timing differences and the H2AUb and H3K27me3 histone marks deposited by Polycomb Repressive Complexes (PRCs)20,23,36, which were associated with relatively early replicating regions in the 2-cell embryos compared to ESCs (Fig. 2g–i and Supplementary Figs. 2b, c and 3a, b). Together, these analyses revealed that inherited maternal histone marks deposited by PRCs are associated with the temporal organization of DNA replication in 2-cell stage embryos.
Abundant local parental differences in the replication timing of 2-cell embryos
To explore whether asynchronous DNA replication timing of the genomes inherited from the maternal oocyte and paternal sperm, henceforth referred to by their parental genome origin, could explain the smaller S/G1 deviation in log2 fold signal between 2-cell embryos and ESC (Fig. 1h–j and Supplementary Fig. 1f, g), we separated the genomes originating from each of the gametes based on single nucleotide variations between the two parental strains. This allowed us to independently visualize and compare the replication timing profiles of the maternal and paternal genome (Fig. 3a). We observed consistent differences in the S/G1 deviation in the maternal and paternal genomes of 2-cell embryos. These differences also exist in embryos that were not synchronized with Nocodazole (Supplementary Fig. 3c) and when the opposite mouse strains were used for the cross (Supplementary Fig. 3d). Measures of the relative Euclidian distances and hierarchical clustering between Repli-seq values of individual 2-cell and ESC parental genomes revealed a distinct DNA replication program in each of the 2-cell parental genomes that was unlike that of ESCs (Fig. 3b and Supplementary Fig. 3e). Next, we performed k-means clustering of the genome-wide Repli-seq enrichment within the maternal and paternal 2-cell and ESC genomes. Based on this, we observed that a large fraction of the genome exhibited consistent earlier replication in the maternal genome compared to the paternal genome, while the opposite was the case for another large fraction (Fig. 3c, d and Supplementary Data 7). These findings in the 2-cell embryo are in contrast to our observations in ESCs, where we observed a strong correlation between the replication timing profiles of the two parental genomes, consistent with previous reports (Fig. 3e)11,12. Notably, separation of parental genomes did not lead to an increase in the S/G1 deviation in log2 fold signal we observed in the zygotes and only partially increased the S/G1 deviation of 2-cell embryos, which was still considerably smaller than that of ESCs (Fig. 1e, f and Supplementary Fig. 3f, g). These data support that the lower S/G1 deviation observed in zygotes and 2-cell embryos compared to ESCs is not primarily caused by asynchrony between the maternal and paternal genomes. The more uniform progression of replication during early embryogenesis, deviating from the classical temporal pattern, could be the result of actual uniformity or that replication timing patterns are below our effective resolution.
a Heatmaps showing normalized Repli-seq read densities (upper), binarized replication status (middle) and mean binarized replication status (bottom) of parental genomes in 2-cell embryos at selected loci. b Distance matrix showing the relative Euclidian distances between Repli-seq enrichment profiles of parental genomes in 2-cell (n = 37) and ESC samples (n = 20). c Heatmaps of genome-wide signal enrichment and variation in individual Repli-seq samples from the each parental genome of S- (n = 37) and G1-phase (n = 7 Maternal, n = 6 Paternal) 2-cell embryos. K-means clustering of 40 kbp bins containing signal from both parental genomes (n = 46,468). d Heatmaps of genome-wide binarized replication status in individual Repli-seq samples from each parental genome of 2-cell embryos, ordered as in c. Rightmost heatmap shows differences in mean binarized replication status between parental genomes. Green squares and purple triangles indicate clusters with notable early/late maternal replication. e Genome-wide relationship between mean normalized S-phase Repli-seq of parental genomes in 2-cell embryos or ESCs (R-values: Spearman’s correlation coefficients).
We next investigated whether genomic regions that harbor high transcriptional induction at the 2-cell stage are replicated early across both parental genomes. Indeed, regions of high transcriptional induction are replicated early in both parental genomes from 4-cell stage onwards, indicating influence from the major embryonic genome activation26 (Fig. 4a, b and Supplementary Fig. 3h). In addition, we explored the relationship between the parental genome-specific replication scores at three maternally and 15 paternally imprinted genes (validated and allele-specific expression at day E3.5)40, and while we found a tendency for earlier replication of paternally relative to maternally imprinted genes, it was not significant and was limited by the few maternally imprinted genes in this category (Supplementary Fig. 3i, j). Given the importance of maternal oocyte-derived factors in the development of the embryo, we examined the enrichment of histone marks in relation to replication status differences between the parental genomes. Notably, the marks H2AUb and in particular H3K27me3 deposited by Polycomb Repressive Complexes (PRCs) were enriched in early-replicating maternal regions (Fig. 4c, d and Supplementary Fig. 4a–d). Furthermore, we observed a highly significant overlap with Polycomb Associating Domains (PADs) (observed for 6097 40 kbp bins compared to 3839 expected by chance) previously identified in late-stage oocytes41 (Supplementary Fig. 5a). Indeed, we observed that the groups of genomic regions with comparably early maternal replication (Defined in Fig. 3d and marked by green squares), were characterized by transcriptional induction in the embryo and high maternally inherited H3K27me3 and H2AUb levels (Fig. 4e, f). Conversely, regions with comparably late maternal replication (Fig. 3d, purple triangles), were characterized by transcriptional downregulation in the embryo and low maternally inherited H3K27me3 and H2AUb levels (Fig. 4e, f). The PRC-regulated genomic regions undergoing early replication in the maternal 2-cell genome did not replicate early in the paternal genome, suggesting that this feature is specific to early embryos (Supplementary Fig. 3a, b).
a Bar diagrams showing the correlation between mean replication status of 2-cell maternal and paternal embryos and transcript density from oocyte and embryo stages, correlation measured using Spearman’s rank. b Heatmaps showing transcript density difference from single oocyte or embryo RNA-seq. FPKM-normalized. Green squares and purple, defined in Fig. 3d. c Tile plot of the genome-wide correlation of histone mark and chromatin accessibility in oocytes (lowermost blue rhombs) relative to mean parental 2-cell replication status and the difference between them, correlation measured using Spearman rank. d Genome-wide histone mark and chromatin accessibility signal from oocytes, FPKM-normalized. Green squares: and purple triangles, defined in Fig. 3d. e Transcript densities at 40 kbp bins within maternal early and late replicating subpopulations. Whiskers represent 1.5x interquartile range, and the bar indicates the median value. (p-values, two-sided Mann-Whitney U-tests, Benjamini-Hochberg corrected for multiple testing). f Upper and lower quartiles (horizontal bars) and medians (black bars) of histone marks, histone variants, and chromatin accessibility signal from oocytes, within maternal early and late replicating subpopulations defined in Fig. 3d. Brackets: the number of biological replicates (p-values, two-sided Mann–Whitney U-tests comparisons of early and late bins for each replicate, Benjamini-Hochberg corrected for multiple testing). g Genome-wide H3K27me3 signal from MII oocytes, 2-cell and 8-cell embryos, FPKM-normalized. Green squares and purple triangles, defined in Fig. 3d. h Genome-browser tracks of H3K27me3 from MII oocytes, 2-cell and 8-cell embryos, with overlaid mean maternal (red) and paternal (blue) 2-cell replication status. Arrows: persisting embryonic maternal H3K27me3, *emerging embryonic H3K27me3, #Maternal H3K27me3 loss. Source data are provided as a Source Data file.
We also analyzed the association of replication timing with histone marks, the histone variant H3.3 and ATAC-seq data from mouse sperm cells42,43. While markers of active chromatin (ATAC-seq, H3.3, H3K27Ac, H3K4me3) were enriched in parts of the genome with both early maternal and paternal replication (Supplementary Fig. 5b), we did not observe strong selective enrichment at relatively early or late paternal clusters. Generally, paternal replication timing showed a less pronounced relationship to tested markers, than maternal replication timing (Supplementary Fig. 5c).
