Abstract
Fucosterols have been widely studied for their antioxidant, anticancer, and anti-inflammatory properties. However, they have not yet been studied in the field of dentistry. This study aimed to determine whether pretreatment of dentin with fucosterol before resin restoration enhances bond stability in resin-dentin hybrid layers. After applying 0.1, 0.5, and 1.0 wt% fucosterol to demineralized dentin, microtensile bond strength (MTBS) and nanoleakage tests were performed before and after collagenase aging, and the surface was observed using scanning electron microscope (SEM). The fucosterol-treated group showed better bond strength and less nanoleakage both before and after collagenase aging, and the corresponding structures were confirmed using SEM. MMP zymography confirmed that the activity of MMPs was relatively low along the concentration gradient of fucosterol, and the FTIR analysis confirmed the production of collagen crosslinks. In addition, fucosterol exhibits cytotoxicity against Streptococcus mutans, the main cause of dental decay. The results of this study suggest that fucosterol pretreatment improves bond strength and reduces nanoleakage at the resin-dentin interface, possibly through a mechanism involving collagen cross-link formation via the inhibition of endogenous and exogenous MMP activity. This study demonstrates the potential of fucosterol as an MMP inhibitor in dentin, which contributes to long-term resin-dentin bond stability and can be used as a restorative material.
Similar content being viewed by others
Introduction
Resin restorations have been studied and widely used for decades since they were first developed in the 1950s; however, they continue to have limitations that have not been resolved. In particular, resin restorations have a relatively short lifespan compared to other restoration types, such as amalgam or gold, due to a higher risk factor for secondary caries due to nanoleakage1,2. While amalgam and gold are maintained mechanically in the cavity, resin-dentin develops collagen-mediated biomechanical bonds3. Resin has the advantage of being applicable in a short time, but it also has the disadvantage of being vulnerable to in vivo influences, and the skill of the operator affects the lifespan of the restoration4. The key to biomechanical bonding of resin restorations is the hybrid layer between the resin and dentin5.
When dentin is acid-eroded, the inorganic material is removed, and the uncovered organic layer of collagen is infiltrated by resin monomers; this layer of collagen and resin is called the hybrid layer. The tightness of the hybrid layer plays a pivotal role in the resin-dentin bond, with collagen, the primary organic component of dentin, being central to this crucial process6.
However, collagen in the hybrid layer is slowly degraded over time by exogenous collagenases from oral bacteria or endogenous matrix metalloproteinases (MMP) from dentin, which enables the bacteria to penetrate the gaps. Nanoleakage eventually leads to discoloration and secondary caries, which are major drawbacks of resin restorations7,8.
Therefore, much research has been conducted to keep the hybrid layer robust. This could be achieved by treatment of dentin with endogenous MMP inhibitors, endogenous and exogenous MMP inhibition through the ethanol wet bonding technique, induction of bond stability through remineralization within the hybrid layer, and treatment with various collagen crosslinkers9,10,11,12.
However, despite numerous efforts, the fragility of the hybrid layer is still a problem that has not been addressed in resin restorations. Furthermore, the applicability of these substances to the human body is uncertain. Therefore, while searching for a natural product-derived material that could be applied to the human body, the effects of fucosterol, which has been studied as one of the candidates for disease treatment, were noted for its antibacterial activity and role as an MMP inhibitor13,14,15.
Fucosterol, a phytosterol and plant sterol, was first identified by chromatography of pea leaves in the 1960s in a study on sterols in higher plants16,17. Fucosterol is abundantly present in algae, mainly in marine plants such as Ecklonia cava and Ecklonia stolonifera, and has been shown to play an antioxidant role by inhibiting various inflammatory mediators in HaCaT cell lines18. In addition, in various cancer cell lines, fucosterol prevents skin aging, ovarian cancer, lung cancer, breast cancer, and leukemia through ER stress or cell cycle regulation, and in a mouse model of liver cancer, it exerted antitumor effects through metabolic inhibition19,20,21,22,23. It has also been studied at the cellular level for a variety of systemic diseases, including diabetes, bacterial infections, fungal infections, hyperlipidemia, depression, and dementia prevention13,24,25,26,27,28.
Therefore, fucosterol has great potential as a target for the development of novel therapeutic agents. However, the role of fucosterol in dentistry has not yet been investigated, and further research is needed. In this study, to investigate the role of fucosterol as an endogenous and exogenous (antibacterial) MMP inhibitor at the resin-dentin interface, dentin was pretreated with fucosterol before resin restoration, the microtensile bond strength (MTBS) of the resin-dentin hybrid layer was measured and observed using SEM observation after nanoleakage tests, its ability to inhibit endogenous MMPs was determined using MMP zymography, and the production of collagen cross-links was determined using Fourier transform infrared spectroscopy (FTIR) analysis. The cytotoxicity of fucosterol on human dental pulp stem cells (HDPSCs) was also measured.
The null hypotheses of this study were as follows: (1) the MTBS measurements and nanoleakage of the resin-dentin hybrid layer before and after collagenase aging would not differ from that of the control; (2) the antibacterial activity of fucosterol would not differ from that of the control; and (3) the inhibitory activity of fucosterol against dentinal endogenous MMPs would not differ from that of the control.
