Abstract
Background
Because spontaneous remission is common in IMN, and there are adverse effects of immunosuppressive therapy, it is important to assess the risk of progressive loss of renal function before deciding whether and when to initiate immunosuppressive therapy. Therefore, this study aimed to establish a risk prediction model to predict patient prognosis and treatment response to help clinicians evaluate patient prognosis and decide on the best treatment regimen.
Methods
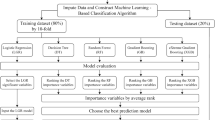
From September 2019 to December 2020, a total of 232 newly diagnosed IMN patients from three hospitals in Liaoning Province were enrolled. Logistic regression analysis selected the risk factors affecting the prognosis, and a dynamic online nomogram prognostic model was constructed based on extreme gradient boost, random forest, logistic regression machine learning algorithms. Receiver operating characteristic and calibration curves and decision curve analysis were utilized to assess the performance and clinical utility of the developed model.
Results
A total of 130 patients were in the training cohort and 102 patients in the validation cohort. Logistic regression analysis identified four risk factors: course ≥ 6 months, UTP, D-dimer and sPLA2R-Ab. The random forest algorithm showed the best performance with the highest AUROC (0.869). The nomogram had excellent discrimination ability, calibration ability and clinical practicability in both the training cohort and the validation cohort.
Conclusions
The dynamic online nomogram model can effectively assess the prognosis and treatment response of IMN patients. This will help clinicians assess the patient’s prognosis more accurately, communicate with the patient in advance, and jointly select the most appropriate treatment plan.
Similar content being viewed by others
Background
Idiopathic membranous nephropathy (IMN) is the most common pathologic type of adult nephrotic syndrome (NS) [1]. IMN is common in the middle-aged and elderly, the incidence has gradually increased in China in recent years, and there is a trend of younger age [2]. IMN is the second or third primary glomerulonephritis leading cause of end-stage renal disease (ESRD) in the USA and Europe [3], and approximately one third of IMN patients have a progressive disease course. The most frightening long-term consequence of IMN is progressive loss of kidney function. And among 60% of untreated patients, there are about 35% patients eventually develop to ESRD within 10 years [4,5,6,7].
In recent decades, some clinical [8, 9], pathological [10, 11], and genetic [12, 13] parameters have been identified as biomarkers for predicting the prognosis of IMN, and the Kidney Disease Improving Global Outcomes (KDIGO) 2021 clinical practice guideline have taken 24 h urinary protein (UTP), estimate glomerular filtration rate(eGFR), serum anti-phospholipase A2 receptor antibody (sPLA2R-Ab), serum albumin (ALB) and others as indicators that may be used to divide patients into categories of low, moderate, high, and very high risk of progressive loss of kidney function [14]. However, there are often inconsistencies in various indicators in clinical practice, such as high level proteinuria in patients with low titer sPLA2R-Ab, or high level proteinuria with nomal serum ALB. And there is currently no model that combines all of these clinical considerations. Therefore, there is an urgent need to develop a model that takes into account clinical factors, and use the cut-off value of the model to identify high-risk patients with poor prognosis, which is conducive to clinical application. In addition, if a patient is initiated immunosuppressive therapy according to the KDIGO 2021 clinical practice guideline, but the outcome of treatment is unclear. If a model can be used to predict patient outcomes and response to treatment, the model may help clinicians assess patient prognosis in advance, and can fully discuss with patients to determine the best treatment options to maximize the benefit of patients.
The nomogram is a useful and accessible tool for physicians to predict the disease progression, to plan for individualized treatment, and to decide the interval for follow-up [15, 16]. Nomograms have been previously developed for IMN [17,18,19,20], but most of the nomograms lack of external validity [17, 19], and no dynamic online nomogram related to IMN prognosis is found at present to our knowledge. Machine learning has recently been used to produce a prediction model for practice. Machine learning can help model information based on statistics, potentially revealing hidden dependencies between predictors and diseases. Previous studies have shown that machine learning algorithms such as extreme gradient boost (XGBoost), random forest (RF) and logistic regression (LR) have been used to predict or identify kidney disease [21, 22]. The purpose of this study is to establish a dynamic online nomogram model based on machine learning model, in order to accurately identify the prognosis and treatment response of IMN patients, and to help clinicians formulate personalized treatment plans.
