Abstract
Background
Biliary colic (BC) is a frequent hepatobiliary disorder encountered in emergency departments. Acupuncture may be effective as an alternative and complementary medicine for BC. Nonetheless, rigorous trials investigating its efficacy are lacking. Therefore, the aim of this study protocol is to determine whether acupuncture provides immediate relief of pain and associated symptoms in BC patients.
Method
Eighty-six participants who aged from 18 to 60 years with BC will be recruited in the First People's Hospital of Longquanyi District, Chengdu (West China Longquan Hospital Sichuan University). All participants will be allocated into two treatment groups including acupuncture group and sham acupuncture group using a 1:1 ratio. Each group will only receive a single 30-min needle treatment while waiting for their test results after completing the routine examination for BC. The primary outcome of the study is to assess the change in pain intensity after the 30-min acupuncture treatment. The secondary outcomes of the study include the change in pain intensity at various time points, the degree of gastrointestinal symptoms at different time points, the level of anxiety experienced during pain episodes at different time points, the score of Pain Anxiety Symptoms Scale-20 (PASS-20), the score of Fear of Pain Questionnaire-III (FPQ-III), and the score of Pain Catastrophizing Scale (PCS), among others.
Discussion
The results of this research will provide substantial evidence regarding the efficacy of acupuncture in alleviating symptoms associated with BC.
Trial registration
ClinicalTrials.gov, ChiCTR2300070661. Registered on 19 April 2023.
Similar content being viewed by others
Background
Biliary Colic (BC) is a symptom of an acute attack of gallstones. The prevalence of gallstones has been displayed to be 10%-20% patients in worldwide. Furthermore, more than 20% of patients with gallstones will develop BC symptom [1]. It is estimated that complications occur in approximately 1%–3% of patients with symptomatic gallstones annually [2]. BC is characterized by persistent pain in the upper part of the abdomen, back, or right shoulder, as well as nausea and vomiting, which are caused by the contraction of the gallbladder pushing bile across the blockage [3].
The affective component of pain includes feelings of distress, sadness, anxiety, and depression in response to noxious stimuli [4]. Depression and anxiety have been linked to prolonged duration of acute pain [5]. Likewise, BC, being an acute pain, may be clinically accompanied by emotional symptoms such as anxiety and depression. Studies on pain-induced anxiety have demonstrated that acute pain intensity is positively correlated with anxiety status [6, 7]. Literature on pain-induced depression has suggested that acute pain may contribute to depressive symptoms following surgery [8].
An evidence-based clinical practice guideline for the remission of BC has recommended non-steroidal anti-inflammatory drugs (such as diclofenac and indomethacin), spasmolytics (such as butylscopolamine), opioids (such as buprenorphine), and cholecystectomy (within 24 h after the diagnosis of biliary colic) [9,10,11]. However, pharmacological treatments may have side effects, including serious gastrointestinal complications, cardiovascular events, hypersensitivity, itch, nausea, and vomiting, among others [12,13,14,15,16,17]. Additionally, cholecystectomy may be accompanied with post cholecystectomy syndrome [18, 19]. Thus, complementary alternative therapy is considered essential to improve BC symptoms.
Acupuncture, as part of traditional Chinese medicine, potentially relieves the pain-related disorders [20,21,22]. Some clinical studies have proved that acupuncture can alleviate pain-related symptoms of BC [23, 24]. Moreover, our team found that acupuncture for BC is effective through the imaging mechanism of acupuncture at GB34 in the early stage. However, the research has mainly focused on the mechanism of pain, and the sample size was relatively small, despite patients with BC often experiencing emotional problems [25, 26]. Therefore, thus far, it is unclear whether acupuncture has analgesic effects on BC owing to the methodological limitations in control groups, randomization, and blinding, among other factors.
Hence, a randomized, sham-controlled trial will be conducted to assess the potential of acupuncture in improving pain and related symptoms of BC.