Results
Analysis of microtensile bond strength (MTBS) on collagenase aging
The MTBS results before and after collagenase aging are presented in Table 1. Comparing the MTBS values we observed, the mean values of the control and FUCO 0.1 wt% groups decreased after collagenase aging (p < 0.05). Within all groups before and after collagenase aging, the MTBS was significantly higher in the FUCO group than in the CON group (p < 0.05). In the collagenase-aged group, the MTBS value increased in proportion to the amount of fucosterol (p < 0.05).
Analysis of fracture surfaces after MTBS using scanning electron microscope (SEM)
The dentin-side fracture surfaces of the specimens in the experimental groups after the MTBS test, both before and after collagenase aging, were observed using SEM (Figs. 1 and 2). In the SEM image of the before collagenase aging group (Fig. 1), the collagen network was covered by the composite resin, making it difficult to observe it. In contrast, the after collagenase aging group (Fig. 2) appeared generally dry and rough, with the composite resin layer detached from several places, revealing a more pronounced collagen network.
Scanning electron microscope (SEM) images of the fracture surface after the MTBS test before collagenase aging. The first column (panels A, E, and I) is the ethanol-treated dentin specimen (control), the second (panels B, F, and J), third (panels C, G, and K), and fourth (panels D, H, and L) columns are fucosterol-treated dentin specimens (FUCO 0.1 wt%, FUCO 0.5 wt%, and FUCO 1.0 wt% respectively). The white arrows indicate resin tags. Each row (BFJ, CGK and DHL) represents a different magnification (1000 ×; 2000 ×; and 5000 ×, respectively). The scale bar in each row represents 50, 20, and 10 μm, respectively. The same results are displayed at different magnifications in each column.
Scanning electron microscope (SEM) images of the fracture surface after MTBS test after collagenase aging. The first column (panels A, E, and I) is the ethanol-treated dentin specimen (control), the second (panels B, F, and J), third (panels C, G, and K), and fourth (panels D, H, and L) columns are fucosterol-treated dentin specimens (FUCO 0.1 wt%, FUCO 0.5 wt%, and FUCO 1.0 wt% respectively). The white arrows indicate resin tags. Each row (BFJ, CGK and DHL) represents a different magnification (1000 ×; 2000 ×; and 5000 ×, respectively). The scale bar in each row represents 50, 20, and 10 μm, respectively. The same results are displayed at different magnifications in each column.
In the CON group, an adhesive structure was observed throughout, whereas in the FUCO group, dentin, collagen network, and composite resin were intertwined. As the concentration of fucosterol increased, the thickness of the resin layer and the number of resin tags increased. Multiple resin tags were observed at FUCO 0.5 wt% and 1.0 wt%, with the highest number at FUCO 1.0 wt%.
Nanoleakage of the hybrid layer on collagenase aging
To investigate the nanoleakage patterns in the hybrid layer before and after collagenase aging in the CON and FUCO groups, the hybrid layer was observed using SEM after silver staining (Figs. 3 and 4). The highest amount of nanoleakage was observed in the CON group both before and after collagenase aging, and nanoleakage decreased with FUCO 0.1 and 0.5 wt%. However, there was no significant difference between the nanoleakage in the FUCO 0.5 wt% and FUCO 1.0 wt% groups.
Scanning electron microscope (SEM) images of the nanoleakage evaluation before collagenase aging. The first column (panels A and E) is the ethanol-treated hybrid layer (control), the second (panels B and F), third (panels C and G), and fourth (panels D and H) columns are fucosterol-treated hybrid layers (FUCO 0.1 wt%, FUCO 0.5 wt%, and FUCO 1.0 wt% respectively). Each row represents a different magnification (3000 ×; 8000 ×, respectively). The scale bar in each row represents 10, and 5 μm, respectively. The same results are shown at different magnifications (see each column). Capital letters C, H, and D represent composite resin, hybrid layer, and dentin, respectively.
Scanning electron microscope (SEM) images of the nanoleakage evaluation after collagenase aging. The first column (panels A and E) is the ethanol-treated hybrid layer (control), the second (panels B and F), third (panels C and G), and fourth (panels D and H) columns are fucosterol-treated hybrid layers (FUCO 0.1 wt%, FUCO 0.5 wt%, and FUCO 1.0 wt% respectively). Each row represents a different magnification (3000 ×; 8000 ×, respectively). The scale bar in each row represents 10, and 5 μm, respectively. The same results are shown at different magnifications (see each column). Capital letters C, H, and D represent composite resin, hybrid layer, and dentin, respectively.
Cytotoxicity of fucosterol in HDPSCs
The cytotoxicity of fucosterol on human dental pulp stem cells (HDPSCs) after 24 h of treatment was evaluated using the MTT assay (Fig. 5). The graph shows the dose-dependent cytotoxic effects of fucosterol on HDPSCs. The viability of HDPSCs was 80% for the medium containing up to 100 μg/mL fucosterol, and the viability decreased to 50% between 100 and 200 μg/mL concentrations (p < 0.001). The MTT assay results showed that 200–400 μg/mL fucosterol killed most HDPSCs (p < 0.001).
Viability of human dental pulp stem cells (HDPSCs) exposed to different concentrations of fucosterol for 24 h. The results are expressed as relative cell viability (%) compared with the control group (1% DMSO-containing medium). Each value represents the mean of the results, and the error bars represent the standard deviation of the mean. The results of MTT show a significant decrease in cell viability following treatment with 100, 200, and 400 μg/mL fucosterol compared to CON, 12.5, 25, and 50 μg/mL (p < 0.001).