Methods
Study cohorts
This was a retrospective analysis of multicenter study in 3 hospitals in Liaoning Province, northeast of China, which included 232 cases from September 2019 to December 2020. The training cohort included 130 patients from Shengjing Hospital of China Medical University, and the validation cohort was 102 patients from the First Affiliated Hospital of Jinzhou Medical University and the General Hospital of Angang Group (Fig. 1). The inclusion criteria for IMN were as follows: (i) patients with MN diagnosed by renal biopsy or a positive anti-PLA2R antibody test with NS. (ii) age 18 to 75 years. (iii) a follow-up time ≥ 24 months and with complete data which was obtained in our institution. The exclusion criteria were as follows: (i) secondary membranous nephropathy (SMN), including those with autoimmune disease, infection, malignancy, drug and heavy metal poisoning related MN. (ii) corticosteroids or immunosuppressants were applied before the start of the study. (iii) follow-up periods less than 24 months, or with missing data. iiii. patients with serious mental illness that is difficult to cooperate with treatment, and pregnant or lactating women. This study had been approved by the Ethics Committee of Shengjing Hospital affiliated to China Medical University, and informed consent was waived because it was a retrospective study(the ethics number: 2023PS847K).
Clinical data collection
The baseline and follow-up data were extracted from patients’ records in hospital’s electronic medical system, including demographic characteristics, clinical variables, laboratory results. According to the manufacturer’s recommendation, detection of sPLA2R-Ab titer was performed using ELISA (E200908BU, Euroimmun, Germany), and a value ≥ 20 RU/ml was considered as positive. Renal biopsy was performed, and the biopsy sample examined by light microscopy, immunofluorescence, and electron microscopy. Membranous lesions from IMN cases were classified into four stages based on the criteria of Ehrenreich and Churg [23].
Treatment options
The treatment strategy was based on the KDIGO 2021 clinical practice guideline [14]. Renin-angiotensin-aldosterone system (RAAS) inhibitors consist primarily of angiotensin-converting enzyme inhibitor (ACEi)/angiotensin-II receptor blocker (ARB). Immunosuppressant therapy includes cyclophosphamide (CTX) or calcineurin inhibitor (CNI). Targeted therapy refers to CD20 monoclonal antibody therapy, mainly including rituximab and obinutuzumab. Other immunosuppressant treatments include mycophenolate mofetil, leflunomide, tripterygium wilfordii and others.
Outcome
The clinical endpoint was non-remission of proteinuria at 24 months. Complete remission (CR) was defined as achieving a normal level of proteinuria excretion of no more than 0.3 g per 24 h and with a stable eGFR. Partial remission (PR) was defined as proteinuria between 0.3 g and 3.5 g per 24 h, or a reduction in proteinuria of at least 50% compared with baseline and with a stable eGFR [24, 25]. Patients who did not meet any of those criteria were categorized as non-remission (NR). A stable eGFR was defined as an eGFR that remained unchanged or declined less than 15% during the period of follow-up.
Model construction and performance evaluation
In the training cohort, we used univariate’ and multivariate’ logistic regression to screen for major risk factors with non-remission urine protein based on the patient’s baseline measurements, and constructed a nomogram based on XGBoost, RF, LR machine learning algorithms. Five key metrics are used to assess the effectiveness of the model: area under the receiver operating characteristic (AUROC), sensitivity, specificity, accuracy and F1-score.
Internal and external validation of the model
The nomogram was subjected to 1000 bootstrap resamples for internal validation to assess its predictive accuracy, and was performed by a visual calibration plot. The discriminative ability of the model was determined by AUROC, which ranges from 0.5 to 1, and the AUROC was compared using Z test. Finally, to estimate the clinical utility of the model, the decision curve analysis (DCA) was performed by calculating the net benefits for a range of threshold probability. The external validity of the model was evaluated by the AUROC, calibration and decision curve analysis in an independent cohort.
Statistical analysis
All the statistical analyses were done by SPSS26.0 and R 4.2.1. Normally distributed continuous variables were expressed with their means and standard deviations whereas non-normal continuous variables were expressed by their medians and interquartile ranges (IQR). Categorical variables were expressed with frequencies and percentages. The statistical significance between two cohorts was determined by T test or the Wilcoxon rank sum test for continuous variables and Chi-square test for categorical variables. Results with P<0.05 were considered statistically significant.