Methods/design
Study design
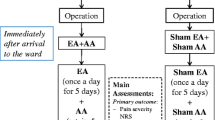
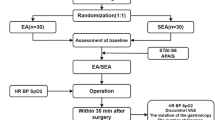
The single center, sham-controlled, blinded, randomized trial of acupuncture treatment for BC will be executed in the First People's Hospital of Longquanyi District of Chengdu (West China Longquan Hospital Sichuan University) from May 2023 to July 2024. The protocol of trial has been approved by the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (No. 2023KL-008). And it has been registered with an identifier (ChiCTR2300070661) at ClinicalTrials.gov (https://www.chictr.org.cn/). The trial will be performed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist [27]. Moreover, informed consent will be obtained from all patients prior to beginning the trial. All BC patients will accept acupuncture treatment while patients are waiting for the test results after completing routine examinations. If patients with BC continue to experience pain after acupuncture treatment, conventional treatment will be administered, and the pain relief time will be recorded. The study flow chart is shown in Fig. 1, and the study time schedule is presented in Table 1.
Participants
Eighty-six patients with BC will be recruited from the emergency department of the First People's Hospital of Longquanyi District of Chengdu (West China Longquan Hospital Sichuan University). All patients will be screened by Specialists and acupuncturists according to the diagnostic criteria from the Recommendations for EASL clinical practice guidelines on the prevention, diagnosis and therapy of gallstones (2016) [9].
Inclusion criteria
Participants who meet the following inclusion criteria will be eligible for the study: (i) those who meet the diagnostic criteria; (ii) mild tenderness in the right upper quadrant, negative or positive Murphy's sign; (iii) a numerical rating scale (NRS) score of ≥ 3 points; (iv) BC patients aged between 18 to 60 years, without any gender restrictions; (v) gallbladder stones demonstrated by radiology or ultrasonography; (vi) no history of acupuncture treatment within the past month; (vii) not participating in any other clinical studies; and (viii) willing to voluntarily sign the informed consent form.
Exclusion criteria
Participants who meet the following exclusion criteria will be excluded from the study: (i) a history of jaundice; (ii) presence of bile duct stones, acute cholecystitis, acute suppurative cholecystitis, gangrenous cholecystitis, incarcerated cholecystitis, gallbladder perforation with diffuse peritonitis, or BC complicated with acute pancreatitis; (iii) administration of analgesics such as antispasmodic, non-steroidal anti-inflammatory drugs, or choleretic before acupuncture; (iv) presence of serious complications or primary diseases involving the brain, cardiovascular, liver, kidney, endocrine, or hematopoietic systems; (v) presence of serious digestive system diseases, such as peptic ulcer, upper gastrointestinal bleeding, gastric tumor, Crohn's disease, irritable bowel syndrome, etc.; and (vi) participation in other clinical trials within the last three months.
Recruitment procedures
BC patients in emergency department of the First People's Hospital of Longquanyi District (West China Longquan Hospital Sichuan University) will be diagnosed by emergency physician. Simultaneously, researchers will be responsible for the screening and presenting the inclusion criterion, the operational and allocated details of intervention (acupuncture group and sham acupuncture group), and the potential risks of this trial. All subjects will have the right to withdraw from the trial at any time. Additionally, all subjects will be required to sign an informed consent form before the trial commences.
Randomization and allocation
Baseline evaluation will be conducted for eligible subjects. The random sequence will be generated using SAS® 9.4 software (SAS Institute Inc., North Carolina, USA). The random sequence will be recorded on sealed and opaque envelopes, with the random numbers of the two groups inside. Moreover, these envelopes will be opened sequentially by a researcher assistant who is not involved in the data collection or acupuncture treatment. In addition, the researcher assistant will randomly assign the subjects to the acupuncture group and the sham acupuncture group with ratio of 1:1.
Blinding
The researcher assistants, outcome assessors, statisticians and subjects will be blinded to the group assignment. All subjects will receive acupuncture treatment lying on the bed in a quiet clinic. The control group will use a non-penetrating sham apparatus, similar in appearance to acupuncture needles, to achieve blinding. Furthermore, all subjects will be asked about their experience after the acupuncture session to assess whether they have been successfully blinded.
Interventions
Acupuncture group
Yanglingquan (GB34) is acupoint of the gallbladder meridian of foot-shaoyang (GB), which is frequently used for Biliary disease [28, 29]. Therefore, right GB34 will be chosen as the acupuncture point to treat BC. Acupuncture treatment will be performed by acupuncturists with more than 3 years of clinical experience. The acupuncturist will disinfect the skin around the right GB34 with a 70% isopropyl alcohol swab when the subjects lie on the treatment bed in a supine position. Disposable sterile acupuncture needles (0.25 mm in diameter and 40 mm in length, Hwatuo, Suzhou, China) will then be inserted in the right GB34 (Fig. 2B). The needle will be lifted and thrust approximately 30–50 mm and twisted and rotated approximately 90° to 180° angle for 60–90 times/min to achieve deqi sensation, including soreness, numbness, heaviness, fullness, and aching. The deqi sensation will be evaluated using the Chinese version of the modified Massachusetts General Hospital Acupuncture Sensation Scale (C-MASS). All subjects will receive one 30-min acupuncture treatment during their waiting time after routine examination, and the manipulations will be executed two times, once every 10 min, for 60 s each time.