Antibacterial evaluation of fucosterol using live/dead staining and MTT assays
To investigate the antibacterial effect of fucosterol, Streptococcus mutans was cultured on dentin discs treated with different concentrations of fucosterol, and a live/dead assay was performed (Fig. 6A). In the CON group, green fluorescence was dominant, indicating live bacteria, whereas the FUCO group showed red fluorescence, indicating that most of the bacteria were dead. The MTT assay results confirmed the antibacterial effect observed in the confocal images and allowed quantification of the effect (Fig. 6B).
(A) Confocal images of bacterial live/dead assay according to the fucosterol concentration. Streptococcus mutans as observed under a confocal microscope at 488 nm (green) and 568 nm (red) wavelengths (All images have the same magnification, and each scale bar represents 10 µm). (B) Viability of Streptococcus mutans exposed to different concentrations of fucosterol for 24 h. Each value represents the mean of the results, and the error bars represent the standard deviation of the mean. MTT results show a significant decrease in the survival of bacteria exposed to FUCO 0.1, 0.5, and 1.0 wt% compared to CON (p < 0.001). *** indicates significance at p < 0.001, and ** indicates significance at p < 0.05.
Evaluation of collagen cross-links formation using Fourier-transform infrared spectroscopy (FTIR) analysis
To determine whether treatment of dentin specimens with fucosterol resulted in the formation of collagen cross-links in dentin, the control (ethanol) and fucosterol-treated specimens (0.1, 0.5, and 1.0 wt%) were analyzed using FTIR. The FTIR spectra of the control (E) and FUCO (F) samples are shown in Fig. 7. To observe the amide-bound portion, the region was enlarged and presented in the 2000–400/cm range. Notably, the peaks indicating the dentin collagen network, such as amide I (1635/cm), II (1541/cm), III (1234/cm), and CH2 bending (1450/cm), were at their highest level at FUCO 1.0 wt%. Similarly, the C–OH (1080/cm), P–O stretching bands (1029/cm), and HPO42− (530–534, 550/cm) peaks, which can be regarded as collagen-related peaks, also appeared stronger with increasing fucosterol concentrations.
FTIR spectra of dentin surface samples treated with control (Ethanol), 0.1, 0.5, and 1.0 wt% fucosterol. The amide I (1635/cm), II (1541/cm), III (1234/cm), and CH2 bending (1450/cm) peaks represent the dentinal collagen network at FUCO 1.0 wt%. Similarly, the PO4 (1080 and 557/cm) peaks can be regarded as collagen-related peaks. (E, CON; F, FUCO).
Evaluation of MMP activity in dentinal tubules using in situ zymography
To determine whether fucosterol inhibits MMPs, MMP zymography was performed using confocal laser scanning microscopy (Fig. 8). The first column shows an image of the resin-dentin hybrid layer obtained using light microscopy. The second column shows the fluorescence signals and the third column shows the combined results of their merging. In the control specimen, a high green fluorescence intensity was observed in some hybrid layers and most dentinal tubules. A significant decrease in green fluorescence intensity was observed starting at 0.1 wt% FUCO, and a corresponding decrease in the green fluorescence intensity was observed with increasing fucosterol concentration.
Discussion
Concerns about tooth fracture and mercury have led to a decrease in the use of amalgam and an increase in the use of composite resins, which are more aesthetically pleasing and have increased durability due to breakthrough material developments1,2,3. Unfortunately, composite resin has unresolved issues related to its long-term stability, including secondary caries and discoloration due to polymerization shrinkage, nanoleakage, and restorative failure. To overcome these problems, numerous studies have been conducted on the hybrid layers where the hydrophilic dentin and hydrophobic resin interact and bond, which is crucial to achieving long-term stability9,10,11,12. Collagen is the most important component of the hybrid layer and the main organic component of dentin. Collagen shrinkage or degradation weakens the resin-dentin bond, and the main factors involved in this degradation are exogenous bacterial MMPs (collagenase) and endogenous dentinal MMPs (collagenase and gelatinase)7,29,30,31. Therefore, controlling endogenous and exogenous dentinal MMPs is an important strategy for the preservation and long-term stabilization of the collagen network in the hybrid layer, which was the focus of this study and the rationale behind the choice of fucosterol as the study target. MMPs are mainly present in dentin, including MMP-1, which is involved in the degradation of type I collagen, the main component of dentin organic matter, and MMP-2,8,931,32.
Fucosterol has been investigated as a therapeutic agent for the treatments18,19,21,22,33,34,35,36. Most of the studies on fucosterol have focused on the pro-inflammatory cytokine molecular pathways in the inflammatory response. In HaCaT keratinocytes, fucosterol inhibited reactive oxygen species (ROS) that activate pro-inflammatory cytokines, and in RAW 264.7 macrophages, it inhibited cyclooxygenase (COX), interleukin-6 (IL-6), TNF-α, NF-κB, and MAPK18,37. In mouse models of liver and lung injury, fucosterol treatment inhibited IL-6, TNF-α, NF-κB, and MAPK-related pathways, consistent with previous findings38,39. Cell signaling molecules or external factors such as inflammatory responses and cell stress activate the MAPK, phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), and NF-κB pathways, which are pathways that control survival and cell proliferation40. When these pathways are activated, gene expression, apoptosis, and proliferation are regulated at the molecular and cellular levels. During this process, proteases involved in cellular mitosis and apoptosis are activated, of which MMPs are one41. Fucosterol prevents the degradation of collagen, presumably by inhibiting the activity of MAPK, PI3K/Akt, and NF-κB in the upstream steps of the pathway.