Results
Patient characteristics
232 IMN patients were enrolled in this study, and the characteristics were presented in Table 1. The training cohort included 85 males, and with the median age being 48 years and the median proteinuria was 7.0 g/d. The characteristics were compared between the training and validation cohorts, and it showed that there were significant differences in age, uric red blood cell (URBC), ALB, serum creatinine (Scr), blood urea nitrogen (BUN), and total cholesterol (TCHO). In the training cohort, 26 patients did not undergo renal biopsy due to personal willingness or physical condition, while the majority of patients with renal biopsy presented in stages 2 and 3. The patient’s treatment plan was based on the KDIGO 2021 clinical practice guideline, more than 60% of patients received RAAS inhibitors or immunosuppressant therapy in the training or validation cohort, and there were no significant differences in treatment regimens between the two groups. The follow-up time was 24 months, and the incidence of the endpoint of the IMN progression was 31.5% and 31.4% in the training and validation cohorts. In addition, we retrospectively analyzed the adjustment of the treatment regimen during the follow-up of the patients in the training cohort, it was found that 30 patients did not achieve remission even after changing the immunosuppressant regimen, and 7 of them made two adjustments to the treatment regimen and still had persistent urine protein.
Feature selection
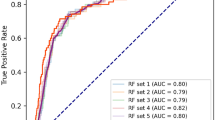
As shown in Table 2, after the multivariable’ analysis, we identified four major risk factors: course ≥ 6 months, UTP, D-dimer, and sPLA2R-Ab. These four variables were used to construct XGBoost, RF and LR machine learning models to predict the prognosis of IMN patients. The performance evaluation results of the three models were shown in Table 3, and the ROC curve and confusion matrix were used to evaluate the model discrimination ability, as shown in Fig. 2. The performance difference between the models were significant, and the RF model had the best performance, with the highest AUROC (0.869), sensitivity (0.700), specificity (0.897), precision (0.700), accuracy (0.769) and F1-score (0.700).
Model construction and comparison
To make the model more practical and easier to visualize, we developed a nomogram using the four predictors (course ≥ 6 months, UTP, D-dimer, and sPLA2R-Ab) (Fig. 3). For each predictive factor in the nomogram, the point was read out by drawing a line straight upward from each predictor to the point axis. The total point was calculated by summing up each point located in the total point axis, which was further converted to probability. Furthermore, a dynamic online nomogram was available via an internet interface at https://progression.shinyapps.io/DynNomapp/ (Fig. 4).
The internal validation of the model
In the training cohort, the C-index for the nomogram was 0.835 (95% CI 0.762–0.908) and the ROC curve displayed in Fig. 5A. Z test showed that the discriminative ability of the nomogram prediction was significantly higher than that of individual predictions (course ≥ 6 months, UTP and D-dimer, Table 4). The calibration plot of the nomogram was plotted in Fig. 6A and demonstrated a good correlation between observed and predicted progression with a mean absolute error of 0.047. The DCA of the nomogram was presented in Fig. 7A, and showed that if the threshold probability of was between 10 and 88% or greater than 90%, using the nomogram to predict the IMN progression added more net benefit.
The calibration curves for the nomogram. A completely accurate prediction model will generate a plot where the probability of the actual observed and predicted corresponding completely and fall along the 45°line. The apparent calibration curve represents the calibration of the model in the development data set, while the bias-corrected curve is the calibration result after correcting the optimism with the 1000 bootstrap-resampling. The closer the apparent calibration curve is to the bias-corrected curve, the more accurately the model predicts prognosis. A: In the training cohort; B: In the validation cohort
The DCA curves analysis for IMN prognosis nomogram in (A) the training and (B) the validation cohorts. The y-axis tested the net benefit. The thin gray line indicates that all patients with IMN are assumed to have non-remission of urine protein at 2 years, while the thick black line indicates that all patients with IMN are assumed to have a remission of proteinuria. The thick red line represented the risk nomogram. In training group, the decision curve showed that if the threshold probability of a patient is between 0.01 to 0.88 or greater than 0.9, using the nomogram in the present study to predict IMN prognosis adds more benefit
The external validation of the nomogram
In the validation cohort, the C-index was 0.874 (95% CI 0.801–0.946, Fig. 5B) and z test showed that the nomogram discrimination was better than the individual indicator (course ≥ 6 months, D-dimer, and sPLA2R-Ab). In addition, in order to evaluate the good calibration ability of the nomogram, we also calculated other evaluation metrics beyond the AUROC based on the cut-off value and the threshold of 0.5, including the sensitivity, specificity, precision, and F1-score, and the results showed that it may be better to assess the patient’s ability to calibrate the mode based on cut-off values (Table 5). A calibration curve (Fig. 6B) also showed high consistency between predicted prognosis probability and actual prognosis proportion. The DCA curve showed that the use of the nomogram increased the net benefit and had a strong clinical utility in predicting IMN prognosis (Fig. 7B).