Given the extensive rewriting of histone marks during the early development of preimplantation embryos, we also related this to H3K27me3 profiles from 2-cell and 8-cell embryos41 (Fig. 4g, h). Although 2-cell and 8-cell H3K27me3 correlates well with maternal early replication, this enrichment generally originated from the oocyte, while embryo-specific H3K27me3 generally occurred at maternal late replicating regions. Altogether, this indicates that genome-wide epigenetic diversity across parental genomes is associated with an asynchronous parental-genome-specific DNA replication program in the early embryo that is replaced at later stages of embryo development.
Early Maternal replication timing is independently associated with the A-compartment and H3K27me3
Given the strong relationship between replication timing and nuclear compartmentalization seen in other cell types33,44,45, we investigated whether replication timing of the maternal and paternal genomes in 2-cell embryos are associated with genome compartmentalization, which emerges in embryos at this stage9,10. We used the PC1 score46 from previously published 2-cell embryo HiC data9 as an indicator for the local genomic propensity for being in the generally active A versus the repressed B nuclear compartments. When visualizing this together with the mean binarized replication status of the maternal and paternal genome in ESC, we observed a strong similarity between the PC1 score and replication timing in both the maternal and paternal genomes (Fig. 5a). This observation suggests the compartmentalization of the 2-cell embryos to a large extent predicts ESC replication timing. In the 2-cell embryos there was an overall association with the PC1 score, although on several loci we observed considerable local deviation to the replication timing of the individual parental genomes (Fig. 5a). To assess these deviations on a global scale, we plotted the mean binarized replication status relative to the PC1 score separately for the maternal and paternal genomes (Fig. 5b). Replication timing was significantly correlated to PC1 scores in each genome (p < 2.2 × 10−16, Spearman’s ρ test), and while we observed a strong tendency for high PC1 scores (A-compartment) to be associated with early maternal and paternal replication, low PC1 scores (B-compartment) were associated with both early and late maternal replication. Given the association between maternally inherited H3K27me3 and early replication, we next visualized the three-way relationship between H3K27me3, PC1 scores, and mean replication status in the maternal and paternal genomes (Fig. 5c). Notably, this analysis revealed a considerable subset of early replicating regions in the B-compartment (low PC1 score) for the maternal genome. These regions were highly enriched in H3K27me3, indicating that chromatin presence in the A-compartment and enrichment of H3K27me3 are independently associated with early replication. In contrast, no such relationship was observable in the paternal genome (Fig. 5c, right panel).
a Genome-browser tracks of mean maternal and paternal 2-cell (top) and ESC (middle) replication status and the PC1 from 2-cell embryo HiC data. b Scatter plots showing the mean 2-cell replication status for the maternal (left) and paternal (right) genomes and PC1 from 2-cell embryo HiC data (R-values, Spearman’s correlation coefficients). c Heatmaps showing the three-way relationship between MII oocyte (left) and Sperm (right) H3K27me3 signal (X-axis), PC1 (Y-axis) from HiC data in 2-cell embryos and the mean 2-cell replication status (color) for the maternal (left) and paternal (right) genomes. d Heatmaps showing the density of maternal early and late replicating regions, defined by genome-wide binarized replication status (Fig. 3d). X-axis: chromosome coordinates, Y-axis: autosome number. e Heatmaps of average enrichment at all chromosomes from individual Repli-seq samples (Y-axis); diploid (top), maternal (middle) and paternal (bottom) genome of 2-cell embryos. X-axis: chromosome coordinates. f Heatmaps of genome-wide mean binarized replication status (top), difference (middle) and LAD density in the maternal and paternal genomes of 2-cell embryos (bottom), clusters defined in Fig. 3c. g Heatmaps of maternal (upper) and paternal (lower) LADs in 2-cell embryos. X-axis: chromosome coordinates, Y-axis: autosome number. h Heatmaps of maternal late (upper left) and early (upper right) replicating regions, defined in Fig. 3d, as well as the density of maternal (lower left) and paternal (lower right) LADs in 2-cell embryos relative to mean maternal/paternal binarized replication status. i Heatmaps of maternal late (left) and early (right) replication, defined in Fig. 3d, within (top) or outside (bottom) LADs in the maternal genome of 2-cell embryos relative to mean enrichment of the H2AUb and H3K27me3 histone marks in MII oocytes. j Heatmaps and genome-browser Repli-seq tracks of maternal and paternal genomes in 2-cell embryos related to various features at selected loci. From top, normalized Repli-seq read densities, binarized replication status, mean binarized replication status, histone variant, chromatin accessibility, and histone mark profiles in oocytes, maternal and paternal LADs in 2-cell embryos. Green and orange highlights indicate maternal early and late replication, respectively. k Schematic of identified relationships between maternal and paternal replication in 2-cell embryos and key genomic features. Source data are provided as a Source Data file.
Oocyte-specific PRC marks establish boundaries with LADs, defining the transitions in early to late maternal replication
Our mapping of replication timing across whole chromosomes uncovered an enrichment of maternally-late replicating regions within the 30-megabase pericentromeric region of the autosomes (Fig. 5d). In contrast, no such effect was observed within the paternal chromosomes (Supplementary Fig. 5d). This observation was consistently observed across individual embryos (Fig. 5e) and was not a characteristic feature in ESCs (Supplementary Fig. 5e). The centromeric regions of the chromosomes in 2-cell embryos congregate at one pole of the nucleus near to the nuclear lamina47. Therefore, we hypothesized that Lamin-Associated Domains (LADs) might influence the organization of replication timing at the chromosome level. To test this, we utilized recently published genome-wide LAD data from the maternal and paternal genomes of 2-cell embryos48. Our analysis revealed a strong association between LADs and replication timing in the 2-cell embryos (Fig. 5f), and a particularly pronounced enrichment of LADs in the pericentromeric regions (Fig. 5g). Interestingly, both parental genomes exhibited a pronounced enrichment of LADs in the late replicating regions (Fig. 5f, h). However, the distinct partitioning of late replication in the pericentromeric region was exclusively observed in the maternal genome, while no relationship between the paternal pericentromere and late replication was apparent, despite the accumulation of paternal 2-cell LADs in this region (Fig. 5g and Supplementary Fig. 5d). Thus, the pericentromeric DNA is organized within LADs of the 2-cell nucleus and asynchronous parental genome replication timing is occurring within this domain.
To gain a deeper understanding of the coexistence between maternal late replication of LAD-associated regions and our previous observations of histone marks deposited by PRCs enriched in early-replicating maternal regions, we assessed the combined relationship among PRCs-deposited marks, LADs, and replication timing. Interestingly, our findings revealed that among the histone marks deposited by PRCs, H2AUb but more prominently H3K27me3 were most enriched in the part of the genome that do not overlap with LADs (Fig. 5i). In contrast, maternal early replication was predominantly partitioned into non-overlapping LAD regions, which also exhibited high enrichment for marks deposited by PRCs, especially H3K27me3 (Supplementary Fig. 5f–k). When we analyzed individual loci we found clear late to early replication timing transitions, which overlapped with boundaries between LADs and H3K27me3 (Fig. 5j). These results provide the first demonstration of a large-scale asynchronous parental genome-specific replication program that is influenced by higher-order genome organization, such as LADs, transcription, as well as the inheritance of maternal marks deposited by PRCs (Fig. 5k).
Discussion
The spatiotemporal pattern of DNA replication has been widely recognized as associated with epigenetic marks, transcriptional activity, and the organization of the 3D genome. Across eukaryotes, a DNA replication program exists, which features constant timing of multi-megabase regions ensuring their replication at specific times during the S phase. These regions are flanked by timing transition regions where DNA replication is progressing49.