Sham acupuncture group
The right non-acupuncture point located at the lateral edge of the tibia, approximately 1–2 cm away from Zusanli (ST36), will be selected as the sham point [30]. A non-penetrating sham apparatus with a blunt tip, specifically the Park sham device, will be used to lightly touch the skin at the sham point [31] (Fig. 2A). The procedures and manipulations in the non-acupuncture group will be identical to those in the acupuncture group. Subjects will receive treatment alone in a single room to prevent any interference from family members or other individuals.
Outcome measures
Primary outcome measures
The primary endpoint is the change of pain intensity from baseline to 30 min after acupuncture by evaluating the NRS. The NRS is a 10-point scale, with zero representing "no pain" and 10 representing "worst possible pain". The pain intensity of BC subjects will be presented by marking a “√” on the line of NRS.
Secondary outcome measures
The secondary end points will be evaluated as following:
-
i)
The pain intensity will be assessed the change of NRS score from baseline to the 10 min, 20 min and 40 min after needle manipulation.
-
ii)
The degree of gastrointestinal symptoms, such as nausea, vomiting and pantothenic acid, will be evaluated for change from baseline by the visual analogue scale (VAS) at 10 min, 20 min, 30 min and 40 min after needle manipulation [32].
-
iii)
The level of anxiety during episodes of pain will be measured by changes in the VAS at 10 min, 20 min and 30 min after needle manipulation, compared to baseline [33]. The VAS will be used to assess the anxiety level of patients with BC during pain attacks.
-
iv)
The pain-related emotional responses, such as anxiety and fear, will be assessed using the Pain Anxiety Symptoms Scale-20 (PASS-20), the Fear of Pain Questionnaire-III (FPQ-III), and the Pain Catastrophizing Scale (PCS) at baseline [34,35,36,37]. The PASS-20 will be used to evaluate whether patients with biliary colic experience anxiety during the non-pain periods.
-
v)
The patients' anticipated responses to acupuncture will be evaluated using the Acupuncture Expectancy Scale (AES) at baseline [38].
-
vi)
The patient’s satisfaction for acupuncture will be monitored by the 5-point Likert scale at 30 min after needle manipulation [39].
-
vii)
The evaluation of needle sensation will be conducted using the Chinese version of the modified Massachusetts General Hospital Acupuncture Sensation Scale (C-MASS) at 10 min, 20 min and 30 min after needle manipulation [40].
-
viii)
The use of rescue analgesia will be recorded 10 min after the completion of acupuncture. The recurrence rate of BC and any adverse events occurring within 72 h after needle manipulation will be obtained through a telephone follow-up.
Safety assessment
The information of adverse events (AEs) related to interventions including infection, bloody swelling, stabbing pain, broken needle, hemorrhage, palpitation, syncope, headache will be monitored and recorded in the case report forms (CRF) by a research assistant. Serious AEs will be handled by emergency medicine physicians or acupuncturists. Moreover, serious AEs will be reported to the Ethics Committee of Hospital of Chengdu University of Traditional Chinese medicine.
Data collection and quality control
The data will be collected alone on CRF by the same evaluator and will be imported into the computer respectively by two research assistants who are not involved in the trial to avoid bias in the results. All personnel involved in the study will be required to complete professional training to ensure the quality of the trial. This training will cover details of the trial, including the introduction of inclusion and exclusion criteria, completion of CRF, acupuncture procedures, and data entry methods.
Sample size calculation
The primary outcome of this study is the mean difference in NRS scores for BC between the baseline and the endpoint. Sample size calculations will be based on a previous neuroimaging study of acupuncture in BC [41], which reported mean differences in NRS scores of -1.74 ± 1.543 for the acupuncture group and -0.66 ± 0.986 for the sham acupuncture group. Accordingly, a total of 76 patients will be calculated using a 2-sided test with a 5% significance level and 90% power. To account for a 10% dropout rate, this trial will ultimately enroll 86 patients.
