Abstract
Tumor-infiltrating lymphocyte (TIL) therapy represents a groundbreaking advancement in the solid cancer treatment, offering new hope to patients and their families with high response rates and long overall survival. TIL therapy involves extracting immune cells from a patient's tumor tissue, expanding them ex vivo, and infusing them back into the patient to target and eliminate cancer cells. This revolutionary approach harnesses the power of the immune system to combat cancers, ushering in a new era of T cell-based therapies along with CAR-T and TCR-therapies. In this comprehensive review, we aim to elucidate the remarkable potential of TIL therapy by delving into recent advancements in basic and clinical researches. We highlight on the evolving landscape of TIL therapy as a prominent immunotherapeutic strategy, its multifaceted applications, and the promising outcomes. Additionally, we explore the future horizons of TIL therapy, next-generation TILs, and combination therapy, to overcome the limitations and improve clinical efficacy of TIL therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
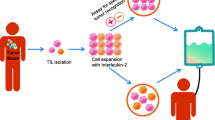
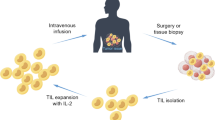
TIL therapy was originally developed by Rosenberg’s group starting in 1986 [1]. The premise involves extracting immune cells, known as TILs, from a patient's tumor, cultivating and expanding these cells in a laboratory, and then reintroducing them into the patient's body to target and destroy cancer cells. TIL therapy recognizes the uniqueness of each patient's cancer by recognizing the cancer neoantigens, activating the T cell immune response to eradicate tumor cells (that is called “cytotoxicity”). This precision medicine approach leads to higher response rates and increased chances of remission, which is nothing short of revolution in the oncology field. It is especially remarkable considering that Dr. Rosenberg discovered TIL’s cytotoxicity ~ 40 years ago without many of the molecular toolkits that are becoming available only in the past 15 years.
Over the past few decades, substantial research efforts have been dedicated to the development of technologies for expanding tumor-infiltrating lymphocytes (TIL) [1,2,3,4,5], and T cells in general, along with the formulation of clinical procedures [6, 7]. TIL therapy has progressed to a point where it is on the cusp of an approval as a biological drug—lifileucel by Iovance (https://www.iovance.com) is upon BLA review by the FDA [8]. There are crucial areas that demand further advancement, including the evaluation of its potency, enhancing clinical effectiveness in both “hot” and “cold” cancers, optimizing the manufacturing process, reducing the overall production cost, and improving the accessibilities of this modality.
To successfully integrate TIL therapy into clinical practice (that is the “standard of care”), it is essential to expand its therapeutic applications in solid tumor types with severe unmet medical needs, establish optimal clinical protocols of treatment procedures, streamline manufacturing processes, and conduct pertinent clinical trials. Furthermore, ongoing research into next-generation TIL therapy is already underway, and the accumulation of real-world data will serve to foster and substantiate further advancements in this field.
In this review, we will provide a highlight of the fundamentals, development, and limitations of current TIL therapy. We will also discuss the recent development of the next-generation TIL therapy.
Current status of adoptive cell therapy (ACT)
The advantages of TIL over CAR-T and TCR-T in solid cancer treatments
Adoptive cell therapy (ACT) represents a dynamic frontier in cancer treatment, harnessing the potential of the immune system to target and kill tumor cells. This approach encompasses various strategies, including tumor-infiltrating lymphocytes (TIL), gene-modified T cells expressing novel T cell receptors (TCR), and chimeric antigen receptors (CAR). These therapies have demonstrated considerable promise in various tumor types, and multiple clinical trials are conducted worldwide to further optimize this treatment modality [9].
CAR-T therapy has shown remarkable efficacy and success in treating hematological malignancies, such as B-cell acute lymphoblastic leukemia (ALL), certain types of non-Hodgkin lymphoma, and leukemia. Multiple CAR-T products have received regulatory approval and are gaining tremendous success in clinical applications [10,11,12,13,14,15]. However, CAR-T therapy has several considerable limitations: (1) it is associated with significantly severe side effect, such as cytokine release syndrome (CRS), acute respiratory distress syndrome (ARDS), and neurological toxicities [16]; (2) CAR-T therapy is highly effective for blood cancers; however, its effectiveness in treating solid tumors remains poor (Table 1).
Conversely, TCR-T therapy shows potential in targeting a wide range of solid tumors. It can be tailored to specific tumor antigens, including those derived from intracellular proteins presented by MHC molecules, making it more suitable for targeting solid tumors. In fact, it has demonstrated clinical efficacy in treating solid tumors rather than blood cancers, as discussed in numerous research and review articles on TCR-T therapies [17,18,19,20]. The FDA has accepted the Biologics License Application (BLA) for a TCR-T cell therapy, specifically afami-cel, for the treatment of advanced synovial sarcoma, granting it priority review (https://www.adaptimmune.com). The limitations of TCR-T therapy include: (1) The current field lacks very specific antigen targets, (2) there are potentially fatal side effects in addition to CRS and neurological toxicities when off-target toxicity (that is, TCR recognizes antigens expressed in normal tissues) is induced, (3) HLA restriction—HLA types need to be matched in order for alpha–beta TCR to function, and (4) the manufacturing process can be complex (Table 1).