Through our exploration using single-embryo Repli-seq, we uncovered a low S/G1 deviation in zygotes. This deviation was only partially restored in the 2-cell embryo when compared to mouse embryonic stem cells (ESCs). We propose that this observation suggests an unconventional DNA replication program in the zygote, characterized by the initiation of a high number of replication origins throughout the genome. Previous microscopy-based work found that replication in zygotes was structured and that paternal pronuclei replicate earlier than maternal pronuclei, and we would have anticipated this to result in a more pronounced zygotic replication timing pattern in sequencing-based data too. Future improvements in DNA amplification and sequencing techniques, may provide higher yield and resolution, enabling further investigation into whether distinct replication timing patterns exist at a smaller scale. The partial recovery of the S/G1 signal in 2-cell embryos, compared to ESCs, may indicate a gradual establishment of the replication program as embryonic development unfolds. This would be consistent with the gradual unfolding of the embryonic genome activation and the formation of A/B compartments, during embryogenesis9,32. The use of Nocodazole to synchronize the 2-cell embryos in our Repli-Seq protocol may limit our interpretation, as it could potentially lead to aneuploidy50,51,52, however, we also performed Repli-seq analysis on a subset of unsynchronized 2-cell embryos, which demonstrated comparable results to those obtained from Nocodazole-treated embryos. (Supplementary Fig. 3c).
Our studies uncovered features that are specific to early embryos after fertilization. They include the lack of synchrony observed in the maternal and paternal genomes of the 2-cell embryo, as well as the link to histone modifications inherited from the gametes. Thus, replication timing programs are not entirely initiated de novo. Post-translational modifications of histones undergo dynamic changes specific to each parental genome following fertilization53. We observed a pronounced enrichment of maternally inherited marks deposited by PRCs in the regions that replicate early on the maternal allele. In particular, the H3K27me3 enrichment specific for embryos occurred mainly in maternal late replicating regions, potentially indicating that the early maternal replication is associated with chromatin states inherited from the oocyte. This suggests that the persistence of PRCs-deposited histones from oocytes may play a role in organizing the replication program or be influenced by it, although we acknowledge the possibility that these chromatin features may be an indirect consequence of a separate underlying mechanism.
Furthermore, we made observations regarding the spatial arrangement of DNA replication in the pericentromeric regions of the parental chromosomes, which served to further distinguish the events occurring within the parental genomes (Fig. 5d). We consistently observed late replication in maternal pericentromeric regions across most chromosomes in the studied 2-cell embryos (Fig. 5d, e and Supplementary Fig. 5d), which was accompanied by the absence of histone marks deposited by PRCs. Interestingly, this is not the first report of unconventional genome organization in the embryo compared with somatic or ESCs. Up to the 4-cell stage, the centromeric regions of the maternal and paternal chromosome congregate at one pole, while the long chromosome arms and telomeric regions extend towards the opposite pole47. Additionally, in mice, the zygote segregates the parental genomes by two separate bipolar spindles during mitosis54, although additional work is required to confirm whether this is also the case in all human embryos55. Nevertheless, the function behind this nuclear segregation and the observed differences in replication timing remains unclear. However, this phenomenon may indicate a functional separation of parental genomes, possibly necessitating each genome to fulfill distinct roles during early embryogenesis.
Given recent reports of heightened replication stress during the cleavage stages of embryogenesis, the exploration of parental-specific DNA replication stress and stress-induced DNA breaks across the parental genomes becomes a matter of great interest. Moreover, the fragile nature of the pericentromere56,57,58,59,60,61 raises intriguing questions about the potential impact of these replication dynamics on the occurrence of gross chromosomal rearrangements, which are relatively common during early embryogenesis62,63,64. Investigations into the role of parental genomes in the formation of such rearrangements hold promise for advancing our understanding of embryonic failure and infertility.
In the concluding phases of manuscript preparation, additional studies addressing replication timing in early embryos have been published15,16. Although their primary focus is distinct from ours, they present some concordant findings. These studies identified that parthenotes replicate with a timing program that correlates closer to the maternal replication program than the paternal one. Furthermore, they show that DNA replication programs become more defined as the embryo develops. In the zygote, there is little to no replication timing program, with no bias towards parental LADs/iLADs. The embryonic replication program emerges in conjunction with the formation of A and B-compartments, the activation of the embryonic genome, and the observation that late replication is associated with the nuclear lamina.
In summary, our study unveils a notable link between replication timing and genome organization in the early embryo. Specifically, we have discovered a remarkable excess of late replicating regions in the maternal pericentromeric region when compared to paternal chromosomes. The identification of distinct replication timing patterns between maternal and paternal chromosomes emphasizes the intricate nature of replication regulation and its connection to genome organization. The observed association between replication timing, LADs, and maternal histone marks deposited by PRCs reveals an intriguing interplay between epigenetic modifications, nuclear architecture, and replication dynamics.
Methods
mESC cell culture
F121-9 mESC line (RRID: CVCL_VC42, a gift from Joost Gribnau) was cultured on 6-well plates coated with 0.01% poly-l-ornithine (Sigma P3655) for 1 h. Subsequently, the plates were coated with 300 pg/ml of laminin (Fisher Scientific 10152421) for 1 h.
The mESCs were maintained in 2i and LIF media65, which was refreshed every 24 h.
Sample preparation for single-cell RT profiling of mESC cells
Sample preparation of mouse ESCs for single-cell Repliseq12,66 was performed following previously established protocols. In brief, single F121-9 (CVCL_VC42) cells (a gift from Professor Joost Gribnau - Erasmus MC, Rotterdam, Netherlands) were re-suspended in 1 ml of 1% fetal bovine serum (FBS) in phosphate-buffered saline (PBS). Subsequently, the cells were fixed by slowly adding 3 ml of ice-cold 100% ethanol and incubating at −20 °C for 30 min. Following fixation, 1 × 106 cells were washed and resuspended in 700 µl of propidium iodide (PI) staining solution (350 µl of 1 mg/ml PI, 70 µl of 10 mg/ml RNase A, and 7 ml of 1% FBS-PBS).
Flow cytometric sorting was performed using a Sony SH800 to isolate single cells in the G1 or S phase, based on their PI staining profile. The single cells were sorted directly into individual wells of a 96-well plate containing 6 µl of single-cell lysis and fragmentation buffer (3 µl of proteinase K, 50 µl of 10x Single Cell Lysis and Fragmentation Buffer (Sigma; L1043), and 447 µl of autoclaved double-distilled water (ddH2O)). Following collection, the plate was centrifuged and stored at −80 °C until Repli-Seq processing.
Spermatozoa cryopreservation
All experiments involving animals were performed under the authorization of the Danish Animal Ethical Committee (“Dyreforsøgstilsynet”) permission 2021-15-0201-009 and are compliant with all relevant national ethical regulations regarding animal research under the requirement of Directive 2010/63/EU. All animals were exposed to 12 h of light (6:00 til 18:00) and were provided with water and standard mouse chow ad libitum and cages maintained at 20–24 °C and 45–65% humidity under the care of veterinarians. For cryopreservation of mouse sperm67, 10 0.25 mL clear plastic straws (Minitube GmbH; 13407/0010) were labeled for each mouse. 4 proven fertile male mouse (CAST/EiJ n = 2, C57BL/6N n = 2), aged between 3 and 8 months, were sacrificed following one week of solitary housing. The cauda epididymis and vas deferens were dissected and placed in 1 mL of Dulbecco’s phosphate-buffered saline (DPBS). Under a dissection microscope, adipose and vascular tissues were carefully removed. Both cauda epididymides were then transferred to a 35 mm dish containing a 120 µl droplet of gCPA sperm cryopreservation media (in-house) covered with NidOil (Nidacon; NO-100).