Statistical analysis
The independent samples t-test or Wilcoxon rank-sum test will be used to analyze continuous variables in the demographic characteristics of the two groups, based on their normality. Categorical variables in the demographic characteristics will be analyzed using chi-squared or Fisher's exact tests. If there is a significant difference in demographic characteristics such as age, gender, and family history, they will be included as covariates in the efficacy analysis. The analysis will include all randomized BC patients based on the intention-to-treat (ITT) principle. Subgroup and sensitivity analyses may also be performed.
The change of pain intensity from baseline to 30 min after acupuncture will be analyzed by using analysis of covariance with acupuncture group and sham acupuncture treatment as factors and baseline pain intensity as a covariate. Repeated measures ANOVA will be used to assess the difference in the secondary outcomes at different time points. The degree of patients’ expectations and satisfaction with acupuncture will be evaluated using linear regression.
Discussion
BC is one of the 64 diseases that are suitable for acupuncture treatment, as recommended by the World Health Organization [42]. The studies of acupuncture treatment for BC have reported that acupuncture has play therapeutic effect on BC [43,44,45,46]. However, the quality of evidence supporting the use of acupuncture for BC is limited due to methodological limitations. Furthermore, some studies have shown that sham acupuncture, such as needle insertion into wrong or non-points, or superficial needling at non-points, may produce efficacious results [47, 48]. Therefore, it is necessary to use non-penetrating needling as the control group to produce a smaller, nonspecific effect [49].
Several clinical studies have proposed that acupuncture on GB34 could significantly improve the symptoms of patients with BC [50, 51]. Furthermore, GB34 is located on the lower limbs of the patient, making it difficult for them to observe the treatment procedure and needle site while in a supine position. Therefore, the selection of acupoints is likely to increase the likelihood of patient-blinding.
In this trial, there are several limitations that need to be acknowledged. Firstly, blinding of acupuncturists is not possible due to the unique nature of acupuncture procedures. Secondly, the small sample size may lead to inconclusive results. Finally, there is a potential for a relatively high dropout rate in the sham acupuncture group.
To summarize, this trial aims to investigate the efficacy and safety of acupuncture in relieving pain and pain-related symptoms in patients with BC by controlling the quality of acupuncturist’s operation and implementing assignment blinding. The results of this trial are expected to provide an alternative treatment option for patients with BC and contribute to the development of guideline recommendations.
Trial status
The trial is currently recruiting participants. The first subject has not been recruited so far and the investigators are still collecting.
Availability of data and materials
Not applicable.
Abbreviations
- BC:
-
Biliary colic
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- GB34:
-
Yanglingquan
- GB:
-
Gallbladder meridian
- C-MASS:
-
Chinese version of the modified Massachusetts General Hospital Acupuncture Sensation Scale
- ST36:
-
Zusanli
- NRS:
-
Numerical rating scale
- VAS:
-
Visual analogue scale
- PASS-20:
-
Pain Anxiety Symptoms Scale-20
- FPQ-III:
-
Fear of Pain Questionnaire-III
- PCS:
-
Pain Catastrophizing Scale
- AES:
-
Acupuncture Expectancy Scale
- AEs:
-
Adverse events
- CRF:
-
Case report forms
- ITT:
-
Intention-to-treat
References
Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers. 2016;2:16024.
Gutt C, Schläfer S, Lammert F. The treatment of gallstone disease. Dtsch Arztebl Int. 2020;117(9):148–58.
Baiu I, Hawn MT. Gallstones and biliary colic. JAMA. 2018;320(15):1612.
Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691.
Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–44.
Kapoor S, White J, Thorn BE, Block P. Patients Presenting to the Emergency Department with acute pain: the significant role of pain catastrophizing and state anxiety. Pain Med. 2016;17(6):1069–78.
Walsh M. Pain and anxiety in A&E attenders. Nurs Stand. 1993;7(26):40–2.
Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42(5):521–30.
European Association for the Study of the Liver (EASL), Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65(1):146–81.
Johnston MJ, Fitzgerald JE, Bhangu A, Greaves NS, Prew CL, Fraser I. Outpatient management of biliary colic: a prospective observational study of prescribing habits and analgesia effectiveness. Int J Surg. 2014;12(2):169–76.
Gao K, Zheng C, Han H, Guo C. A multicenter randomized prospective study of early cholecystectomy for pediatric patients with biliary colic. J Gastrointest Surg. 2021;25(3):713–9.
