Abstract
Background
Biallelic variants in SPARC are extremely rare, and have been reported in only a few cases of autosomal recessive osteogenesis imperfecta (OI) type XVII. Here, we describe an individual with a SPARC homozygous missense variant (c.787G > A; p.Glu263Lys) and expand on the phenotype.
Case presentation
The proband had a history of multiple fractures, osteopenia, severe thoracolumbar levoscoliosis, rib fusion, global hypotonia, conductive hearing loss, and was non-ambulatory. Several of his features were similar to previously described cases, such as early neuromuscular concerns, scoliosis, long bone and vertebral compression fractures, and delayed motor milestones, suggesting these are consistent across SPARC-related osteogenesis imperfecta (OI). However, the proband sustained fractures at a younger age with a more severe course compared to most previous reports. He also had bony fusion of several ribs and hearing loss, which have not been reported in SPARC-related OI.
Conclusions
Overall, the proband supports the current phenotype of SPARC-related OI, but also expands the phenotypic variability.
Similar content being viewed by others
Background
Osteogenesis Imperfecta (OI) is a group of inherited conditions which affect skeletal development and cause bone fragility. OI is genetically heterogeneous, but most cases are due to heterozygous pathogenic variants in COL1A1 and COL1A2 [1]. However, there are multiple rarer genetic causes of OI. One example is OI due to biallelic pathogenic variants in SPARC, termed OI type XVII (OMIM 616507) [2].
The SPARC (secreted protein, acidic, cysteine-rich) gene encodes osteonectin, or basement membrane protein 40 (BM-40) [3] Osteonectin has been shown to bind collagen and regulate cell matrix interactions [3, 4]. It is abundantly expressed in mineralized tissues [2]. Osteonectin-null mice have decreased numbers of osteoblasts and osteoclasts with a severe osteopenia phenotype [4]. Based on the role of osteonectin in collagen production, collagen assembly, and bone mineralization [3], it is not surprising that variants in SPARC lead to an OI phenotype.
In 2015, Mendoza-Londono et al. [5] described the first two individuals with a severe form of OI attributed to bi-allelic missense mutations in SPARC, subsequently defined as OI type XVII (OMIM 616507) [2]. Since that time, Hayat et al., Durkin et al., Selina et al., and Storoni et al. [6,7,8,9] have described six additional individuals. All but one of the affected individuals have homozygous variants in SPARC; one patient has compound heterozygous variants (summarized in Table 1). Seven unique variants have been described, including nonsense, missense and intronic variants. The variant c.497G > A (p.Arg166His) has been described in two individuals [5, 6]; Arg166 is highly conserved among species and has previously been shown to be essential for binding collagen [10]. Substitution of these residues reduces the ability to bind to collagen type I [10]. Mendoza-Londono et al. also reported that the migration of collagen type I alpha chains produced by skin fibroblasts of their affected patients was mildly delayed, suggesting overmodification of collagen alpha chains [5].
Given the small number of individuals reported to date there are limited data on the phenotypic spectrum of OI type XVII. In addition, one individual reported by Hayat et al. [6] passed away at age 22 months, limiting knowledge of age-related outcomes. However, based on these few reports [5,6,7,8,9], the current consistent phenotypic findings of OI type XVII include severe scoliosis, fractures, and normal cognition. Most reports mention motor delays and some discuss hypotonia or muscle weakness in the affected individuals. There was variability of the sclerae with the sclerae being reported as white, gray, blue, and blue-gray. Most individuals had normal dentition, although one individual had dentinogenesis imperfecta. No individuals have been reported with hearing loss. Here, we present an additional affected individual with a homozygous missense variant in SPARC and unique features.
Case presentation
The child was born at 36 weeks via induced vaginal delivery due to severe oligohydramnios, and the pregnancy was complicated by maternal nephrotic syndrome. The pregnancy was otherwise uncomplicated and routine ultrasounds were reported as normal. The child was of Asian Indian descent and parents were first cousins. Birth weight was 2.36 kg (3rd centile), birth length was 47 cm (18th centile), and birth head circumference was 34 cm (21st centile). At 9 weeks of age, he was noted to have global hypotonia and was unable to lift his head.
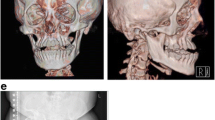
There were no other significant concerns until 6 months of age when he had his first known fracture, a femur fracture. He subsequently had multiple additional identified fractures (Fig. 1): left femur between 1–2 years; right femur, left femur, rib, and right tibia between 2–3 years; left femur, right humerus, right radius, right ulna, and right tibia after 3 years. He required surgery with instrumentation in both of his femurs and in his right forearm. He developed a severe 47-degree levoscoliosis of the thoracolumbar spine and bilateral coxa valga. He had a thoracic cage abnormality (Fig. 1) with bony fusion of several ribs with the development of multilevel loss of vertebral body height/compression fractures and biconcave vertebrae. Radiographs showed delayed bone age and generalized osteopenia. He also had restrictive lung disease due to the scoliosis, and mild obstructive sleep apnea. Magnetic resonance imaging of the brain showed mild periventricular white matter volume loss.
The patient was started on bisphosphonate therapy (pamidronate) at 31 months of age, although he continued to sustain fractures. He also received calcium and vitamin D supplementation.
He had bilateral undescended testes and required a right sided orchiopexy, and a left sided orchiectomy due to testicular torsion at 14 months of age. Additionally, the patient had failure to thrive with recurrent vomiting requiring gastrostomy tube placement at 19 months of age. At the time of last evaluation, at 3 years of age, the patient continued to receive feedings through the gastrostomy tube and was only able to take a few teaspoons of purees and some water by mouth per day. A swallow study showed penetration with thin liquids but no frank aspiration.