The role of cholesterol in aging is also worth noting. Although the role of cholesterol in MMP expression in human skin is not fully understood, several studies have suggested that cholesterol upregulates MMPs by modulating ERK and JNK activity. Cholesterol reduces the expression of MMP-9 by preventing extracellular ERK and JNK activation in HaCaT cells and regulates MMP-1 expression through modulation of ERK and JNK activity in human dermal fibroblasts14,42. Furthermore, cholesterol depletion activates MMP-2 through the MAPK/ERK kinase (MEK) and JNK pathways in human dermal fibroblasts15,42. The structure of fucosterol, a phytosterol, is surprisingly identical to that of cholesterol, except for the ethylidene group. The similarity in their molecular structures suggests that they may have antagonistic, synergistic, or different functions. Fucosterol lowers cholesterol by competing with cholesterol absorption, and acts as an antioxidant by scavenging free radicals through its unique ethylidene group43,44. In a study on the antidiabetic effects of fucosterol, Jung et al.45 found that the hydrophobic ring system and aliphatic side chain of fucosterol are tightly bound in the specificity pocket via nonpolar amino acid residues on aldose reductase, whereas the anion-binding site on aldose reductase interacts with 3-hydroxyl groups and double bonds. In other words, the amphipathic nature of fucosterol may indicate a higher affinity and binding capacity for the active site of the enzymes in vivo; therefore, it is quite possible that fucosterol binds to the cytokine receptor involved in NF-κB-MAPK signaling and inhibits the activation of the downstream pathway. In fact, systems pharmacology and in silico analyses of fucosterol have further confirmed that fucosterol shows significant binding affinity for liver X-receptor-β (LXR-), glucocorticoid receptor (GR), tropomycin receptor kinase B (TrkB), toll-like receptor 2/4 (TLR2/4), β-secretase 1 (BACE1), and others46. Both tropomycin receptor kinase B (TrkB) and toll-like receptor 2/4 (TLR2/4) cell membrane receptors that trigger the activation of MAPKs and NF-κB in a cascade.
Based on the previous studies on fucosterol and the results in Figs. 5 and 8, we speculate that molecular pathways involving MAPKs and NF-κB might play a role in the downregulation of MMPs following fucosterol treatment in teeth or HDPSCs. However, MMP activity was observed in the dentinal tubules of real teeth using in situ zymography; therefore, we cannot completely rule out the possibility that the environment of the dentinal tubules and odontoblasts may differ from that of HDPSCs. In addition, stem cell-specific signaling may affect other pathways (Fig. 9).
Schematic representation of fucosterol-MMP inhibition mechanism based on the results. Stabilization of resin-tags by collagen in the mixed resin-dentin layer is key to the stability of resin restorations. Fucosterol not only has antimicrobial activity against S. mutans, including exogenous MMPs, but also reduces endogenous MMP-9 in the dentin layer. This event prevents the degradation of collagen, allowing the resin-dentin hybrid layer to remain stable.
Stabilization of resin-tags by collagen in the mixed resin-dentin layer is key to the stability of resin restorations. Fucosterol not only has antimicrobial activity against S. mutans, including exogenous MMPs, but also reduces endogenous MMP-9 in the dentin layer. This event prevents the degradation of collagen, allowing the resin-dentin hybrid layer to remain stable.
In this study, we confirmed the potential of fucosterol as an MMP inhibitor in dentin that contributes to long-term resin-dentin bond stability and can be used in dental restoration approaches. However, further investigation is essential to elucidate the mechanisms underlying the relationship between the inhibition of intracellular MMP-9 expression and signaling molecules by fucosterol. In addition, the cytotoxicity data against HDPSCs revealed that cytotoxicity was observed at lower concentrations than those used on the teeth. Presumably, for fucosterol dissolved in ethanol, rather than in water, to affect dental pulp stem cells in a dentin environment, higher concentrations are required than those applied directly to cells in the laboratory. In other words, this discrepancy is probably due to differences in the environment in which fucosterol dissolved in ethanol/DMSO solvent was applied directly to HDPSCs. Furthermore, based on the hypothesis that fucosterol would move into the cells through the dentinal tubules and affect the expression of MMP-9, one would expect that it would be more favorable to apply fucosterol in the total-etch technique. Without the washing process, the fucosterol may not be sufficiently penetrated by the smear layer. However, applying fucosterol to the 2-STEP bonding system is not in line with our original intention, which was to contribute to the development of primers containing fucosterol; however, whether it applies to the 2-STEP bonding system is something that needs further study by controlling the concentration of fucosterol. Despite these limitations, this study is the first to apply fucosterol, which has been investigated as a therapeutic candidate for several diseases, in a dentin environment and analyzed its effects. Surprisingly, the fucosterol-pretreated group outperformed the control group in all analyses, and these findings may provide new insights into achieving long-term stability of resin restorations, a long-lasting problem.
