Abstract
Aim
Rechallenge of [177Lu]Lu-PSMA-617 radioligand therapy (RLT) was proposed for patients who initially responded to PSMA-RLT experiencing partial remission, but relapsed into progression after a certain period of remission. However, only limited data is available regarding this approach. In this study, we analyzed the efficacy and safety profile of one or more series of [177Lu]Lu-PSMA-617 RLT rechallenge in patients from a prospective registry (REALITY Study, NCT 04833517) after they initially benefited from PSMA-RLT.
Methods
Forty-seven patients with metastatic castration-resistant prostate cancer (mCRPC) who had biochemical response to initial [177Lu]Lu-PSMA-617 RLT followed by disease progression received at least one (up to three) series of [177Lu]Lu-PSMA-617 RLT rechallenge. Biochemical response rates based on prostate-specific antigen (PSA) serum value, PSA-based progression-free survival (PFS) and overall survival (OS) were calculated. Adverse events of the treatment were assessed according to ‘common terminology criteria for adverse events’ (CTCAE).
Results
After one series of RLT rechallenge, a PSA decline of at least 50% was achieved in 27/47 patients (57.4%). The median PFS of all patients was 8.7 mo and the median OS was 22.7 mo, each calculated from the administration of the first rechallenge series. Patients who responded (PSA decline > 50%) to the rechallenge showed a median OS of 27.3 mo. Regarding PFS, a significant correlation (r = 0.4128, p = 0.0323) was found for these patients comparing initial and rechallenge RLT. Ten patients received a second and 3 patients received a third rechallenge series with 8/10 and 3/3 patients responding to repeated RLT rechallenge. No severe deterioration of adverse events rated by CTCAE criteria was observed.
Conclusion
[177Lu]Lu-PSMA-617 RLT rechallenge is associated with significant PSA response and encouraging survival outcome as well as a very favourable safety profile and should therefore be considered as a straight-forward treatment option in mCRPC patients, who previously benefited from PSMA-RLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Prostate cancer remains one of the most prevalent forms of malignant diseases in men worldwide in 2022 [1]. Patients with prostate cancer frequently progress into the advanced stage of metastatic castration-resistant prostate cancer (mCRPC) which is resistant to physical or chemical castration by androgen deprivation therapy (ADT) and is attributed with a poor prognosis [2,3,4]. In this scenario treatments with androgen receptor signaling inhibitor (ARSI) [5, 6], taxane-based chemotherapy [7, 8], 223Ra treatment [9] or PARP-inhibitors [10, 11] are established treatment options. In recent years, radioligand therapy (RLT) targeting prostate-specific membrane antigen (PSMA) has been demonstrated to be effective, safe and well-tolerated in several retrospective and prospective studies on mCRPC [12,13,14,15,16,17,18]. Based on the phase 3 VISION trial PSMA-RLT with 177Lu was finally approved by both the FDA and EMA [17, 19, 20]. However, patients who initially responded excellently to PSMA-RLT and showed a temporary period of remission tend to relapse into progression. In this rather late stage of disease, only limited treatment options for patients remain. Rechallenge of PSMA-RLT might be a reasonable approach, particularly in patients who previously had an excellent response to this treatment. However, data on this topic are scarce and only reported for a limited number of patients [21,22,23]. In this study, we analyzed the efficacy and safety profile of one or more rechallenge series of [177Lu]Lu-PSMA-617 RLT in patients from a prospective registry (REALITY Study, NCT 04833517) who initially responded to PSMA-RLT and experienced partial remission.
Materials and methods
Study design and patient population
The aim of this study was to evaluate the clinical value of [177Lu]Lu-PSMA-617 RLT rechallenge in mCRPC. Rechallenge was defined as the retreatment with one or more series of [177Lu]Lu-PSMA-617 (each series comprising multiple sequential treatment cycles) in patients who responded to the initial [177Lu]Lu-PSMA-617 series, followed by a relevant progression-free time period and renewed progression. Response was characterized as a decrease in prostate-specific antigen (PSA) serum value ≥ 50% during treatment, whereas an increase of PSA value ≥ 25% was assessed as progression. After progression occurred, the patients received a rechallenge series of [177Lu]Lu-PSMA-617 RLT. The primary endpoint comprised evaluation of PSA response rate and outcome. The secondary endpoint included the analysis of adverse events. The study design is displayed in Fig. 1.