Using a pair of watchmaker scissors, the epididymides were cut several times to release the sperm. The dish was placed in a CO2 incubator at 37 °C with 5% CO2. To aid sperm dispersion and release, the dish was gently swirled once every minute for a total of 3 min.
Ten 10 µl aliquots of the sperm suspension were taken from the dish and deposited onto the lid of a 35 mm culture dish. Clear plastic straws were prepared for freezing by drawing up HTF medium (in-house) into the straw using a syringe, followed by a small amount of air, and then the 10 µl aliquot of sperm. Additional air was introduced into the straw before sealing it with a metal ball (Minitube GmbH; 13400/9970).
All ten straws were placed in the gaseous phase of liquid nitrogen for a duration of 10 min. Subsequently, the straws were slowly immersed into the liquid phase of nitrogen before being transferred to a −150 °C freezer.
In vitro fertilization of mouse oocytes
In vitro fertilization of mouse oocytes67 was performed by first thawing a single straw of cryopreserved sperm from either CAST/EiJ or C57BL/6N mice for 10 min in a water bath set to 37 °C. After thawing, the sperm was dispersed into 90 µl of TYH + MBCD (in house, for 50 mL solution, combine the following with milliQ H2O; 358.6 mg NaCl (Sigma; S-5886), 17.8 mg KCl (Sigma; P-5405), 14.65 mg MgSO4.7H2O (Sigma; M-7774), 8.1 mg KH2PO4 (Sigma; P-5655), 0.25 ml Na-Pyruvate (Gibco; 11360-039), 50 mg Glucose (Sigma; G-6152), 12.55 mg CaCl2.2H2O (Sigma; C-7902), 49.55 mg Methyl-β-cyclodextrin (Sigma; C-4555), 1 ml Penicillin G/Streptomycin (Gibco; 15140-122) and 50 mg Polyvinualcohol (Sigma; P8136)) sperm pre-incubation medium in a 35 mm dish coated with NidOil (Nidacon; NO-100). The dish was equilibrated at 37 °C for 20 min in a 5% CO2 in air. Alternatively, a droplet of fresh sperm was taken from the epididymis of a freshly sacrificed male mouse and incubated in 90 µl of TYH + MBCD sperm pre-incubation medium in a 35 mm dish coated with NidOil (Nidacon; NO-100).
Simultaneously, a 30 mm fertilization dish was prepared by adding 90 µl droplet of 2 mM reduced glutathione (Sigma, G4251) in HTF (in house, for 100 mL solution, combine the following with milliQ H2O; 593.8 mg NaCl (Sigma; S-5886), 35 mg KCl (Sigma; P-5405), 4.9 mg MgSO4.7H2O (Sigma; M-7774), 5.4 mg KH2PO4 (Sigma; P-5655), 57 mg CaCl2 (Sigma; C-5670), 210 mg NaHCO3 (Sigma; S-5761), 0.34 ml Na-Lactate (Sigma; L-7900), 3.7 mg Na-Pyruvate (Sigma; P-4562), 50 mg Glucose (Sigma; G-6152), 49.55 mg Methyl-β-cyclodextrin (Sigma; C-4555), 0.5 ml Penicillin G/Streptomycin (Gibco; 15140-122), 400 mg BSA (Sigma; A-7030) and 0.04 ml Phenol red (Sigma; P-0290)) and covering it with NidOil. The dish was equilibrated to 37 °C in a 5% CO2 in air environment.
Female C57BL/6N or CAST/EiJ mice were superovulated by intraperitoneal injection of either 5 or 7 IU of PMSG (Prospec; HOR-272), followed by 5 or 7 IU of hCG (Sigma; CG5) 48 h later. The dosage of PMSG and hCG administered depended on the age of the mice, mice aged between 4–5 weeks received 5 IU, while those that were 6–8weeks or older received 7 IU. After 15 h from the second injection, the mice were euthanized, and the oviducts were dissected and placed into the warm NidOil of the fertilization dish. Cumulus-enclosed oocytes were transferred from the ampoule into the fertilization droplet. Following a 30-minute pre-incubation, between 1 and 10 µl of the most motile sperm was extracted from the pre-incubation droplet and added to the fertilization dish containing the cumulus-enclosed oocytes. The dish was then incubated for three hours. Meanwhile, a wash dish was prepared by creating four droplets of 90 µl of Advanced KSOM embryo media (Sigma; MR-101) in a 60 mm dish. The dish was covered with NidOil and equilibrated at 37 °C in a 5% CO2 environment. The embryos were washed through four wash droplets to remove the cumulus cells and sperm. For subsequent experimentation (detailed below), embryos were selected at random, ensuring no intentional bias regarding the sex of the embryos.
EdU labeling of single mouse embryos
To determine the duration of the first and second S phase, we conducted pulse labeling of single zygotes and 2-cell stage embryos using the Click-iT EdU Cell Proliferation Kit for Imaging (ThermoFisher Scientific; C10340), as follows:
Embryos were obtained at the zygote stage (no prior treatment) across three independent experiments using nine 4–5-week-old C57BL/6N females. Additionally, 2-cell stage embryos were obtained in three more independent experiments, also using nine 4–5-week-old C57BL/6N females. In all experiments, cryopreserved sperm from CAST/EiJ mice was used for IVF (as described above). The 2-cell stage embryos were labeled with EdU following release from Nocodazole synchronization. At hourly intervals, individual zygotes or 2-cell embryos were transferred to pre-equilibrated droplets of Advanced KSOM embryo media (Sigma; MR-101) containing 500 µM EdU (provided in Click-iT EdU Cell Proliferation Kit for Imaging (ThermoFisher Scientific; C10340)). The droplets were maintained at 37 °C in a 5% CO2 environment. After a 30-minute incubation period, the embryos were washed with Advanced KSOM media (Sigma; MR-101) and fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature. Subsequently, the embryos were transferred to a wash buffer consisting of 5% BSA in PBS and incubated on a rocking stage at 4 °C for 48 h. To permeabilize the embryos, they were treated with 0.5% Triton X-100 in PBS for 20 min at room temperature, followed by rinsing in PBST (0.1% Tween 20 in PBS) for 20 min at room temperature. The embryos were then subjected to the Click-iT EdU cell proliferation reaction cocktail, according to the manufacturer’s instructions. They were incubated in the reaction cocktail for 1 h. After incubation, the embryos were rinsed in a wash buffer containing 20 µg/ml DAPI (ThermoFisher Scientific; D1306) for 30 min.
For imaging, embryos were mounted using SlowFade Diamond Antifade Mountant (ThermoFisher Scientific; S36967) between two No.1 coverslip. Confocal microscopy imaging was performed using an LSM900 confocal laser scanning microscope (Zeiss) equipped with a 40x Plan-Apochromat objective. The Alexa 647 fluorophore was excited using a 639-nm laser, and DAPI was excited using a 405-nm laser. Image processing was performed using Zen software and ImageJ68 software for quantification of corrected total fluorescence.