Conclusions
The null hypothesis that the MTBS measurements and nanoleakage levels in the resin-dentin interfaces before and after collagenase aging would not differ between the control and fucosterol groups was rejected. The fucosterol-treated group showed better bond strength and less nanoleakage than the control group both before and after collagenase aging. In addition, the antibacterial effect of fucosterol against S. mutans was validated, and its ability to inhibit endogenous dentinal MMP activity was confirmed. Consequently, this study confirmed the potential of fucosterol as an MMP inhibitor in dentin that contributes to long-term resin-dentin bond stability and can be used in dental restoration approaches.
Materials and methods
Preparation of fucosterol solution
Experimental solutions of 0.1, 0.5, and 1.0 wt% fucosterol (Sigma Aldrich, St. Louis, MO, USA) in pure ethanol were used in this experiment.
Bonding procedures
The in vitro procedures were carried out using extracted human 40-caries-free molar teeth after obtaining ethical approval from the Ethical Committee of the Pusan National University Dental Hospital (IRB No: 2022-02-007–002). All samples were disinfected with 0.5% chloramine in distilled water and used immediately for the experiment. A total of 40 human molars were randomly assigned 10 to each of four groups: a control group (CON) and a fucosterol-pretreated group (FUCO 0.1, 0.5, and 1.0%).
To expose the occlusal dentin surface and subsequently apply the restorative resin, half of the crown structure was removed using a low-speed saw (Accutom-100, Struers, Cleveland, OH, USA) before bonding. Each specimen was polished to obtain a uniform smear layer using 600-, 1000-, 2000-, and 4000-grit silicon carbide papers for 10 s under running water.
The occlusal surface of each specimen was etched with 37% phosphoric acid for 15 s and then rinsed with a simultaneous jet of water and air for 10 s. Subsequently, three experimental solutions containing different fucosterol concentrations (0.1, 0.5, and 1.0 wt%) were applied for 1 min to the dentin surfaces of 30 randomly selected samples of FUCO groups. Pure ethanol was applied to ten samples from the CON group. After all dentin surfaces of the specimens were air-dried, All-Bond Universal (Bisco Inc., Schaumburg, IL, USA) was applied and light-cured for 20 s using an LED unit (Valo, Ultradent Products, South Jordan, UT, USA). A 5 mm-thick resin (Filtek Z350 XT, 3 M, Maplewood, MN, USA) composite block was built on the surfaces in five 1-mm-thick increments, with each increment being light-cured for 20 s. Each procedure was performed by a single investigator. The resin-dentin blocks were kept in distilled water at 37 °C for 24 h.
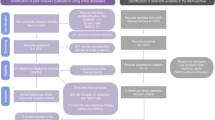
Of the 10 molars per group, (1) 2 molars for in situ Zymography; these 2 molars were sliced into 10 × 10 × 0.5 mm plates, yielding approximately 5 specimens per tooth, i.e. 10 stained specimens per group. (2) The remaining 8 molars were all sawed into 10 × 1.0 × 1.0 mm sticks, yielding approximately 10 specimens per tooth, i.e. 80 specimens per group. 40 of these specimens were subjected to collagenase aging. Thirty of the 40 specimens were then used for microtensile bond strength test and three were silver stained for nanoleakage. The same was done for all four groups. The dimensions of each resin-dentin stick were measured using a digital caliper to compute the microtensile bond strength (MTBS). A schematic image of the experiments after these bonding procedures is shown in Fig. 10.
Schematic representation of experiments after bonding procedures. After disinfecting a freshly extracted tooth, the root area is molded into a resin block. Then, half of the crown is cut out, and polished, and the resin is stacked according to the bonding procedure. Next, the specimens are cut vertically using a low-speed saw and divided into two groups according to whether they are collagenase-aged or not. For in situ zymography, the collagenase aging group was not included.
Microtensile bond strength (MTBS) test
To evaluate the tensile force of each stick, a universal testing machine (Instron, Norwood, MA, USA) was used at a crosshead speed of 0.5 mm/min. Premature failures were not included in the MTBS test results. Half of the collected resin-dentin sticks were measured using the MTBS after 24 h. The other half of the resin-dentin sticks were subjected to collagenase aging for one month before MTBS testing. The MTBS test was also performed on the collagenase-aged resin-dentin samples in the same manner.
Collagenase aging
Collagenase solution was prepared by adding bacterial (Clostridium histolyticum) collagenase I and II (Sigma Aldrich, St. Louis, MO, USA) to artificial saliva to obtain a concentration of 0.1 mg/mL. Half of the resin-dentin sticks were immersed in collagenase-containing artificial saliva and stored for a month in the dark at 37 °C, and the collagenase solution was replaced every 3 days. The collagenase solution was stored at – 20 °C before use.
Nanoleakage assessment
Based on the protocol of Tay et al.47, three resin-dentin sticks from each group were collected and processed with a 50% ammoniacal silver nitrate solution. The sticks were soaked in a silver tracer solution for 24 h in the dark and rinsed with distilled water. After rinsing, the samples were soaked in a photodeveloping solution for 8 h under fluorescent light, coated with epoxy resin, and polished using silicon carbide papers of up to 4000 grit. The test samples were observed under a scanning electron microscope (SEM, S-4700, Hitachi, Tokyo, Japan). Since the purpose of the experiment was to observe the microtensile bond strength and nanoleakage of the specimen after collagenase aging, the resin-dentin stick format was chosen to allow the collagenase aging solution to penetrate evenly from all sides. If the size of the sample was a flat plate, the collagenase aging solution might not be able to penetrate the middle part of the plate that is attached to the bottom during incubation in the solution. However, if the size of the sample to be stained is small, it may be an unfavorable condition for staining because dentin is porous and highly permeable, and silver penetration is also diffusion-dependent48.