All patients were enrolled in the ‘prospective registry to assess outcome and toxicity of targeted radionuclide therapy in patients with mCRPC in clinical routine’ (REALITY Study; NCT04833517) between January 2016 and September 2022. In this time frame, 341 mCRPC patients were treated with [177Lu]Lu-PSMA-617 RLT within this registry. In total, n = 47 patients of the initial cohort could be identified fulfilling the following inclusion criteria (Fig. 2): (I) biochemical response to the initial [177Lu]Lu-PSMA-617 RLT series; (II) maintained response after initial RLT series; (III) [177Lu]Lu-PSMA-617 RLT rechallenge after relevant progression-free time period and renewed progression. Accordingly, 163/341 patients were excluded due to missing biochemical response during the initial series of [177Lu]Lu-PSMA-617 RLT. Fifty-six of these 178 patients were subsequently excluded because after temporarily responding but not maintaining response up to the end of the initial series. Out of the remaining 122 patients, 47 received [177Lu]Lu-PSMA-617 RLT rechallenge after relevant progression-free time period and renewed progression and were finally included in the analysis. All patients of the cohort were heavily pretreated with androgen-deprivation therapy (ADT), androgen-receptor signaling inhibitors (ARSI) and/or chemotherapy. Detailed patient characteristics are compiled in Table 1.
The initial series of PSMA-RLT encompassed administration of a median of 3 cycles (range: 1–8 cycles) with a median administered activity of 6.20 GBq/cycle (range: 4.33–9.10 GBq/cycle) [177Lu]Lu-PSMA-617. Detailed information on the initial PSMA-RLT series is provided in Table S1 in the supplemental material. The initial series of PSMA-RLT was discontinued when patients experienced remarkable biochemical response with only limited tumor load remaining. The mean PSA decline observed after the initial series of PSMA-RLT was 85.5 ± 14.4% (median: 91.5%; range: 53–99%). The rechallenge, i.e. the second [177Lu]Lu-PSMA-617 RLT series was initiated after a PSA-based progression-free survival (PFS) of median 10.8 months. Sufficient PSMA expression was verified by PSMA-targeted positron emission tomography/ computed tomography (PET/CT) and defined as markedly higher tumoral tracer uptake compared to the healthy liver. The administration of [177Lu]Lu-PSMA-617 RLT was applied on a compassionate use basis, following the regulations of the German Pharmaceutical Act § 13 (2b). The protocol of this study was in accordance with the declaration of Helsinki. All patients provided written consent after being informed about the general risks and potential negative side effects of this treatment and agreed to the publication of any data resulting from the study in anonymized form. The study was approved by the local institutional review board (ethics committee permission number 140/17).
Treatment details of PSMA-RLT rechallenge
The radiolabeling of PSMA-617 with 177Lu and the quality control of [177Lu]Lu-PSMA-617 were accomplished based on the standard procedures described by Kratochwil et al. [24]. The radionuclide 177Lu was provided by IDB Holland BV (Baarle-Nassau, Netherlands) and PSMA-617 by ABX advanced biochemical compounds GmbH (Radeberg, Germany).
Administered activities were adjusted to the characteristics of each individual patient considering tumor burden, location of metastases, diffuse involvement of bone marrow, course of disease, general physical condition, body weight, body surface, renal function, and blood cell count. 30 min prior to infusion of [177Lu]Lu-PSMA-617, the patient received hydration by 500 mL 0.9% NaCl intravenously and additionally a cooling of the salivary glands was applied. Infusion of [177Lu]Lu-PSMA-617 was administered over a period of one hour.