Discussion
As a quantitative tool for risk and benefit assessment, clinical prediction model can provide more intuitive and rational information for doctors, patients and medical policy makers. In recent years, a number of nomograms with IMN had been established [17,18,19,20], which were used to predict progression or relapse of patients with IMN, and to distinguish malignancy-associated membranous nephropathy from IMN. Compared to the above researches, the endpoint of this study was the non-remission of proteinuria at 2-year follow-up, in order to evaluate the patient’s response to treatment. Furthermore, we constructed a dynamic online nomogram model, which was multi-indexed, simple and operable, without cumbersome formulas and calculations, and the external validation also showed the universality and applicability of the model. We only needed to slide and select the value of each variable to obtain the probability of non-remission of proteinuria in the patient. The most important thing was that there was no manual intervention in the whole process, which avoided accidental errors.
According to current reports, this is the first dynamic online nomogram based on baseline parameters to predict treatment response in patients with IMN. The nomogram has been validated internally and externally to show that it has good discrimination, calibration ability and clinical net benefit. And based on the nomogram, the clinician can preliminarily judge the patient’s prognosis and response to treatment after 2 years, fully communicate with the patient, and choose the most suitable personalized treatment plan for the patient.
The results of the retrospective study indicated that course ≥ 6 months, UTP, D-dimer, and sPLA2R-Ab were significant independent predictors of poor response in patients with IMN. What makes our study unique is that it links D-dimer, a marker of thromboembolic complications in IMN, to prognosis and confirms that D-dimer is an independent risk factor for urine protein remission in IMN. D-dimer is a specific product of cross-linked fibrin under the action of plasmin [26], which can be used as an important molecular marker to reflect the plasma hypercoagulability state and the activation of the fibrinolytic system in vivo [27, 28]. IMN is an immune-mediated inflammatory disease with a high risk of thromboembolic complications due to damage to vascular endothelial cells, activation of the coagulation system, and weakening of the fibrinolytic system [29, 30]. Persistent proteinuria in patients with IMN presenting with nephrotic syndrome may lead to secondary venous thrombosis, increasing the risk of infection and acute kidney injury, and thus leading to poor prognosis in patients with IMN [29, 31]. Therefore, the IMN patients with high levels of D-dimer may indicate a high risk of thrombotic events and critical condition in IMN patients, and need to initiate anticoagulation and immunosuppressive therapy as soon as possible [32, 33].
IMN is a slowly progressive immune and inflammation-associated renal disease [34]. We also found that patients with a long course of disease had a poor response to treatment, which may be due to the persistence of chronic inflammation, resulting in increased deposition of immune complexes on the outside of the glomerular basement membrane, massive formation of basement membrane “spike” and thickening of the basement membrane, thereby aggravating renal injury and leading to poor prognosis [35, 36]. Moreover, studies had shown that immune-inflammation index and monocyte-lymphocyte ratio were reliable markers which might be used to predict prognosis for IMN patients [37, 38].
Previous studies and well-known researchers agree that the prognosis of IMN patients is closely related to UTP and sPLA2R-Ab levels. The heavier UTP and the higher sPLA2R-Ab level, the worse the prognosis for patients with IMN. Higher proteinuria level is significantly associated with a higher risk of reduction in renal function [39, 40]. Persistent proteinuria is an independent risk factor for progression of IMN to ESRD. The results of the present study cohort indicated that the 24-h proteinuria level was an independent predictor for a poor renal outcome, which was consistent with the present reports.