Bjorkman DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications. Am J Med. 1999;107(6A):3S-10S.
Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18(4):204–12.
Ikegaya H, Saka K, Sakurada K, Nakamura M, Yoshida K. A case of sudden death after intramuscular injection of butylscopolamine bromide. Leg Med (Tokyo). 2006;8(3):194–7.
Shiraishi T, Nakamura M, Horiuchi T, Takazawa T. Anaphylaxis caused by butylscopolamine bromide: a case report. JA Clin Rep. 2020;6(1):26.
Wang Z, Jiang C, Yao H, Chen O, Rahman S, Gu Y, et al. Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain. 2021;144(2):665–81.
Shim H, Gan TJ. Side effect profiles of different opioids in the perioperative setting: are they different and can we reduce them? Br J Anaesth. 2019;123(3):266–8.
Ben Hmida W, Jerraya H, Nasseh S, Haloui N, Khalfallah M, Nouira R. The complications of subtotal cholecystectomy: a case report. Int J Surg Case Rep. 2021;83:105950.
Jaunoo SS, Mohandas S, Almond LM. Postcholecystectomy syndrome (PCS). Int J Surg. 2010;8(1):15–7.
Sun Y, Liu Y, Liu B, Zhou K, Yue Z, Zhang W, et al. Efficacy of acupuncture for chronic prostatitis/chronic pelvic pain syndrome : a randomized trial. Ann Intern Med. 2021;174(10):1357–66.
Hinman RS, McCrory P, Pirotta M, Relf I, Forbes A, Crossley KM, et al. Acupuncture for chronic knee pain: a randomized clinical trial. JAMA. 2014;312(13):1313–22.
Grissa MH, Baccouche H, Boubaker H, Beltaief K, Bzeouich N, Fredj N, et al. Acupuncture vs intravenous morphine in the management of acute pain in the ED. Am J Emerg Med. 2016;34(11):2112–6.
Tang S. Analgesic observation of 108 cases of biliary colic treated with eye acupuncture. J Guangxi Univ Tradit Chin Med. 2000;04:41–2.
Tan X. Clinical observation of 76 cases of biliary colic treated with acupuncture. Jiangxi Tradit Chin Med. 1995;S1:125–6.
Ning Sun. A study on the central mechanism of acupuncture at Yanglingquan acupoint in the treatment of GSD patients based on fNIRS and fMRI technology. Chengdu Univ Tradit Chin Med. 2022.
YuanFang Zhou. The central mechanism of acupuncture for immediate relief of pain in patients with biliary colic based on functional near-infrared spectroscopy. Chengdu Univ Tradit Chin Med. 2022.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Ding Y, Gu C, Shen L, Wu L, Shi Z, Chen Y. Effects of acupuncture combined general anesthesia on endorphin and hemodynamics of laparoscopic cholecystectomy patients in the perioperative phase. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33(6):761–5.
Zhang Y, Jia Y, Xiao D. ERCP nephrolithotomy combined Shugan Lidan decoction and electroacupuncture at Yanglingquan (GB34) in treatment of common bile duct calculi. Chin J Tradit Chin Med. 2018;36(02):509–12.
Zeng F, Qin W, Ma T, Sun J, Tang Y, Yuan K, et al. Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. Am J Gastroenterol. 2012;107(8):1236–47.
Park J, White A, Stevinson C, Ernst E, James M. Validating a new non-penetrating sham acupuncture device: two randomised controlled trials. Acupunct Med. 2002;20(4):168–74.
Egerton-Warburton D, Meek R, Mee MJ, Braitberg G. Antiemetic use for nausea and vomiting in adult emergency department patients: randomized controlled trial comparing ondansetron, metoclopramide, and placebo. Ann Emerg Med. 2014;64(5):526-532.e1.
Kober A, Dobrovits M, Djavan B, Marberger M, Barker R, Bertalanffy P, et al. Local active warming: an effective treatment for pain, anxiety and nausea caused by renal colic. J Urol. 2003;170(3):741–4.
McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7(1):45–50.
McNeil DW, Rainwater AJ 3rd. Development of the fear of pain questionnaire–III. J Behav Med. 1998;21(4):389–410. https://doi.org/10.1023/a:1018782831217.
Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139–49.
Kim S, Bae DW, Park SG, Park JW. The impact of Pain-related emotions on migraine. Sci Rep. 2021;11(1):577.