All 47 patients received a rechallenge, i.e. a second series of [177Lu]Lu-PSMA-617 RLT comprising a median of 2 cycles (range: 1–6 cycles) with a median administered activity of 7.0 GBq/cycle (range 4.25–9.25 GBq/cycle). A second rechallenge i.e. a third series of [177Lu]Lu-PSMA-617 RLT applied to 10 patients, comprised a median of 2 cycles (range: 1–5 cycles) with a median administered activity of 7.63 GBq/cycle (range: 4.4–9.1 GBq/cycle). Three patients of the cohort also received a third rechallenge i.e. a fourth series of [177Lu]Lu-PSMA-617 consisting of a median of 2 cycles (range: 2–3 cycles) administering a median activity of 7.9 GBq/cycle (range: 4.5–9.3 GBq/cycle). The mean interval between the second and third series and between the third and fourth series was 9.7 ± 5.4 months and 15.1 ± 3.0 months, respectively.
PSMA-RLT rechallenge series were discontinued when patients experienced remarkable biochemical response with only limited residual tumor burden (depicted by post-therapeutic [177Lu]Lu-PSMA-617 scintigraphy or [68Ga]Ga-PSMA-11 PET/CT) or when progression was observed.
Response evaluation and outcome
Response was biochemically assessed by measurement of PSA serum value during and after each series of [177Lu]Lu-PSMA-617 RLT. Following the recommendations of the prostate cancer working group 3 (PCWG3) [25] an increase of PSA value ≥ 25% was defined as progressive disease (PD). A decrease of PSA ≥ 50% was rated as partial remission (PR), while a change of PSA ranging from + 25% to -50% was rated as stable disease (SD). Patients who experienced a PR were characterized as responders, and patients with SD or PD as non-responders.
PSA-based progression-free survival (PFS) was calculated specifically for each rechallenge with the start of the corresponding series and the endpoint of either biochemical PD or last study visit. Overall survival (OS) was calculated starting at the date of initiating the second PSMA-RLT series (first rechallenge) and ending either at the occurrence of death from any cause or last contact. The cut-off date of the study was 31st October 2023. All statistical analyses were performed using PRISM 9 software (GraphPad Software, San Diego, USA). Level of significance was defined as p-value < 0.05. PFS and OS were determined using the Kaplan-Meier method (log-rank test). Spearman correlation was used to calculate the relation between PFS of the initial series and the first rechallenge series.
Recording of adverse events
To assess adverse events during and after the RLT series the ‘common terminology criteria for adverse events’ (CTCAE), version 5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf; last accessed 7th May 2024), were used. Renal impairment, anemia, thrombocytopenia, leukopenia, fatigue and xerostomia were analyzed. While anemia, thrombocytopenia, leukopenia and renal impairment were evaluated by frequent blood cell count and glomerular filtration rate (GFR), xerostomia and fatigue were assessed by using a questionnaire following CTCAE terminology.
Results
PSA response
Preceding the administration of the first [177Lu]Lu-PSMA-617 RLT rechallenge a mean serum PSA value of 346 ± 845 ng/mL (range: 1.00–5475 ng/mL) was observed, which decreased to 75 ± 112 ng/mL (range 1.00–663 ng/mL) under therapy, implying a mean PSA decline of 46.6% ± 47.1%. In total, 27/47 patients (57.4%) experienced partial remission (PR), 17/47 (36.2%) stable disease (SD) and 3/47 (6.4%) progressive disease (PD). A representative example of a patient with PR after rechallenge RLT is presented in Fig. 3.