It is well documented that PLA2R and its autoantibodies are closely related to the prognosis of IMN [41,42,43]. Compared with glomerular PLA2R deposition, serum anti-PLA2R antibody levels are more closely correlated with renal outcome [44]. The KDIGO 2021 glomerular disease management guidelines recommend longitudinal monitoring of sPLA2R-Ab levels at 6 months after start of treatment may be useful for evaluating treatment response in patients with MN, and can be used to adjust the treatment strategy [14]. Consistent with current findings, we also confirmed a significant association between baseline sPLA2R-Ab levels and renal outcome in IMN patients.
Unfortunately, we did not find satisfactory results for common prognostic markers of IMN, such as serum albumin [14, 45]. First, the nomogram predicts that most patients with non-remission will have refractory MN and will endpoint with the time outcome, while previous studies have mostly ended with event outcomes, which may lead us to different results. And secondly, we suspect that this may be due to the liver’s strong ability to synthesize albumin, and of course there is some correlation with our small sample size, and we hope to conduct further studies on large sample sizes to illustrate the association between them. Furthermore, our findings did not find that treatment regimens had a significant effect on urine protein outcomes. However, RAAS inhibitors, immunosuppressant, and CD20 monoclonal antibody therapy all showed low odds ratios in univariable’ logistics regression, implying that certain patients might benefit from these treatments. Therefore, based on our model, we recommend that when assessing the outcome of patients with urine protein, if the probability of non-remission of urine protein is high and the likelihood of disease progression is high, we should actively communicate with the patient and take intervention to achieve a good prognosis.
The present study developed a dynamic online nomogram model for the early prediction of poor treatment response in IMN patients, and we can formulate individualized treatment and management plans, and determine whether it is appropriate to initiate immunosuppressive therapy to reduce the risk of progression to ESRD. But, there are several limitations to the present study. First, this study covered data from three study centers, the failure to establish a unified testing platform resulted in differences in validation and training cohorts baseline data. Conversely, it also verified the universality and applicability of the prognostic model. Second, recent studies showed that chronic tubulointerstitial inflammation was considered as a risk factor for poor renal prognosis in patients with IMN [19, 46]. This retrospective study had a small sample size, and the urinary α1/β2-microglobulin were not been included the association between chronic tubulointerstitial inflammation and poor prognosis of IMN had not been studied. Therefore, there is a need for a prospective, multicenter, large- scale cohort to explore this correlation.
Conclusions
In conclusion, we developed a dynamic online model for assessing patient prognosis and treatment response in patients with IMN and validated the model using independent patient cohorts. The nomogram is easy to use and can identify patients with IMN who are at high risk of poor response to treatment and a poor prognosis, and may help clinicians formulate an individualized treatment plan for patients and discuss when to start immunosuppressive therapy for a good prognosis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ACEi:
-
Angiotensin-converting enzyme inhibitor
- ALB:
-
Albumin
- ARB:
-
Angiotensin II receptor blocker
- AUROC:
-
Area under the receiver operating characteristic
- BUN:
-
Blood urea nitrogen
- CI:
-
Confidence interval
- C-index:
-
Concordance index
- CNI:
-
Calcineurin inhibitor
- CR:
-