He initially passed his newborn hearing screen, but at age 3 years, was found to have mild conductive hearing loss. At 3 years of age he continued to have diffuse hypotonia, gross and fine motor delays, and joint hypermobility. He was non-ambulatory and mostly remained in a supine position. He was able to support himself sitting for about 5 min at a time and could roll over. He was receiving physical and occupational therapy at the time of evaluation at 3 years of age. There was no evidence of cognitive delays and he had normal speech for his age.
Trio whole exome sequencing was performed clinically and a homozygous missense variant was identified in SPARC (c.787G > A; p.Glu263Lys). The laboratory classified this variant as pathogenic according to the American College of Medical Genetics and Genomics criteria. The variant has been previously reported in the literature in an affected individual with OI type XVII, and is absent from population databases (Exome Aggregation Consortium). Multiple functional prediction algorithms predict this variant to be damaging. The parents had no history of fractures, but the proband’s brother, found to be a carrier of the SPARC variant, had a fracture of his foot at 5 years of age after an incorrect step off a curb, with normal healing, without any additional features indicative of OI.
Discussion
Our case represents another individual with OI type XVII caused by biallelic variants in SPARC, and expands upon the phenotype that has been previously reported in the literature [5,6,7,8,9]. The clinical features of our patient and previously reported cases are summarized in Table 1. SPARC (secreted protein acidic and rich in cysteine) which is located on chromosome 5 (5q31-q33) encodes osteonectin, which is critical for calcification of collagen in bone [5, 11]. The autosomal recessive inheritance pattern with homozygous variants of the few reported patients, in addition to a similar phenotype in osteonectin-null animal models [4], support loss of function of SPARC as causative for an OI phenotype.
Our case provides additional evidence that SPARC-related OI is a moderate-severe form of OI. Compared to other reported cases, our patient experienced fractures on average at a younger age and had additional features, including hearing loss and a thoracic cage deformity with bony fusion of several ribs. Our case supports variable expressivity with the potential for other modifying factors, as our patient and individual 2 presented by Mendoza-Londono et al. [5] carry the same homozygous variant, but have slightly varying phenotypes. Compared to that individual [5], the herein reported patient appears to have a more severe presentation, as he is non-ambulatory, has severe scoliosis, and an earlier age of fracture onset.
There is no previous evidence of individuals heterozygous for a pathogenic SPARC variant presenting with symptoms. The brother of our patient was found to be heterozygous for the SPARC variant and had a history of one fracture with minimal trauma. He did not have any other manifestations of OI. Although the fracture history could raise questions about the possibility of a manifesting carrier, there are no other reports of heterozygous individuals having an OI phenotype (including the parents of our patient), and heterozygous Sparc knockout mice appear unaffected [4].
Conclusion
SPARC-related OI is a rare disorder with some phenotypic variability based on current information. At this time, there are still only a few cases reported and further reports will be helpful to better clarify the phenotype spectrum.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- OI:
-
Osteogenesis imperfecta
References
Lim J, Grafe I, Alexander S, Lee B (2017) Genetic causes and mechanisms of osteogenesis imperfecta. Bone 102:40–49
Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 616507: 12/19/2022: World Wide Web URL: https://omim.org/
Rosset EM, Bradshaw AD (2016) SPARC/osteonectin in mineralized tissue. Matrix Biol 52–54:78–87
Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E (2000) Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest 105:915–923
Mendoza-Londono R, Fahiminiya S, Majewski J, Care4Rare Canada Consortium, Tétreault M, Nadaf J et al (2015) Recessive osteogenesis imperfecta caused by missense mutations in SPARC. Am J Hum Genet 96:979–985
Hayat A, Hussain S, Bilal M, Kausar M, Almuzzaini B, Abbas S et al (2020) Biallelic variants in four genes underlying recessive osteogenesis imperfecta. Eur J Med Genet 63:103954
Durkin A, DeVile C, Arundel P, Bull M, Walsh J, Bishop NJ et al (2022) Expanding the phenotype of SPARC-related osteogenesis imperfecta: clinical findings in two patients with pathogenic variants in SPARC and literature review. J Med Genet 59:810–816
Selina A, James D, Madhuri V (2023) A novel biallelic splice site variant in the SPARC gene causing severe osteogenesis imperfecta. Indian J Pediatr 90:626
Storoni S, Celli L, Zhytnik L, Maasalu K, Märtson A, Kõks S et al (2023) Novel pathogenic variants in SPARC as cause of osteogenesis imperfecta: two case reports. Eur J Med Genet 66:104857
Sasaki T, Hohenester E, Göhring W, Timpl R (1998) Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J 17:1625–1634
Goldblum SE, Ding X, Funk SE, Sage EH (1994) SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci U S A 91:3448–3452
Acknowledgements
We thank the family for their participation in this report.
Funding
No funding for support of this report.
Author information
Authors and Affiliations
Contributions
BMD, AS and DAS were major contributors in writing the manuscript. CH obtained consent from the family. All authors contributed to obtaining and interpreting clinical data and reviewing the medical literature. All authors read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable; written informed consent was obtained from the parent of the patient.
Consent for publication
Informed consent to publish was obtained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dunleavy, B.M., Schildt, A.J., Harrington, C. et al. Osteogenesis imperfecta type XVII: expansion of the phenotype. Egypt J Med Hum Genet 25, 7 (2024). https://doi.org/10.1186/s43042-024-00475-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00475-9