Sample preparation for RT profiling of single mouse embryos (Single-Embryo Repli-Seq)
To generate zygotes for Single-Embryo Repli-Seq, oocytes were collected (as described above) from nine 4–5-week-old C57BL/6N females across three independent experiments. In one of these experiments, the oocytes were fertilized with fresh sperm collected from the epididymis of a single 13-week-old CAST/EiJ male. In the remaining two experiments, the oocytes were fertilized using cryopreserved sperm from CAST/EiJ mice, as detailed above. After IVF, the zygotes were categorized into G1 or S phase. G1 phase was determined by two pronuclei detection, while S phase was determined by timing established using prior EdU incorporation experiments. Additionally, in a separate experiment, oocytes were collected from nine 5–6-week-old C57BL/6N females and fertilized via IVF using fresh sperm from the epididymis of a 12-week-old CAST/EiJ male. A subset of these zygotes were selected at various time points, ranging from the formation of the two pronuclei up to about 4 h before cell division. To achieve this, we modified our in vitro fertilization protocol, to improve the synchronization of zygotes development by incubating the oocytes with sperm for a duration of 1 h. The Zona pellucida and polar bodies were removed using a dissection microscope equipped with laser biopsy, and naked zygotes were washes in advanced KSOM (Sigma; MR-101) and transferred to a low-adhesion 96-well PCR plate. Each well contained 6 µl of single-cell lysis and fragmentation buffer (to prepare 500 µl, add 3 µl proteinase k (Sigma; P-4850) and 50 µl 10× Single cell lysis and fragmentation buffer (Sigma; L1043) to 447 µl of autoclaved ddH2O).
For 2-cell embryos, oocytes were collected from six 4–5-week-old C57BL/6N females and ten 5–8-week-old CAST/EiJ females across three independent experiments. In the first two experiments, the oocytes from C57BL/6N females were fertilized with fresh sperm taken from two 12–13-week-old CAST/EiJ males. In the subsequent experiment, the oocyes from CAST/EiJ females were fertilized with fresh sperm from a single 10-week-old C57BL/6N male. Fifteen hours after combining sperm with cumulus-enclosed oocytes, the resulting zygotes were transferred using a micropipette into a 90 µl droplet of Advanced KSOM embryo media (Sigma; MR-101) containing 40 nM Nocodazole. The droplet was placed in a 30 mm dish covered with NidOil (Nidacon; NO-100). This step synchronized the zygotes in the G2/M phase from which they were released after 4 h and facilitating the precise selection of embryos in G1 or S phase. Following the synchronization period, the zygotes were washed through seven droplets of Advanced KSOM embryo media, each covered with NidOil. These droplets were pre-equilibrated to 37 °C in a 5% CO2 environment. A subset of embryos was additionally chosen without Nocodazole treatment. In this instance, oocytes from six C57BL/6N females were fertilized using fresh sperm from a 13-week-old CAST/EiJ male mouse. The resulting 2-cell stage embryos were selected as being in G1 or S phase based on their timing from division. Under normal circumstances, the zygotes divide within 1 h after release from the block. G1 phase embryos were promptly processed by removing the zona pellucida and polar bodies (if present) using a dissection microscope. The resulting naked embryos were washed and transferred to a low-adhesion 96-well PCR plate containing 6 µl of single-cell lysis and fragmentation buffer. The embryo plates were centrifuged and subsequently stored at −80 °C until preparation for single-cell sequencing.
For the processing of single S-phase embryos, the same procedure was followed. However, selection of S-phase embryos was based on prior EdU labeling experiments, which determined the time of entry and exit from the S phase after release from Nocodazole synchronization. Specifically, early S-phase cells were selected 3 h after release, mid-S-phase cells were selected 5 h after release, and late-S-phase cells were selected 6 h after release.
Sample preparation and sequencing for single-embryo RT profiling
Single embryos or single mouse embryonic stem cells (mESCs) were processed for single-cell Reli-Seq12,66. Whole-genome amplification was performed using the SeqPlex Enhanced DNA Amplification Kit (Sigma; SEQXE-50XN).
First, the samples were thawed and briefly centrifuged, then incubated on a thermal cycler for 1 h at 55 °C, followed by a 4 min incubation at 99 °C. After the incubation, 1 µl of library preparation buffer was added to each well, which were then briefly vortexed and centrifuged. The plate was then incubated at 95 °C for 2 min and then held at 4 °C. The samples were then transferred to ice, and 0.5 µl of library preparation enzyme was added before incubation using a thermal cycler, programmed as follows: 16 °C for 20 min, 24 °C for 20 min, 37 °C for 20 min, 75 °C for 5 min and then held at 4 °C.
Immediately following this, 22.5 µl of DNA amplification master mix (15.9 µl ddH2O, 6 µl 5X amplification mix and 0.6 µl DNA polymerase for SeqPlex) was added to each sample on ice and samples were then incubated on a thermal cycler with the following program: 94 °C for 2 min, followed by 24 cycles of 94 °C for 15 s and 70 °C for 5 min, then 70 °C for 30 min before holding at 4 °C. The resulting amplification was then subject to a bead clean up.
For the bead clean up, 51 µl of AMPure XP beads (Beckman Coulter; A63881) was added to each well and incubated for 15 min at room temperature. The samples were transferred to a magnet stand and left for 5 min after which the supernatant was discarded. The plate was removed from the magnet stand, and 100 µl of 80% ethanol was added to each well and mixed by vortexing. The plate was placed back on the magnet stand for 5 min, the ethanol supernatant was removed, and the beads were allowed to dry for 3 min. The beads were then resuspended in 15 µl of ddH2O and placed back on the magnet stad for 5 min, and then supernatant was then collected in a fresh plate.
The samples were quantified using the Qubit dsDNA HS assay kit (ThermoFisher; Q32851) and 800 ng of Amplified DNA was used in the adaptor digestion mix (2 µl 10X Primer Removal Buffer, 0.4 µl Primer Removal Solution, 0.94 µl Primer Removal Enzyme and made up to a total volume of 10 µl with ddH2O). The reaction was incubated for 1 h at 37 °C, followed by 65 °C for 20 min. The resulting reaction was the subject to a bead clean up using 36 µl of AMPure XP beads as before.
The samples were quantified using the Qubit dsDNA HS assay kit and the quantification of the samples was further confirmed by checking the product size using the Agilent Tapestation 2200 High Sensitivity system (Agilent; 5067). For library preparation, we utilized the KAPA Hyper Prep Library Preparation Kit (Roche; KK8502) in combination with IDT for Illumina TruSeq DNA UD Indexes (Illumina; 20023784). The end repair and A-tailing steps were performed using 40 ng of whole-genome amplified DNA. The reaction volume was scaled down to 1/5th of the recommended reaction volume and vortexed and briefly spun down before incubating on a thermocycler at 20 °C for 30 min, followed by 65 °C for 30 min, and then held at 4 °C. Adaptor ligation was performed in the same plate as the end repair and A-tailing reaction. The reaction mixture consisted of 12 µl end repair and A-tailing reaction product, 0.5 µl IDT for Illumina TruSeq DNA UD Indexes, 1.5 µl PCR-grade water, 6 µl ligation buffer, and 2 µl DNA ligase. The reaction mixture was vortexed, spun down, and incubated at 20 °C for 15 min. Post-adaptor ligation cleanup was performed using 0.8X Agencourt AMPure XP beads (Beckman Coulter; A63881), following the instructions provided with the KAPA Hyper Prep Kit. Library amplification was carried out by scaling down the recommended manufacturer’s protocol to 1/5th and using 11 µl of the bead-cleaned post-ligation product. Amplification was performed using a thermocycler with an initial denaturation step of 98 °C for 45 s, followed by 5 PCR cycles at 98 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. After the final cycle, an extension step was included, and the samples were incubated at 72 °C for 1 min, followed by holding at 4 °C. The samples were cleaned according to the KAPA Hyper Prep Kit post-amplification cleanup instructions using 0.9X Agencourt AMPure XP beads. The final libraries were quantified using the Qubit dsDNA HS kit and assessed for library size using the Agilent Tapestation 2200 High Sensitivity system. The libraries were sequenced on a NextSeq550 platform using a 150-bp single-end read format.