Complete remission
- CTX:
-
Cyclophosphamide
- Cysc:
-
Cystatin C
- DCA:
-
Decision curve analysis
- eGFR:
-
Estimate glomerular filtration rate
- ESRD:
-
End stage renal disease
- HGB:
-
Hemoglobin
- IMN:
-
Idiopathic membranous nephropathy
- IQR:
-
Interquartile ranges
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- LR:
-
Logistic regression
- NR:
-
non-remission
- NS:
-
Nephrotic syndrome
- PR:
-
Partial remission
- RF:
-
Random forest
- RAAS:
-
Renin-angiotensin-aldosterone system
- Scr:
-
Serum creatinine
- SMN:
-
Secondary membranous nephropathy
- sPLA2R-Ab:
-
Serum anti-phospholipase A2 receptor antibody
- TCHO:
-
Total cholesterol
- TG:
-
Triglycerides
- UA:
-
Uric acid
- URBC:
-
Uric red blood cell
- UTP:
-
24 h urinary protein
- UWBC:
-
Uric white blood cell
- XGBoost:
-
Extreme gradient boost
References
Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, Sethi S, Tong A, Vivarelli M, Wetzels J. Membranous nephropathy. Nat Rev Dis Primers. 2021;7(1):69. https://doi.org/10.1038/s41572-021-00303-z
Hu R, Quan S, Wang Y, Zhou Y, Zhang Y, Liu L, Zhou XJ, Xing G. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10(1):10994. https://doi.org/10.1038/s41598-020-67910-w
Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis. 2000;35(1):157–65. https://doi.org/10.1016/S0272-6386(00)70316-7
Hladunewich MA, Troyanov S, Calafati J, Cattran DC. The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol. 2009;4(9):1417–22. https://doi.org/10.2215/CJN.01330209
Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy–a modern view. Clin J Am Soc Nephrol. 2014;9(3):609–16. https://doi.org/10.2215/CJN.04160413
Diaz M, Agraz I, Soler, MJ. Anti-phospholipase A2 receptor antibody and spontaneous remission in membranous nephropathy. Clin Kidney J. 2019;12(1):33–5. https://doi.org/10.1093/ckj/sfy079
Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697–704. https://doi.org/10.1681/ASN.2009080861
Zhang J, Fan Z, Wang P, Zhang AH. Phospholipase A2 receptor antibodies and clinical prognosis in patients with idiopathic membranous nephropathy: an updated systematic review and meta-analysis. Kidney Blood Press Res. 2023;48(1):102–13. https://doi.org/10.1159/000529415
Liu J, Zha Y, Zhang P, He P, He L. The association between serum complement 4 and kidney disease progression in idiopathic membranous nephropathy: a multicenter retrospective cohort study. Front Immunol. 2022;13:896654. https://doi.org/10.3389/fimmu.2022.896654
Yeter HH, Isik Gonul I, Eraslan E, Karacalik C, Ogut B, Guz G. Effects of phospholipase A(2) receptor and thrombospondin type-1 domain-containing 7A expression in glomerular basement membranes on treatment response and renal outcome in membranous nephropathy. Clin Exp Nephrol. 2021;25(5):488–500. https://doi.org/10.1007/s10157-020-02011-6
Zhang Y, Liu Y, Liang L, Liu L, Tang X, Tang L, Chen P, Chen J, Wang Z, Cao W, et al. Effect of glomerular mannose-binding lectin deposition on the prognosis of idiopathic membranous nephropathy. Kidney Blood Press Res. 2020;45(5):713–26. https://doi.org/10.1159/000508665
Wang HY, Cui Z, Xie LJ, Zhang LJ, Pei ZY, Chen FJ, Qu Z, Huang J, Zhang YM, Wang X, et al. HLA class II alleles differing by a single amino acid associate with clinical phenotype and outcome in patients with primary membranous nephropathy. Kidney Int. 2018;94(5):974–82. https://doi.org/10.1016/j.kint.2018.06.005
Le WB, Shi JS, Fan Y, Gong SW. HLA alleles and prognosis of PLA2R-related membranous nephropathy. Clin J Am Soc Nephrol. 2021;16(8):1221–7. https://doi.org/10.2215/CJN.18021120
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753–79. https://doi.org/10.1016/j.kint.2021.05.015
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. https://doi.org/10.1016/S1470-2045(14)71116-7
Ohori Tatsuo G, Riu Hamada M, Gondo T, Hamada R. Nomogram as predictive model in clinical practice. Gan Kagaku Ryoho. 2009;36(6):901–6. PMID: 19542708.