Scanning electron microscope (SEM)
For SEM imaging, each resin-dentin beam was initially fixed in Karnovsky’s fixative, post-fixed in 1% osmium tetroxide, and dehydrated in an ascending ethanol series, following a previously described protocol49. All samples were maintained under ambient laboratory conditions for 24 h, followed by scanning electron microscopy (S-4700; Hitachi, Tokyo, Japan).
Human dental pulp stem cells: MTT assay
Human dental pulp stem cells (HDPSCs) were cultured in a modified essential medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum at 37 °C with 5% CO2 in a humidified environment. The HDPSCs in passage 5 were used in this study. HDPSCs were seeded in a 96-well plate at a density of 10,000 cells per well and cultured at 37 °C with 5% CO2 for 24 h. Fucosterol (control, 12.5, 25, 50, 100, 200, and 400 μg/mL) solutions were then added. After 24 h incubation, 10 μL of MTT (Sigma Aldrich, St. Louis, MO, USA) solution (0.5 mg/mL) was added to each well and incubated for 3 h. After incubation at 37 °C in an anaerobic environment, the MTT solution from each well was pipetted out and replaced with 100 μL of dimethyl sulfoxide. After gentle shaking for 5 min, the absorbance of the supernatant was measured at 540 nm using a spectrophotometer (Allsheng, Hangzhou, China). The results are expressed as the relative cell viability (%) compared to the control group (1% DMSO-containing medium) (Supplementary Information).
Bacterial culture and biofilm preparation
Human molars were cut into 1 mm-thick dentin discs using a slow-speed diamond saw, and each specimen was gradually ground using 600-, 1000-, and 2000-grit silicon carbide paper for 10 s under flowing water to obtain a uniform smear layer. Each sample was randomly transferred to a 24-well plate and sterilized with ethylene oxide. Four experimental solutions (control, 0.1, 0.5, and 1.0 wt% fucosterol) were applied to the upper surface of each dentin disc specimen for 60 s using a microbrush. Next, a mixture of 10 μL of Streptococcus mutans cell suspension (1 × 108 CFU/mL) and 1 mL Brain Heart Infusion (BHI) supplemented with 1% sucrose was added to each well. After incubating at 37 °C for 24 h under anaerobic conditions, the biofilm-coated samples were gently immersed twice in 1 mL of sterile phosphate-buffered saline to wash away unattached bacterial cells. The biofilm-coated dentin discs were then transferred to another 24-well plate with the occlusal side facing upward.
Live/dead bacterial assay
Biofilm-coated specimens from each group were stained using a live/dead bacterial viability kit (Thermo Fisher Scientific, Waltham, MA, USA) for 15 min at room temperature. Live and dead bacteria were stained with SYTO-9 and propidium iodide, respectively, according to the manufacturer’s instructions. The excitation wavelengths for SYTO-9 and PI were 488 and 568 nm, respectively, and the specimens were observed using a confocal laser scanning microscope (LSM900, Zeiss, Jena, Germany).
Antibacterial evaluation using MTT assay
Based on the protocol of Li et al.50, biofilm-coated specimens from each group were transferred to a 12-well plate, and 1 mL of MTT (Sigma Aldrich, St. Louis, MO, USA) solution (0.5 mg/mL) was added in each well. After 1 h of incubation at 37 °C in an anaerobic environment, the MTT solution from each well was removed and replaced with 1 mL of dimethyl sulfoxide. After gentle shaking for 10 min, the absorbance of the supernatant was measured at 540 nm using a spectrophotometer.
Fourier-transform infrared spectroscopy (FTIR)
Each dentin disk was incubated with the etching solution (37% phosphoric acid) for 15 s, rinsed with distilled water, and air-dried for 10 s. Then, experimental solutions (control, 0.1, 0.5, 1.0 wt% fucosterol) were applied to each dentin disk for 1 min. The test samples were then examined using a spectrometer (IS50, Thermo Fisher Scientific, Waltham, MA, USA) with a resolution of 4/cm and 32 scans in transmittance mode. The spectral range 4000–400/cm was used to survey peaks and shoulders at 3300/cm (amide A), 3000/cm (amide B), 1635/cm (amide I), 1541/cm (amide II), 1450/cm (CH2 bending), and 1234/cm (amide III) assigned to dentin collagen cross-linking after normalization and baseline correction processes51.
MMP zymography
Based on the protocol described by Li et al.31, a quenched fluorescein-conjugated gelatin mixture (E-12055, Molecular Probes, Eugene, OR, USA) was used for MMP zymography according to the manufacturer’s instructions. Longitudinal sections of resin-dentin samples from each group (CON and FUCOs) were wet-polished using 600, 1000, 2000, and 4000-grit silicon carbide paper. A total of 50 μL quenched fluorescein-conjugated gelatin mixtures were dropped onto the resin-dentin interfaces of each specimen. The specimens were then covered with coverslips, transferred to humidified chambers, and stored at 37 °C for 24 h under light protection. The amount of green fluorescence produced was observed using a confocal laser scanning microscope with excitation at 488 nm and emission at 530 nm.