Single-embryo and single mESC Repli-Seq data analysis
Our single-embryo Repli-Seq data analysis, which was used to analyze all embryos and single mESCs, was based on two previously published protocols11,12,66. We followed the previously established analysis pipeline12,66 up to the generation of log2 fold replication timing (RT) scores, with some modifications. The binarization of Replication Status followed the piecewise copy fit and mixture modeling approach that has previously been established11 with adaptations for our analysis. This relied on the R-packages Zoo, Fbasics, Mixtools, and Copynumber69.
Fastq files for G1 and S phase were checked for quality using FastQC and trimmed for Illumina index and Seqplex adaptor sequences using Cutadapt70, as described in previous studies12,66. For haplotype analysis, sequencing reads were mapped to a modified mm10 reference genome that included N-masked positions for single hybrid strains. The N masked genome was created using the genome preparation command of the SNPsplit package71, which incorporates N-masking into the mm10 reference for the CAST/EiJ genome from the Mouse genome project V7 vcf file72,73,74. Subsequently, this new mm10 N-masked genome was then used to map the trimmed sequencing reads using bowtie275 for parsed haplotype analysis, alternatively unparsed haplotype analysis was performed using the original mm10 reference genome and duplicate reads were marked with Picard (http://broadinstitute.github.io/picard).
To divide the reads into maternal and paternal haplotypes, we used the allele-specific alignment sorter SNPsplit71, which utilized a list of known SNP positions between C57BL/6 and CAST/EiJ mice. Quality control of parsed BAM files was confirmed using SAMStat76,77, and typically, we obtained >50% reads with >30 MAPQ. To calculate log2 fold replication timing scores, a G1 reference file was generated from three or more G1 control cells with a normal karyotype. The karyotype was confirmed using the hidden Markov model of the findCNVs command in the Aneufinder package78, as previously described12,66. In our analysis of mESC, we made the decision to exclude chromosome 3 and chromosome 8 due to aneuploidy.
Computing of RT scores were performed by counting reads in sliding windows of 200 kb at 40 kb intervals normalized to the G1 control according to the standard scRepliseq analysis or haplotype-resolved scRepliseq analysis previously published protocol for unphased and phased haplotype analysis, respectively66. Log2 fold RT scores were converted to binary RT scores using a previously outlined protocol, with binary values determined using the skew approach when the component means from the mixture modeling were <0.2511.
The Replication score for each sample was obtained by calculating the fraction of the genome that had been replicated based on the binary scores for each sample, ignoring bins from masked parts of the genome. A matrix assembly was generated containing Normalized Repli-seq enrichment and binarized Replication Status for each cell. Data aggregation for this was done using the merge command in R (v. 4.1.2)79 and IDs for each bin consisting of chromosome name and start coordinate for all datasets. This was performed separately for three pairwise comparisons, published vs. our ESC data, ESC vs. 2-cell data, and maternal vs. paternal 2-cell data, resulting in output that only included the relevant datasets and covered parts of the genome where data were present in all input samples (Supplementary Data 1,4,6). Subsequent analysis was performed in EaSeq (see below)80 after importing bedgraphs-files of log2-fold enrichment comparisons between S-phase and G1-phase samples as well as bedgraphs of Binarized Replication Status as ‘datasets’.
Genomic data sources and quality control
Previously published Repli-seq data from single ESCs11 were downloaded from the NCBI GEO database81 at GSE102077. Lamin-associated domains in maternal and paternal 2-cell embryos were identified in ref. 48 and bed-files with allele-specific coordinates were downloaded from the NCBI GEO database81 at GSE112551. Late 2-cell embryo HiC data were previously published9, and the genome-wide vector of first principal component (PC1) was provided by the authors as a bedgraph file. PADs and interspersed regions (iPADs) were previously published and derived from the supplementary tables41. Imprinted genes were previously published40, and we used the subset that was validated and found to be expressed in an allele-specific way by the authors. Refseq genome annotations82 for mm10 were downloaded from the UCSC table browser83. Read qualities were analyzed using FastQC84, fastqScreen (v. 0.11.4)84, and MultiQC (v. 1.7)85.
ChIP-seq, Cut&Run, RNA-seq, and ATAC-seq data sourcing and processing
ChIP-seq and Cut&Run data of histone marks and the histone variant H3.3, input, as well as ATAC-seq data from MII or fully grown oocytes, preimplantation embryos, and sperm cells were published in refs. 19,20,23,26,37,38,39,42,43. A complete list can be found in Supplementary Data 6. Single oocyte/embryo RNA-seq was previously published26 and was processed as described in ref. 86. ChIP-seq, Cut&Run, and ATAC-seq reads were analyzed using FastQC and trimmed using Trimgalore87. Reads were mapped using bowtie275, and handled using Samtools88. Blacklisted regions89 were obtained from https://github.com/Boyle-Lab/Blacklist/archive/v2.0.zip and removed using bedtools intersect -v90. Subsequent analysis was performed in EaSeq (see below)80 after importing mapped reads from Bam-files as ‘datasets’. For ChIP-seq, Cut&Run and ATAC-seq this was done using default settings, including deduplication. For RNA-seq removal of duplicates was disabled.
Repli-seq analysis and visualization
Analysis and visualization was done using EaSeq (v. 1.12)80, Microsoft Excel 2016 for simple bar diagrams and line charts, and R (v. 4.1.2)79 for Boxplots and interquartile range plots as well as the beeswarm package (v.0.4.0, https://github.com/aroneklund/beeswarm) for Bee-swarm plots. In EaSeq, Genome browser tracks and annotations were made using the EaSeq plot tools ‘FillTrack’, ‘Annot.’ and ‘RegionMap’ for LADs (available in the beta-testing panel). Scatter plots and heatmaps of ChIP-seq, Cut&Run, ATAC-seq, HiC PC1, and RNA-seq values were made using the EaSeq tools ‘Scatter’ and ‘ParMap’. Assembled matrices (Supplementary Data 4-6) and LAD coordinates we imported as ‘Regionsets’ in EaSeq. Heatmaps of single-embryo log2-fold enrichment comparisons between S-phase and G1-phase samples as well as heatmaps of Binarized Replication Status at individual loci (as well as averages from multiple loci) were generated using the ‘Multi Track’ tool available in the ‘Beta-testing’ panel of EaSeq. ChIP-seq, Cut&Run, and ATAC-seq Signal intensities at the bins in the matrix assemblies were quantified using the EaSeq tool ‘Quantify’, using default normalization (Fragments Per Kilobase per Million reads – FPKM) for scatter plots and clustered heatmaps. RNA-seq read densities were quantified using the EaSeq tool ‘Quantify’ but with normalization disabled to obtain matrices of raw read counts. Following this, a pseudoread was added to RNA-seq read counts, and all quantified RNA-seq counts in a matrix assembly were quantile normalized using the tool ‘Normalize’. These normalized values were used to calculate means for each condition as well as log2 fold difference from the mean using the EaSeq tool ‘Calculate’. Repliseq S-phase/G1 log2 fold differences at imprinted loci were quantified using the EaSeq tool ‘Quantify’ at regions corresponding to 40 kbp centralized around the imprinted genes. Heatmaps of feature density e.g. ChIP-seq, RNA-seq signal, HiC PC1, PAD, iPAD, or LAD-overlap were generated using the tool ‘Z-scatter’. Similarly, plots of features at autosomes were generated using this tool and the autosome number for the Y-axis and start coordinate of each bin for the X-axis. Overlap with LADs, PADs, and iPADs for each matrix assembly was determined using the EaSeq tool ‘Coloc’. Subpopulations were obtained using the ‘Gate’ tool.