Liu L, Wang H, Zhao B, Liu X, Sun Y, Mao Y. Nomogram to predict the progression of patients with primary membranous nephropathy and nephrotic syndrome. Int Urol Nephrol. 2022;54(2):331–41. https://doi.org/10.1007/s11255-021-02859-x
Li M, Wang H, Lai X, Guo D, Jiang C, Fu Z, Liu X. A preliminary nomogram model for predicting relapse of patients with primary membranous nephropathy. Ren Fail. 2023;45(1):2199092. https://doi.org/10.1080/0886022X.2023.2199092
Zhang J, Pan S, Li D, Li Z, Jin L, Zhou Z, Pan M. A nomogram for the prediction of renal outcomes among patients with idiopathic membranous nephropathy. Exp Ther Med. 2020;20(4):3130–7. https://doi.org/10.3892/etm.2020.9063
Chen T, Zhou Y, Zhu J, Chen X, Pan J. Prediction model of renal function recovery for primary membranous nephropathy with acute kidney injury. BMC Nephrol. 2022;23(1):247. https://doi.org/10.1186/s12882-022-02882-9
Chen Q, Zhang Y, Zhang M, Li Z, Liu J. Application of machine learning algorithms to predict acute kidney injury in elderly orthopedic postoperative patients. Clin Interv Aging. 2022;17:317–30. https://doi.org/10.2147/CIA.S349978
Chen S, Jiang L, Gao F, Zhang E, Wang T, Zhang N, Wang X, Zheng J. Machine learning-based pathomics signature could act as a novel prognostic marker for patients with clear cell renal cell carcinoma. Br J Cancer. 2022;126(5):771–7. https://doi.org/10.1038/s41416-021-01640-2
Churg J, Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hypertens. 1973;1(Pt 1):443–8. PMID: 4536436.
Thompson A, Cattran DC, Blank M, Nachman PH. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26(12):2930–7. https://doi.org/10.1681/ASN.2015010091
Yamamoto R, Imai E, Maruyama S, Yokoyama H, Sugiyama H, Nitta K, Tsukamoto T, Uchida S, Takeda A, Sato T, et al. Incidence of remission and relapse of proteinuria, end-stage kidney disease, mortality, and major outcomes in primary nephrotic syndrome: the Japan Nephrotic Syndrome Cohort Study (JNSCS). Clin Exp Nephrol. 2020;24(6):526–40. https://doi.org/10.1007/s10157-020-01864-1
Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113(13):2878–87. https://doi.org/10.1182/blood-2008-06-165845
Favresse J, Lippi G, Roy PM, Chatelain B, Jacqmin H, Ten Cate H, Mullier F. D-dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548–77. https://doi.org/10.1080/10408363.2018.1529734
Tan X, Chen G, Liu Y, Zhou L, He L, Liu D, Liu Y, Zhang F, Li H, Liu H. Serum D-dimer is a potential predictor for thromboembolism complications in patients with renal biopsy. Sci Rep. 2017;7(1):4836. https://doi.org/10.1038/s41598-017-05210-6
Li SJ, Guo JZ, Zuo K, Zhang J, Wu Y, Zhou CS, Lu GM, Liu ZH. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome-a prospective study. Thromb Res. 2012;130(3):501–5. https://doi.org/10.1016/j.thromres.2012.04.015
Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513–20. https://doi.org/10.2215/CJN.10131011
Barbour S, Reich H, Cattran D. Short-term complications of membranous nephropathy. Contrib Nephrol. 2013;181:143–51. https://doi.org/10.1159/000349976
Parker K, Ragy O, Hamilton P, Thachil J, Kanigicherla D. Thromboembolism in nephrotic syndrome: controversies and uncertainties. Res Pract Thromb Haemost. 2023;7(6):102162. https://doi.org/10.1016/j.rpth.2023.102162
Li X, Xie X, Zhao Y, Wang G, Shao H, Zhang X. Some points for the KDIGO 2021 guideline for prophylactic anticoagulation in membranous nephropathy: is it clear enough for us to follow? Nephron. 2023;147(3–4):193–8. https://doi.org/10.1159/000525913
Cai Q, Hendricks AR. Membranous nephropathy: a ten-year journey of discoveries. Semin Diagn Pathol. 2020;37(3):116–20. https://doi.org/10.1053/j.semdp.2020.01.001
Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3(2):957–76. https://doi.org/10.1002/cphy.c120028
Singh J, Jain A, Bhamra R, Rathi V, Dhingra AK. The mechanistic role of different mediators in the pathophysiology of nephropathy: a review. Curr Drug Targets. 2023;24(2):104–17. https://doi.org/10.2174/1389450124666221026152647