Statistical analysis
Statistical analysis was performed on all experimental results, and the mean and standard deviation were calculated by 1-way analysis of variance (ANOVA) (SPSS 12.0; SPSS Inc., Chicago, IL), followed by a multiple comparisons test performed using a Tukey honesty, significant difference (HSD) test.
Data availability
All data associated with this study are presented in the paper.
Abbreviations
- MMP:
-
Matrix metalloproteinases
- MTBS:
-
Micro-tensile bond strength
- SEM:
-
Scanning electron microscope
- CLSM:
-
Confocal laser scanning microscopy
- FTIR:
-
Fourier-transform infrared spectroscopy
- CON:
-
Control group
- FUCO:
-
Fucosterol pretreated group
- HDPSCs:
-
Human dental pulp stem cells
- MTT:
-
Methylthiazol tetrazolium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- LC50 :
-
Lethal concentration 50%
- BHI:
-
Brain Heart Infusion
- ROS:
-
Reactive oxygen species
- COX:
-
Cyclooxygenase
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- I-κB:
-
Inhibitor of nuclear factor kappa B
- c-Fos:
-
Cellular oncogene fos
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
C-Jun N-terminal kinase
References
Moraschini, V., Fai, C. K., Alto, R. M. & dos Santos, G. O. Amalgam and resin composite longevity of posterior restorations: A systematic review and meta-analysis. J. Dent. 43, 1043–1050 (2015).
Beyer, C., Schwahnr, C., Meyerr, G. & Söhnelr, A. What German dentists choose for their teeth: A Web-based survey of molar restorations and their longevity. J. Prosthet. Dent. 125, 805–814 (2021).
Bayne, S. C., Ferracane, J. L., Marshall, G. W., Marshall, S. J. & van Noort, R. The evolution of dental materials over the past century: Silver and gold to tooth color and beyond. J. Dent. Res. 98, 257–265 (2019).
Sugimura, R. et al. Surface moisture influence on etch-and-rinse universal adhesive bonding. Am. J. Dent. 32, 33–38 (2019).
Tjäderhane, L. Dentin bonding: Can we make it last?. Oper. Dent. 40, 4–18 (2015).
Wang, T. & Nakabayashi, N. Effect of 2-(methacryloxy)ethyl phenyl hydrogen phosphate on adhesion to dentin. J. Dent. Res. 70, 59–66 (1991).
Hass, V. et al. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 32, 732–741 (2016).
Burke, F. J., Cheung, S. W., Mjör, I. A. & Wilson, N. H. Restoration longevity and analysis of reasons for the placement and replacement of restorations provided by vocational dental practitioners and their trainers in the United Kingdom. Quintessence Int. 30, 234–242 (1999).
Zheng, P. & Chen, H. Evaluate the effect of different mmps inhibitors on adhesive physical properties of dental adhesives, bond strength and mmp substarte activity. Sci. Rep. 7, 4975 (2017).
Sadek, F. T. et al. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 89, 1499–1504 (2010).
Kim, H., Choi, A., Gong, M. K., Park, H. R. & Kim, Y. I. Effect of remineralized collagen on dentin bond strength through calcium phosphate ion clusters or metastable calcium phosphate solution. Nanomater. Basel. 10, 2203 (2020).
De-Paula, D. M. et al. Influence of collagen cross-linkers addition in phosphoric acid on dentin biomodification and bonding of an etch-and-rinse adhesive. Dent. Mater. 36, e1–e8 (2020).
da Silva, F. E. F. et al. Semisynthesis, in silico study and in vitro antibacterial evaluation of fucosterol derivatives. Steroids. 189, 109137 (2023).
Kim, S. et al. Cholesterol inhibits MMP-9 expression in human epidermal keratinocytes and HaCaT cells. FEBS Lett. 581, 3869–3874 (2007).
Kim, S. et al. Induction of tissue inhibitor of matrix metalloproteinase-2 by cholesterol depletion leads to the conversion of proMMP-2 into active MMP-2 in human dermal fibroblasts. Exp. Mol. Med. 42, 38–46 (2010).
Goad, L. J. & Goodwin, T. W. The biosynthesis of sterols in higher plants. Biochem. J. 99, 735–746 (1966).
Chen, Z. et al. Extraction and quantitation of phytosterols from edible brown seaweeds: Optimization, validation, and application. Foods. 12, 244 (2023).
Fernando, I. P. S. et al. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 233, 116714 (2019).
Singla, R. K. et al. The role of nanomaterials in enhancing natural product translational potential and modulating endoplasmic reticulum stress in the treatment of ovarian cancer. Front. Pharmacol. 13, 987088 (2022).
Li, X. et al. Exploration in the mechanism of fucosterol for the treatment of non-small cell lung cancer based on network pharmacology and molecular docking. Sci. Rep. 11, 4901 (2021).
Pacheco, B. S. et al. Cytotoxic activity of fatty acids from antarctic macroalgae on the growth of human breast cancer cells. Front. Bioeng. Biotechnol. 6, 185 (2018).
Ji, Y. B., Ji, C. F. & Yue, L. Study on human promyelocytic leukemia HL-60 cells apoptosis induced by fucosterol. Biomed. Mater. Eng. 24, 845–851 (2014).