Repliseq clustering
Hierarchical clustering was performed in R (v. 4.1.2)79 on scaled and transformed (R-commands: scale() and t(), default settings) log2-fold Repli-seq enrichment using the command dist(method = ‘euclidean’) to calculate a distance matrix, and a dendrogram was plotted after hierarchically clustering the distance matrix using the command hclust (method = ‘ward.D2’). k-means-clustering was performed in EaSeq, using the tool ‘ClusterP with all additional normalization disabled. Clustering was performed individually for comparisons between 2-cell and ESC data and 2-cell maternal and paternal data.
Statistics
All statistics were performed as described in legends using R (v. 4.1.2)79.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw and processed Repli-seq data generated for this publication have been deposited in NCBI’s Gene Expression Omnibus81 and are accessible under GEO Series accession number GSE237400. A complete list with hyperlinks to previously published data used for this study can be found in Supplementary Data 6. Requests for materials should be addressed to the corresponding authors. Source data are provided with this paper.
Code availability
Scripts for diploid and haplotype-resolved Repli-seq processing were acquired from https://github.com/kuzobuta/scRepliseq-Pipeline and https://github.com/kuzobuta/scRepliseq-Pipeline/tree/master/scripts/haplotype-resolved-analysis. EaSeq and source code is available at http://easeq.net.
References
Hansen, R. S. et al. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc. Natl Acad. Sci. USA 107, 139–144 (2010).
Rhind, N. & Gilbert, D. M. DNA replication timing. Cold Spring Harb. Perspect. Biol. 5, a010132 (2013).
Julienne, H., Zoufir, A., Audit, B. & Arneodo, A. Human genome replication proceeds through four chromatin states. PLoS Comput. Biol. 9, e1003233 (2013).
Pope, B. D. et al. Topologically associating domains are stable units of replication-timing regulation. Nature 515, 402–405 (2014).
Dileep, V. et al. Topologically associating domains and their long-range contacts are established during early G1 coincident with the establishment of the replication-timing program. Genome Res. 25, 1104–1113 (2015).
Rivera-Mulia, J. C. et al. Dynamic changes in replication timing and gene expression during lineage specification of human pluripotent stem cells. Genome Res. 25, 1091–1103 (2015).
Aran, D., Toperoff, G., Rosenberg, M. & Hellman, A. Replication timing-related and gene body-specific methylation of active human genes. Hum. Mol. Genet. 20, 670–680 (2011).
Suzuki, M. et al. Late-replicating heterochromatin is characterized by decreased cytosine methylation in the human genome. Genome Res. 21, 1833–1840 (2011).
Du, Z. et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 547, 232–235 (2017).
Flyamer, I. M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544, 110–114 (2017).
Dileep, V. & Gilbert, D. M. Single-cell replication profiling to measure stochastic variation in mammalian replication timing. Nat. Commun. 9, 427 (2018).
Miura, H. et al. Single-cell DNA replication profiling identifies spatiotemporal developmental dynamics of chromosome organization. Nat. Genet. 51, 1356–1368 (2019).
Marchal, C. et al. Genome-wide analysis of replication timing by next-generation sequencing with E/L Repli-seq. Nat. Protoc. 13, 819–839 (2018).
Takahashi, S. et al. Genome-wide stability of the DNA replication program in single mammalian cells. Nat. Genet. 51, 529–540 (2019).
Nakatani, T. et al. Emergence of replication timing during early mammalian development. Nature 625, 401–409 (2024).
Shuangyi, X. et al. DNA replication in early mammalian embryos is patterned, predisposing lamina-associated regions to fragility. bioRxiv https://doi.org/10.1101/2023.12.25.573304 (2023).
Ferreira, J. & Carmo-Fonseca, M. Genome replication in early mouse embryos follows a defined temporal and spatial order. J. Cell Sci. 110, 889–897 (1997).
Bouniol-Baly, C., Nguyen, E., Besombes, D. & Debey, P. Dynamic organization of DNA replication in one-cell mouse embryos: relationship to transcriptional activation. Exp. Cell Res. 236, 201–211 (1997).
Dahl, J. A. et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537, 548–552 (2016).
Liu, X. et al. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 537, 558–562 (2016).
Zhang, B. et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537, 553–557 (2016).
Xia, W. et al. Resetting histone modifications during human parental-to-zygotic transition. Science 365, 353–360 (2019).
Chen, Z., Djekidel, M. N. & Zhang, Y. Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat. Genet. 53, 551–563 (2021).
Peaston, A. E. et al. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 7, 597–606 (2004).
Franke, V. et al. Long terminal repeats power evolution of genes and gene expression programs in mammalian oocytes and zygotes. Genome Res. 27, 1384–1394 (2017).
Sankar, A. et al. KDM4A regulates the maternal-to-zygotic transition by protecting broad H3K4me3 domains from H3K9me3 invasion in oocytes. Nat. Cell Biol. 22, 380–388 (2020).
Brind’Amour, J. et al. LTR retrotransposons transcribed in oocytes drive species-specific and heritable changes in DNA methylation. Nat. Commun. 9, 3331 (2018).
McLay, D. W. & Clarke, H. J. Remodelling the paternal chromatin at fertilization in mammals. Reproduction 125, 625–633 (2003).
Aguirre-Lavin, T. et al. 3D-FISH analysis of embryonic nuclei in mouse highlights several abrupt changes of nuclear organization during preimplantation development. BMC Dev. Biol. 12, 30 (2012).
Palmerola, K. L. et al. Replication stress impairs chromosome segregation and preimplantation development in human embryos. Cell 185, 2988–3007.e2920 (2022).
Kort, D. H. et al. Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum. Reprod. 31, 312–323 (2016).
Ke, Y. et al. 3D chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell 170, 367–381.e320 (2017).
Moindrot, B. et al. 3D chromatin conformation correlates with replication timing and is conserved in resting cells. Nucleic Acids Res. 40, 9470–9481 (2012).
Sima, J. et al. Identifying cis elements for spatiotemporal control of mammalian DNA replication. Cell 176, 816–830.e818 (2019).
Muller, C. A. & Nieduszynski, C. A. DNA replication timing influences gene expression level. J. Cell Biol. 216, 1907–1914 (2017).
Mei, H. et al. H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat. Genet. 53, 539–550 (2021).
Xu, Q. et al. SETD2 regulates the maternal epigenome, genomic imprinting and embryonic development. Nat. Genet. 51, 844–856 (2019).
Ishiuchi, T. et al. Reprogramming of the histone H3.3 landscape in the early mouse embryo. Nat. Struct. Mol. Biol. 28, 38–49 (2021).
Zhang, C. et al. The chromatin remodeler Snf2h is essential for oocyte meiotic cell cycle progression. Genes Dev. 34, 166–178 (2020).
Santini, L. et al. Genomic imprinting in mouse blastocysts is predominantly associated with H3K27me3. Nat. Commun. 12, 3804 (2021).
Du, Z. et al. Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol. Cell 77, 825–839.e827 (2020).
Jung, Y. H. et al. Maintenance of CTCF- and transcription factor-mediated interactions from the gametes to the early mouse embryo. Mol. Cell 75, 154–171.e155 (2019).
Jung, Y. H. et al. Chromatin states in mouse sperm correlate with embryonic and adult regulatory landscapes. Cell Rep. 18, 1366–1382 (2017).
Ryba, T. et al. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res. 20, 761–770 (2010).
Yaffe, E. et al. Comparative analysis of DNA replication timing reveals conserved large-scale chromosomal architecture. PLoS Genet. 6, e1001011 (2010).
Nichols, M. H. & Corces, V. G. Principles of 3D compartmentalization of the human genome. Cell Rep. 35, 109330 (2021).
Mayer, W., Smith, A., Fundele, R. & Haaf, T. Spatial separation of parental genomes in preimplantation mouse embryos. J. Cell Biol. 148, 629–634 (2000).
Borsos, M. et al. Genome-lamina interactions are established de novo in the early mouse embryo. Nature 569, 729–733 (2019).