Maximum intensity projection of [68Ga]Ga-PSMA-11 PET/CT images of a representative patient with biochemical and molecular imaging response after the initial series (2 cycles) and the rechallenge series (4 cycles) of [177Lu]Lu-PSMA-617 (time interval between the initial series and the rechallenge: 3.25 years)
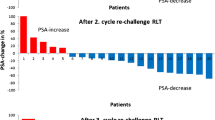
For patients who underwent a second rechallenge series of [177Lu]Lu-PSMA-617 RLT, the mean PSA value decreased by 67.4% ± 29.0%, from 203 ± 213 ng/mL (range: 49–826 ng/mL) to 93 ± 109 ng/mL (range 1.40–385 ng/mL) with PR, SD and PD in 8/10 (80%), 2/10 (20%) and 0/10 (0%) patients, respectively. During a third rechallenge series, applied to 3 patients, a mean PSA value of 379 ± 281 ng/mL (range: 120–678 ng/mL) at start and 72 ± 87 (range: 9.50–171 ng/mL) after completion of the treatment series was observed. All patients (3/3, 100%) showed a PR with a mean PSA decline of 80.9% ± 14.2%. Figure 4 represents a waterfall plot of the individual PSA changes for all rechallenge series. Figure 5 exemplarily shows a patient who received a baseline [177Lu]Lu-PSMA-617 RLT followed by three rechallenge series. Each treatment series induced PR characterized by remarkable PSA decline.
Outcome
The median PSA-based progression-free survival (PFS) and the overall survival (OS), both calculated from the administration of the first rechallenge cycle with [177Lu]Lu-PSMA-617, were analyzed. As depicted in Fig. 6A the median PFS was 8.7 months (CI: 0.5–39.2 months). Data regarding OS displayed in Fig. 6B, showed a median of 22.7 months (CI: 18.2–24.7 months) after administration of the first rechallenge series. While patients who responded to the rechallenge approach, i.e. experienced a PR, showed a median OS of 27.3 months (CI: 16.3–34.6 months), patients with SD or PD showed a significantly shorter (Log-rank test p = 0.0302) median OS of 10.3 months (CI: 8.3–22.7 months). If considering the initial therapy with [177Lu]Lu-PSMA-617 RLT as the starting point for OS calculation, the median OS of the patients was 18.7 months (CI: 10.6–78.4 months). Regarding PFS no significant correlation was observed comparing initial and rechallenge RLT for all patients (r = 0.2744, p = 0.0620, Fig. 7A). However, in a subgroup analysis including responders to rechallenge, a moderate significant positive correlation was found (r = 0.4162, p = 0.0323, Fig. 7B).
Adverse events
The majority of recorded events was categorized as mild or moderate (CTCAE score 1 or 2). Figure 8; Table 2 present the documented adverse events, based on the CTCAE terminology, for the initial series, first and second rechallenge of RLT. A slight increase in CTCAE grade 1/2 events was observed comparing first and second rechallenge treatment series (17.0% for xerostomia, 10.6% for fatigue, 4.3% for leukopenia, 2.1% for thrombocytopenia, 1.7% for anemia and 4.3% for renal impairment). CTCAE grade 3/4 events were rarely experienced. The comparison of initial and rechallenge RLT revealed a minor increase for leukopenia (2.1%), thrombocytopenia (2.1%) and anemia (6.4%), while no change for renal impairment and no case of CTCAE grade 3/4 for xerostomia and fatigue was recorded. To summarize, the frequency and severity of all treatment-related adverse events occurring for patients who received two (N = 10) or three (N = 3) rechallenge series a respective table (Table S2) is presented in the supplemental material. In addition, Figure S1 of the supplemental material shows the individual course of GFR in these patients over the initial and the rechallenge series of RLT.
Discussion
PSMA-targeted RLT has opened up promising treatment perspectives of mCRPC with remarkable response rates, prolonged survival and a favorable side effect profile in prospective studies [12, 14, 17, 26]. Patients with mCRPC initially benefitting and responding to RLT will, however, experience re-progression at some point. Since therapy options are limited at this stage of disease, a rechallenge approach with recurrent application of RLT appears intuitively reasonable. However, data regarding this approach is still scarce. Addressing this issue, we analyzed the cohort of patients from the prospective registry (REALITY Study; NCT04833517) who received RLT rechallenge, focusing on response rate, outcome and toxicity. This study demonstrates a high level of efficacy and safety of this rechallenge approach suggesting it indeed as a valuable and promising treatment option.