Huo, H. & Bao, H. Comparative study on the anti-tumor effect of steroids derived from different organisms in H22 tumor-bearing mice and analysis of their mechanisms. Eur. J. Pharmacol. 15, 176269 (2024).
Jung, H. A., Bhakta, H. K., Min, B. S. & Choi, J. S. Fucosterol activates the insulin signaling pathway in insulin resistant HepG2 cells via inhibiting PTP1B. Arch. Pharm. Res. 39, 1454–1464 (2016).
Tyśkiewicz, K. et al. Antifungal properties of Fucus vesiculosus L. supercritical fluid extract against Fusarium culmorum and Fusarium oxysporum. Molecules. 24, 3518 (2019).
Song, Y., Oh, G. H., Kim, M. B. & Hwang, J. K. Fucosterol inhibits adipogenesis through the activation of AMPK and Wnt/β-catenin signaling pathways. Food. Sci. Biotechnol. 26, 489–494 (2017).
Zhen, X. H. et al. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 768, 131–138 (2015).
Gan, S. Y., Wong, L. Z., Wong, J. W. & Tan, E. L. Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int. J. Biol. Macromol. 121, 207–213 (2019).
Xu, C. & Wang, Y. Cross-linked demineralized dentin maintains its mechanical stability when challenged by bacterial collagenase. J. Biomed. Mater. Res. B Appl. Biomater. 96, 242–248 (2011).
Toledano, M. et al. Effect of bacterial collagenase on resin-dentin bonds degradation. J. Mater. Sci. Mater. Med. 18, 2355–2361 (2007).
de Moraes, I. Q. S. et al. Inhibition of matrix metalloproteinases: A troubleshooting for dentin adhesion. Restor. Dent. Endod. 45, e31 (2020).
Sulkala, M. et al. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral. Biol. 52, 121–127 (2007).
Mao, Z. et al. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cell cycle arrest and targeting of Raf/MEK/ERK signalling pathway. Phytomedicine. 61, 152809 (2019).
Ramos, A. A., Almeida, T., Lima, B. & Rocha, E. Cytotoxic activity of the seaweed compound fucosterol, alone and in combination with 5-fluorouracil, in colon cells using 2D and 3D culturing. J. Toxicol. Environ. Health A 82, 537–549 (2019).
Kim, M. S., Oh, G. H., Kim, M. J. & Hwang, J. K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 89, 911–918 (2013).
Hwang, E. et al. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. (N.Y.) 16, 361–370 (2014).
Jayawardena, T. U. et al. Particulate matter-induced inflammation/oxidative stress in macrophages: Fucosterol from Padina boryana as a potent protector, activated via NF-κB/MAPK pathways and Nrf2/HO-1 involvement. Mar Drugs. 18, 628 (2020).
Mo, W. et al. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 2824139 (2018).
Li, Y. et al. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 195, 515–521 (2015).
Lin, Y., Bai, L., Chen, W. & Xu, S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets 14, 45–55 (2010).
Xie, Y. et al. Nuclear matrix metalloproteinases: Functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discov. 3, 17036 (2017).
Kim, S. et al. Cholesterol, a major component of caveolae, down-regulates matrix metalloproteinase-1 expression through ERK/JNK pathway in cultured human dermal fibroblasts. Ann. Dermatol. 22, 379–388 (2010).
Dominguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals (Elsevier, 2013).
Kırtıl, E., Kılercıoglu, M. & Öztop, H. M. Reference Module in Food Sciences. http://www.sciencedirect.com/science/article/pii/B9780123847300004328 (2016).
Jung, H. A. et al. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem. Biol. Interact. 206, 55–62 (2013).
Hannan, M. A., Dash, R., Sohag, A. A. M. & Moon, I. S. Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Mar. Drugs 17, 1002 (2019).
Tay, F. R., King, N. M., Chan, K. M. & Pashley, D. H. How can nanoleakage occur in self-etching adhesive systems that demineralize and infiltrate simultaneously?. J. Adhes. Dent. 4, 255–269 (2002).
Meerbeek, B. V. The “myth” of nanoleakage. J. Adhes. Dent. 9, 491–492 (2007).
Tay, F. R. & Pashley, D. H. Biomimetic remineralization of resin-bonded acid-etched dentin. J. Dent. Res. 88, 719–724 (2009).
Li, K. et al. Quercetin as a simple but versatile primer in dentin bonding. RSC Adv. 7, 36392–36402 (2017).
Drouet, C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. BioMed. Res. Int. 2013, 490946 (2013).
Acknowledgements
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (2021R1A2C1003240).
Author information
Authors and Affiliations
Contributions
H.K. and Y.I.K.: conceptualization, methodology, investigation, and writing of the original article; J.Y.J., Y.K., M.K.B., K.H.Y., S.Y.Y., H.R.P., and I.R.K.: data curation, validation, and software analysis; Y.I.K.: supervision, project administration. All authors commented on the paper and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committee of Pusan National University Dental Hospital (IRB No: 2022-02-007-002), and all methods were performed by the Declaration of Helsinki guidelines and regulations. Informed consent was obtained from all the participants.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, H., Jung, YJ., Kim, Y. et al. Long-term hybrid stability and matrix metalloproteinase inhibition by fucosterol in resin-dentin bonding biomechanics. Sci Rep 14, 20415 (2024). https://doi.org/10.1038/s41598-024-71715-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71715-6
- Springer Nature Limited