Marchal, C., Sima, J. & Gilbert, D. M. Control of DNA replication timing in the 3D genome. Nat. Rev. Mol. Cell Biol. 20, 721–737 (2019).
Cimini, D., Cameron, L. A. & Salmon, E. D. Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr. Biol. 14, 2149–2155 (2004).
Worrall, J. T. et al. Non-random Mis-segregation of human chromosomes. Cell Rep. 23, 3366–3380 (2018).
Zhang, C. Z. et al. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184 (2015).
Zheng, H. et al. Resetting epigenetic memory by reprogramming of histone modifications in mammals. Mol. Cell 63, 1066–1079 (2016).
Reichmann, J. et al. Dual-spindle formation in zygotes keeps parental genomes apart in early mammalian embryos. Science 361, 189–193 (2018).
Xu, X., Li, L., Zhang, C. & Meng, L. Observation of two separate bipolar spindles in the human zygote. J. Assist Reprod. Genet. 36, 601–602 (2019).
Wolff, D. J., Miller, A. P., Van Dyke, D. L., Schwartz, S. & Willard, H. F. Molecular definition of breakpoints associated with human Xq isochromosomes: implications for mechanisms of formation. Am. J. Hum. Genet. 58, 154–160 (1996).
Barbouti, A. et al. The breakpoint region of the most common isochromosome, i(17q), in human neoplasia is characterized by a complex genomic architecture with large, palindromic, low-copy repeats. Am. J. Hum. Genet. 74, 1–10 (2004).
Scott, S. A., Cohen, N., Brandt, T., Warburton, P. E. & Edelmann, L. Large inverted repeats within Xp11.2 are present at the breakpoints of isodicentric X chromosomes in Turner syndrome. Hum. Mol. Genet. 19, 3383–3393 (2010).
Costa, G., Barra, V., Lentini, L., Cilluffo, D. & Di Leonardo, A. DNA demethylation caused by 5-Aza-2’-deoxycytidine induces mitotic alterations and aneuploidy. Oncotarget 7, 3726–3739 (2016).
Martinez AC & van Wely, K. H. Centromere fission, not telomere erosion, triggers chromosomal instability in human carcinomas. Carcinogenesis 32, 796–803 (2011).
Gravholt, C. H., Friedrich, U., Caprani, M. & Jorgensen, A. L. Breakpoints in Robertsonian translocations are localized to satellite III DNA by fluorescence in situ hybridization. Genomics 14, 924–930 (1992).
Girardi, G., Lingo, J. J., Fleming, S. D. & Regal, J. F. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front. Immunol. 11, 1681 (2020).
Escriba, M. J., Vendrell, X. & Peinado, V. Segmental aneuploidy in human blastocysts: a qualitative and quantitative overview. Reprod. Biol. Endocrinol. 17, 76 (2019).
Babariya, D., Fragouli, E., Alfarawati, S., Spath, K. & Wells, D. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. Hum. Reprod. 32, 2549–2560 (2017).
Hayashi, K. & Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 8, 1513–1524 (2013).
Miura, H. et al. Mapping replication timing domains genome wide in single mammalian cells with single-cell DNA replication sequencing. Nat. Protoc. 15, 4058–4100 (2020).
Nakagata N. Transgenic Mouse Methods and Protocols (eds Hofker M. H., van Deursen J.) (Humana Press, 2011).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Nilsen, G. et al. Copynumber: Efficient algorithms for single- and multi-track copy number segmentation. BMC Genomics 13, 591 (2012).
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 17, 1 (2011).
Krueger, F. & Andrews, S. R. SNPsplit: allele-specific splitting of alignments between genomes with known SNP genotypes. F1000Res 5, 1479 (2016).
Keane, T. M. et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294 (2011).
Doran, A. G. et al. Deep genome sequencing and variation analysis of 13 inbred mouse strains defines candidate phenotypic alleles, private variation and homozygous truncating mutations. Genome Biol. 17, 167 (2016).
Lilue, J. et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat. Genet. 50, 1574–1583 (2018).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Lassmann, T. SAMStat 2: quality control for next generation sequencing data. Bioinformatics 39, btad019 (2023).
Lassmann, T., Hayashizaki, Y. & Daub, C. O. SAMStat: monitoring biases in next generation sequencing data. Bioinformatics 27, 130–131 (2011).
Bakker, B. et al. Single-cell sequencing reveals karyotype heterogeneity in murine and human malignancies. Genome Biol. 17, 115 (2016).
R Core Team. R: A Language And Environment For Statistical Computing (R Foundation for Statistical Computing, 2021).
Lerdrup, M., Johansen, J. V., Agrawal-Singh, S. & Hansen, K. An interactive environment for agile analysis and visualization of ChIP-sequencing data. Nat. Struct. Mol. Biol. 23, 349–357 (2016).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Karolchik, D. et al. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 32, D493–D496 (2004).
Wingett, S. W. & Andrews, S. FastQ Screen: a tool for multi-genome mapping and quality control. F1000Res 7, 1338 (2018).
Ewels, P., Magnusson, M., Lundin, S. & Kaller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Sankar A., Johansen, J. V. & Borup, R. Single oocyte/embryo RNASeq data processing. Protocolexchange https://doi.org/10.21203/rs.2.21804/v2 (2020).
Felix Krueger F. J., P. Ewels, E. Afyounian, & B. Schuster-Boeckler. Trimgalore, GitHub repository. https://github.com/FelixKrueger/TrimGalore (2021).
Li, H. et al. The Sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Acknowledgements
We thank the members of the Hoffmann lab and Center for Chromosome Stability, as well as Mika Zagrobelny Larsen, for support and critical suggestions throughout this work. We thank Ricardo Alonso Laguna Barraza, Rodrigo Dos A Garcia, and Ioana Maria Zah of the Core Facility of Transgenic Mice, The University of Copenhagen, for their technical support. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen. We would like to express our gratitude to David Gilbert and Vishnu Dileep for their valuable guidance on single-cell Repli-Seq methodology. Additionally, we appreciate the insightful discussions and assistance provided by Ichiro Hiratani and Hisashi Miura during the initial phase of establishing the single-cell Repli-Seq methodology. We express our gratitude to Zhenhai Du, Mate Borsos, and Wei Xie for generously sharing the PC1 scores derived from previously published HiC data of mouse embryos. This work was supported by the Danish National Research Foundation DNRF115 (J.A.H, E.R.H., and M.L), the Lundbeck Foundation, R347-2020-2177 (J.A.H.), the Novo Nordisk Foundation, NNF22OC0080710 (M.L.), NNF22OC0074308 (E.R.H.), NNF15COC0016662 (E.R.H.), the Norwegian Research Council’s Center of Excellence Program (M.L. and J.A.D.), the ERC, 724718-ReCAP (E.R.H.), and the Independent Research Council Denmark, DFF-FSS 0134-00299B (E.R.H.).
Author information
Authors and Affiliations
Contributions
J.A.H., E.R.H., and M.L. conceived the study and designed experiments. J.A.H. and J.M.G. planned and handled mouse work, IVF, and embryo collection. Embryo culture, labeling, imaging, and experiments were carried out by J.A.H. Library preparation was conducted by J.A.H. Data analysis and processing was conducted by J.A.H., A.H., and M.L.; J.A.H., J.A.D, E.R.H., and M.L. discussed data and provided critical input. J.A.D., E.R.H., and M.L. prepared the manuscript. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Halliwell, J.A., Martin-Gonzalez, J., Hashim, A. et al. Sex-specific DNA-replication in the early mammalian embryo. Nat Commun 15, 6323 (2024). https://doi.org/10.1038/s41467-024-50727-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50727-w
- Springer Nature Limited