Mao JJ, Xie SX, Bowman MA. Uncovering the expectancy effect: the validation of the acupuncture expectancy scale. Altern Ther Health Med. 2010;16(6):22–7.
Qin Z, Zang Z, Zhou K, Wu J, Zhou J, Kwong JSW, et al. Acupuncture for chronic prostatitis/chronic pelvic pain syndrome: a randomized sham acupuncture controlled trial. J Urol. 2018;200(4):815–22.
Yu DT, Jones AY, Pang MY. Development and validation of the Chinese version of the Massachusetts general hospital acupuncture sensation scale: an exploratory and methodological study. Acupunct Med. 2012;30(3):214–21.
Sun N, He DM, Ye X, Bin L, Zhou Y, Deng X, et al. Immediate acupuncture with GB34 for biliary colic: protocol for a randomised controlled neuroimaging trial. BMJ Open. 2022;12(1):e050413.
World Health Organization. 64 indications for acupuncture recognized by the World Health Organization. Chin Acupuncture Moxibustion. 2008;28(S1):65.
Luo B. Immediate efficacy of acupuncture at Yanglingquan point for relief of biliary colic. Mod Med Health Res Electron J. 2018;2(14):152.
Chen WH, Yu HW. Puncturing on Yanglingquan point for relieving of colic pain bile cyst. Acupunct Res. 2000;01:62–3.
Wang P, Wu YL, Zhang YQ. Observations on the analgesic effect of acupuncture on cholecystagia. Shanghai J Acupunct Moxibustion. 2003;02:16–7.
Wang M. Acupuncture relieved 120 cases of biliary colic. Mod Med J China. 2009;11(02):55.
Moffet HH. Sham acupuncture may be as efficacious as true acupuncture: a systematic review of clinical trials. J Altern Complement Med. 2009;15(3):213–6.
Dincer F, Linde K. Sham interventions in randomized clinical trials of acupuncture–a review. Complement Ther Med. 2003;11(4):235–42.
Lee H, Bang H, Kim Y, Park J, Lee S, Lee H, et al. Non-penetrating sham needle, is it an adequate sham control in acupuncture research? Complement Ther Med. 2011;19(Suppl 1):S41–8.
Kong SD, Jin P, Wang Q. Relief of biliary colic by acupuncture at GB34 in 36 cases. People’s Mil Surg. 2016;59(01):84.
Feng LS, Gu CF, Yu LH, Hu JF, Wang GQ, Chu YY. Pain-relieving effects of acupuncture in treating 260 patients with acute cholecystalgia. J Yunnan Univ Tradit Chin Med. 2014;37(01):43–4+52.
Acknowledgements
We appreciate the ChatGPT platform for helping to polish English language and grammatical check in my manuscript.
Funding
The study is supported by the National Natural Science Foundation of China for the Youth (No. 81904096), Special Project of Central Government Guides Local Science and Technology Development' in Sichuan Provincial Department of Science and Technology (2020ZYD046) and China Postdoctoral Science Foundation (No. 2019M653361). All funding bodies will not be involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YFZ, RRS, XTG and FRL participate in the study design and conception of trial. The manuscript is drafted by YFZ, YQS and XYY. DMH, YZ, YFZ, SBD and CL will be in charge in assisting with patient recruitment. NS will be in charge of random allocation. YFZ will perform acupuncture treatment for BC patients. XYY will conduct quality control on case report forms and trials. LPD and YD will be responsible for the data collection. All authors have read, revised the protocol and approved its publication.
Authors’ information
Professor FRL is master of acupuncture, YFZ, NS and associate professor RRS are doctors of acupuncture, XYY and YQS are Master of acupuncture, XTG work at the Meishan Hospital of Traditional Chinese Medicine, DMH, YZ, YFZ, SBD, CL, LPD and YD work at the First People's Hospital of Longquanyi District, Chengdu (West China Longquan Hospital Sichuan University).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol of study (version 3.0) including the informed consent and CRF, has been approved by the Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (No. 2023KL-008). The informed consent will be signed by subject or legal guardian. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Shen, Y., Ye, X. et al. Acupuncture on GB34 for immediate analgesia and regulating pain-related anxiety for patients with biliary colic: a protocol of randomized controlled trial. BMC Complement Med Ther 23, 224 (2023). https://doi.org/10.1186/s12906-023-04030-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04030-8