Kazan DE, Kazan S. Systemic immune inflammation index and pan-immune inflammation value as prognostic markers in patients with idiopathic low and moderate risk membranous nephropathy. Eur Rev Med Pharmacol Sci. 2023;27(2):642–8. https://doi.org/10.26355/eurrev_202301_31065
Zhang AH, Dai GX, Zhang QD, Huang HD, Liu WH. The value of peripheral blood cell ratios in primary membranous nephropathy: a single center retrospective study. J Inflamm Res. 2023;16:1017–25. https://doi.org/10.2147/JIR.S404591
Chen X, Chen Y, Ding X, Zhou Y, Lv Y, Li D, Chen B, Chen T, Chen C. Baseline proteinuria level is associated with prognosis in idiopathic membranous nephropathy. Ren Fail. 2019;41(1):363–9. https://doi.org/10.1080/0886022X.2019.1605294
Yamaguchi M, Ando M, Katsuno T, Tsuboi N, Maruyama S. Urinary protein and renal prognosis in idiopathic membranous nephropathy: a multicenter retrospective cohort study in Japan. Ren Fail. 2018;40(1):435–41. https://doi.org/10.1080/0886022X.2018.1487864
Yin P, Wang J, Liang W, Zhan L, Liu Y, Lin J, Chen X, He Y, Jian H, Xie Z, et al. Outcomes of primary membranous nephropathy based on serum anti-phospholipase A2 receptor antibodies and glomerular phospholipase A2 receptor antigen status: a retrospective cohort study. Ren Fail. 2020;42(1):675–83. https://doi.org/10.1080/0886022X.2020.1792315
Liu X, Xue J, Guo X, Ding Y, Zhang Y, Zhang X, Huang Y, Huang B, Hu Z, Lu G, et al. A PLA2R-IgG4 antibody-based predictive model for assessing risk stratification of idiopathic membranous nephropathy. J Healthc Eng. 2021;2021:1521013. https://doi.org/10.1155/2021/1521013
Kukuy OL, Cohen R, Gilburd B, Zeruya E, Weinstein T, Agur T, Dinour D, Beckerman P, Volkov A, Nissan J, et al. The prognostic value of anti-PLA2R antibodies levels in primary membranous nephropathy. Int J Mol Sci. 2023;24(10). https://doi.org/10.3390/ijms24109051
Pang L, Zhang AM, Li HX, Du JL, Jiao LL, Duan N, Liu Y, Yu D. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy: a cross-sectional study. Med (Baltim). 2017;96(24):e7218. https://doi.org/10.1097/MD.0000000000007218
Delafosse M, Ponlot E, Hanset N, Estève E, Boffa JJ, Dahan K. Serum albumin still of interest to predict outcomes in membranous nephropathy in the era of phospholipase A2 receptor. Kidney Int Rep. 2020;5(9):1611–2. https://doi.org/10.1016/j.ekir.2020.06.040
Zhang XD, Cui Z, Zhang MF, Wang J, Zhang YM, Qu Z, Wang X, Huang J, Wang F, Meng LQ, et al. Clinical implications of pathological features of primary membranous nephropathy. BMC Nephrol. 2018;19(1):215. https://doi.org/10.1186/s12882-018-1011-5
Acknowledgements
The authors thank all investigators, trial teams and patients for their participation in the study.
Funding
This work was financially supported by project of Liaoning Provincial Department of Science and Technology(Grant No.2020JH 2/10300150). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
F.W. was involved in the study design, conduct of the study, data collection and interpretation, and wrote the first draft of the manuscript. Y.Q.W. was involved in the study design, data collection, conduct of the study, data analysis and interpretation and critical revision of all drafts of the manuscript. J.Y.X., F.M.W. were involved in the study design and data collection. H.L.Z. and N.Y. were involved in the acquisition, analysis, and interpretation. Y.X. performed the data analyses. X.Y., C.C.J., X.S.S., B.R.Z. and H.Z. were involved in the study design, conduct of the study, data collection and interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of Shengjing Hospital affiliated to China Medical University. All methods were carried out in accordance with declaration of Helsinki. The need for informed consent was waived by the Ethics Committee of Shengjing Hospital affiliated to China Medical University because it was a retrospective study(the ethics number: 2023PS847K).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, F., Xu, J., Wang, F. et al. A dynamic online nomogram for predicting renal outcomes of idiopathic membranous nephropathy. BMC Med Inform Decis Mak 24, 173 (2024). https://doi.org/10.1186/s12911-024-02568-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-024-02568-2