In this study, we analyzed a cohort of n = 47 patients receiving PSMA-RLT rechallenge and observed a substantial response rate of 57.4% and a mean PSA decline of 46.6% in the entire rechallenge cohort. Furthermore, we were able to show that the application of multiple rechallenge series during the course of disease was safe and effective. Our results confirm previous studies of small patient cohorts with RLT rechallenge [21,22,23]. Violet et al. evaluated 15 patients who were treated with one series of rechallenge [177Lu]Lu-PSMA-617 RLT. In this analyzed group 73% of the patients showed a PSA decline ≥ 50%. In a cohort of 30 patients, Yordanova et al. reported a benefit of [177Lu]Lu-PSMA-617 RLT rechallenge in 75–90% patients experiencing either stable disease or a response in the first 4 rechallenge cycles. Gafita et al. investigated the feasibility of [177Lu]Lu-PSMA-I&T RLT rechallenge in a small cohort of 8 patients. The authors observed that the second treatment series was effective with a response rate of 37.5%. While these authors already reported satisfying responses on a first rechallenge attempt, our study showed that even up to 3 rechallenge series (in total up to 17 cycles) continued to be effective. All patients who responded favorably to a first rechallenge and received multiple rechallenges also benefited from the second (n = 10) or even a third (n = 3) rechallenge series. This suggests that repeated response can be expected following a first successful rechallenge.
Remarkably, the PFS observed after the first rechallenge RLT (median 8.7 month) was only slightly shorter compared to PFS after initial RLT (median 10.8 month). In addition, for patients responding to rechallenge, a correlation analysis revealed a significant moderate correlation between PFS observed at initial treatment and rechallenge. Specifically, this indicates a relatively long PFS for those patients who showed long PFS on initial RLT.
Similarly promising results were also found for OS. Starting with the application of the first rechallenge a median time of 22.7 mo was observed, a very encouraging result with respect to the advanced stage of disease. Particularly, for patients who achieved a reduction of PSA of at least 50%, a significantly longer OS of 27.3 vs. 10.2 mo for non-responders was observed. The median OS of our cohort is in line with previous studies on RLT rechallenge which reported an OS range between 12 mo and 26.6 mo on smaller cohorts of patients [21,22,23].
The repeated application of an initially successful therapy is a concept already used in clinical practice, for example in the application of chemotherapy, not only in prostate cancer but in other malignant diseases as well [27,28,29,30,31,32]. However, in contrast to RLT, chemotherapy is often used in earlier stages of prostate cancer [33, 34]. The reported OS for rechallenge chemotherapy with docetaxel and cabazitaxel ranges between 13.7 and 43.5 mo [35,36,37,38]. Differences in stage of disease and applied chemotherapy drugs may explain this wide range. Nevertheless, it should be of note, that the respective studies also report on a variety of side effects caused by rechallenge chemotherapy. In this context, the adverse events of RLT rechallenge must also be taken into account. The safety profile of initial and rechallenge RLT was assessed as favorable, meaning for the majority of patients, that initial treatment and rechallenge had similar limited side effects and was overall well tolerated without treatment termination due to adverse events. In line with our findings, Mader et al. who treated patients in an extended PSMA-RLT therapy concept (comprising up to 16 cycles) observed a comparable safety profile with limited toxicity [39].
In summary, rechallenge PSMA-RLT was effective and safe in this retrospective analysis. We found no counter-argumentation against the concept of rechallenging patients with RLT and considering this as a treatment option for mCRPC patients, who previously benefited from initial PSMA-RLT. To strengthen these conclusions, further research in larger cohorts, preferably in a prospective setting, is clearly recommended. Furthermore, therapy approaches combining rechallenge RLT either with systemic therapy or radiosensitizer and RLT with alpha emitters might also be an option in the future [23, 40,41,42,43,44,45,46]. Especially the alpha emitter 225Ac, either as monotherapy e.g. [225Ac]Ac-PSMA-617 or within a tandem radionuclide concept, e.g. combining [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617, has proven to be effective in end-stage mCRPC, especially in patients with insufficient response to monotherapy with 177Lu-labeled PSMA ligands [43,44,45,46]. These concepts might also be worth an evaluation in a rechallenge setting for patients who have previously responded to the initial series. In addition, the use of other beta-emitting radionuclides such as e.g. 161Tb was recently discussed and could be an option for use in rechallenge. These approaches should be investigated and compared in future studies to optimize rechallenge RLT.
Limitations of the present study should be considered which may restrict the interpretation and generalization of the results. First, the study suffers from its retrospective nature and involves only a relatively small number of patients. Second, administered 177Lu activity and number of cycles were individually chosen and no standardized protocol was applied. Further studies are needed to investigate the ideal 177Lu activity to be used for PSMA-RLT rechallenge. Future studies in larger cohorts should also evaluate predictive parameters to further optimize management strategies for end-stage mCRPC. Due to only intermittently performed PSMA PET/CT imaging and missing contrast-enhanced diagnostic CT scans, we only considered PSA-based PFS and were not able to determine a valid radiological PFS.
Conclusion
[177Lu]Lu-PSMA-617 RLT rechallenge is associated with significant PSA response and encouraging survival outcome as well as a very favourable safety profile. Therefore, this concept of rechallenge RLT should be considered as a treatment option in mCRPC patients, who previously benefited from initial PSMA-RLT. Future studies evaluating [177Lu]Lu-PSMA-617 RLT rechallenge in a prospective setting are strongly recommended.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate Cancer epidemiology and risk Factors-A systematic review. Eur Urol. 2023;84:191–206.
Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–92.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Martin GA, Chen AH, Parikh K. A novel use of Olaparib for the treatment of metastatic castration-recurrent prostate Cancer. Pharmacotherapy. 2017;37:1406–14.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G, et al. Clinical outcome of standardized 177Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur J Nucl Med Mol Imaging. 2020;47:713–20.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804.
Hope TA, Calais J. PSMA-targeted radiopharmaceutical therapy in patients with metastatic castration-resistant prostate cancer. Lancet. 2021;397:768–9.
Meyrick D, Gallyamov M, Sabarimurugan S, Falzone N, Lenzo N. Real-World Data Analysis of Efficacy and survival after Lutetium-177 labelled PSMA ligand therapy in metastatic castration-resistant prostate Cancer. Target Oncol. 2021;16:369–80.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate Cancer. N Engl J Med. 2021;385:1091–103.
Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, et al. 177Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: initial 254-patient results from a prospective registry (REALITY study). Eur J Nucl Med Mol Imaging. 2022;49:1075–85.
Hennrich U, Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): the first FDA-Approved Radiotherapeutical for treatment of prostate Cancer. Pharmaceuticals (Basel). 2022;15:1292.
Al-Ibraheem A. Theranostics in developing countries: addressing challenges and potentials from Training to Practice. World J Nucl Med. 2023;22:171–3.
Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester H-J, et al. Early experience of Rechallenge 177Lu-PSMA Radioligand Therapy after an initial good response in patients with advanced prostate Cancer. J Nucl Med. 2019;60:644–8.
Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1073–80.
Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang S-P, Kong G, et al. Long-term follow-up and outcomes of Retreatment in an expanded 50-Patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate Cancer. J Nucl Med. 2020;61:857–65.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-resistant prostate Cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Fizazi K, Herrmann K, Krause BJ, Rahbar K, Chi KN, Morris MJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:597–610.
Palmieri C, Krell J, James CR, Harper-Wynne C, Misra V, Cleator S, et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010;7:561–74.
Ceresoli GL, Zucali PA, De Vincenzo F, Gianoncelli L, Simonelli M, Lorenzi E, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer. 2011;72:73–7.
Bearz A, Talamini R, Rossoni G, Santo A, de Pangher V, Fasola G, et al. Re-challenge with pemetrexed in advanced mesothelioma: a multi-institutional experience. BMC Res Notes. 2012;5:482.
Tonini G, Imperatori M, Vincenzi B, Frezza AM, Santini D. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013;32:92.
Zhuo M-L, Bai H, Wang Z-J, Duan J-C, An T-T, Wu M-N, et al. Rechallenge with pemetrexed-based chemotherapy improves the survival of patients with advanced non-squamous non-small-cell lung cancer. Mol Clin Oncol. 2014;2:953–9.
Hanovich E, Asmis T, Ong M, Stewart D. Rechallenge Strategy in Cancer Therapy. Oncology. 2020;98:669–79.
Williams IS, McVey A, Perera S, O’Brien JS, Kostos L, Chen K, et al. Modern paradigms for prostate cancer detection and management. Med J Austr. 2022;217:424–33.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol. 2021;79:263–82.
Heck MM, Thalgott M, Retz M, Wolf P, Maurer T, Nawroth R, et al. Rational indication for docetaxel rechallenge in metastatic castration-resistant prostate cancer. BJU Int. 2012;110:E635–640.
Oudard S, Kramer G, Caffo O, Creppy L, Loriot Y, Hansen S, et al. Docetaxel rechallenge after an initial good response in patients with metastatic castration-resistant prostate cancer. BJU Int. 2015;115:744–52.
Thibault C, Eymard J-C, Birtle A, Krainer M, Baciarello G, Fléchon A, et al. Efficacy of cabazitaxel rechallenge in heavily treated patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2018;97:41–8.
Pobel C, Auclin E, Teyssonneau D, Laguerre B, Cancel M, Boughalem E, et al. Cabazitaxel multiple rechallenges in metastatic castration-resistant prostate cancer. Cancer Med. 2021;10:6304–9.
Mader N, Nguyen Ngoc C, Kirkgöze B, Baumgarten J, Groener D, Klimek K, et al. Extended therapy with [177Lu]Lu-PSMA-617 in responding patients with high-volume metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50:1811–21.
Maharaj M, Heslop L, Govender T, Korowlay N, Singh A, Choudhary P, et al. The outcome and safety of re-challenge lutetium-177 PSMA (177Lu-PSMA) therapy with low-dose docetaxel as a radiosensitizer-a promising combination in metastatic castrate-resistant prostate cancer (mCRPC): a case report. Nucl Med Mol Imaging. 2021;55:136–40.
Rosar F, Bader H, Bartholomä M, Maus S, Burgard C, Linxweiler J, et al. Addition of Standard Enzalutamide Medication shows synergistic effects on response to [177Lu]Lu-PSMA-617 radioligand therapy in mCRPC patients with imminent treatment failure-preliminary evidence of pilot experience. Cancers (Basel). 2022;14:2691.
Prasad V, Zengerling F, Steinacker JP, Bolenz C, Beer M, Wiegel T, et al. First experiences with 177Lu-PSMA therapy in combination with Pembrolizumab or after pretreatment with Olaparib in single patients. J Nucl Med. 2021;62:975–8.
Rosar F, Krause J, Bartholomä M, Maus S, Stemler T, Hierlmeier I, et al. Efficacy and safety of [225Ac]Ac-PSMA-617 augmented [177Lu]Lu-PSMA-617 radioligand therapy in patients with highly advanced mCRPC with poor prognosis. Pharmaceutics. 2021;13:722.
Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8.
Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79(3):343–50.
Rosar F, Hau F, Bartholomä M, Maus S, Stemler T, Linxweiler J, et al. Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Theranostics. 2021;11(9):4050–60.
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding received.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in the patients described herein were in accordance with the ethical standards of the Institutional and/or National Research Ethics Committees and with the 1964 Helsinki Declaration and its later amendments, or with comparable ethical standards. This report does not include any animal studies. The study was approved by the Institutional Review Board of Ärztekammer des Saarlandes/Saarbrücken (ethics committee permission number 140/17). Written informed consent was obtained from all study participants.
Consent for publication
All patients have given written consent to publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosar, F., Schuler, J., Burgard, C. et al. Efficacy and safety of rechallenge [177Lu]Lu-PSMA-617 RLT after initial partial remission in patients with mCRPC: evaluation of a prospective registry (REALITY study). Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06825-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06825-4