TIL therapy can potentially be used to treat a wide range of cancer types as well as TCR-T therapy; it relies on extracting immune cells from patient’s tumor. TILs are infiltrated into a tumor site. Therefore, these cells have already recognized the tumor as foreign antigen(s). By isolating and expanding, these TILs can be used to target and attack the cancer cells with precision. TILs are extracted from the patient’s own tumor; hence, it is personalized for each patient. This minimizes the risk of rejection or graft-versus-host disease (GVHD) often associated with allogeneic therapies.
TIL therapy, while generally well tolerated and uneventful [21], does have associated risks and potential side effects. Administration may lead to transient dyspnea, chills, and fever immediately following infusion [22]. These adverse events (AEs) are not typically associated with elevated serum levels of circulating cytokines and should not be conflated with cytokine release syndrome, which is commonly observed with other cell therapies such as chimeric antigen receptor (CAR) T cell therapy [23].
Common AEs in TIL therapy include:
Lymphodepletion-related toxicity: Prior to TIL infusion, patients typically undergo a lymphodepleting regimen, which can lead to cytopenias and increased susceptibility to infections [22, 24, 25]. Management strategies include prophylactic antibiotics, antiviral medications, and antifungal agents, as well as supportive care measures such as growth factors for neutropenia.
IL-2-related reactions: Patients may experience fever, chills, or hypotension during or shortly after the TIL infusion due to IL-2 toxicity [24,25,26,27,28]. These reactions are usually manageable with supportive care and symptomatic treatment.
Other potential risks: As with any therapy involving immune modulation, there is always a risk of unforeseen adverse events, and close monitoring during and after treatment is essential.
By implementing these management strategies, we can mitigate the risks associated with TIL therapy and ensure a safer treatment experience for patients. We will include a detailed section on these safety aspects in our discussion to provide a more balanced and comprehensive overview.
TIL therapy has shown promise in achieving long-lasting responses, even in advanced or metastatic cancers [29,30,31]. Some patients have experienced complete and sustained remissions, suggesting that the treatment can provide lasting benefits [32,33,34,35]. TIL therapy is investigated for a wide range of solid tumors, including melanoma, cervical cancer, lung cancer, breast cancer, and more recently gastric intestinal cancers [36,37,38,39]. This makes it a potentially valuable treatment option for a range of cancer types. The FDA has approved a tumor-infiltrating lymphocyte (TIL) therapy called lifileucel (also known as Amtagvi) for the treatment of advanced melanoma, marking the first cellular therapy to treat solid tumors (https://www.iovance.com). Furthermore, TIL therapy can be used in combination with other immunotherapies, such as immune checkpoint inhibitors, to enhance its effectiveness. This combination approach may provide additive or synergistic benefits in treating cancer.
The limitations of TIL therapy include: (1) the current manufacturing process for TIL therapy is labor-intensive, time-consuming, and costly, and (2) tumor tissues are challenging to obtain, particularly in late-stage cancer patients (Table 1).
In summary, CAR-T cell therapy is most suitable for hematological malignancies, TCR-T cell therapy shows promise for solid tumors, and TIL therapy is currently most effective for melanoma and potentially other solid tumors. All offer promising approaches to cancer treatment as emerging T cell-based modalities, each with its own set of advantages and disadvantages. The selection of a specific immunotherapy approach should be carefully tailored to factors such as the type of cancer, the patient’s medical condition, and the treatment’s availability at the healthcare facility. Understanding the unique strengths and limitations of each therapy can guide their application in clinical practice to maximize patient outcomes.
TIL therapy up to date
The impact of prior anticancer treatments on TIL: evaluating effectiveness and characteristics
It is indeed important to consider the impact of prior treatments such as anticancer agents and irradiation on tumor-infiltrating lymphocytes (TILs) when harvesting them for therapy. Research indicates that the impact of chemotherapy and radiation on TILs may not significantly affect their effectiveness and characteristics [40,41,42]. TILs, which are extracted from a patient's tumor, expanded in a laboratory, and reinfused to fight cancer, have shown promising results even in patients who have undergone extensive prior treatments. Studies suggest that these pre-treatments do not greatly diminish the functional capacity of TILs, as the therapy can still induce substantial antitumor responses.
Clinical efficacy of TIL therapy in melanoma and resistance factors
In recent years, as TIL therapy has been developed to a certain level of maturity, a number of clinical trials are underway. Nearly 50 trials led by either academic groups (Table 2a) or biotech companies (Table 2b) are ongoing, targeting both immunologically “hot” solid tumors and more recently “cold” solid tumors. Melanoma has been studied in TIL therapy for 30 years and represents a highly immunogenic indication, with objective response rate (ORR) 36%-56%, progression-free survival (PFS) 3.7–7.5 months, overall survival (OS) 15.9–21.8 months in metastatic melanoma [24, 25, 34, 43,44,45]. Retrospective analysis of a single-center experience of non-selected autologous TIL study conducted by Pillai et al. and Instil Bio (https://instilbio.com) revealed that the ORR was 67%, complete response (CR) rate was 19%, and the disease control rate (DCR) was 86%, which was consistent with that observed in the prior PD-1 inhibitor subgroup (58%, 8%, and 75%, respectively) among 21 patients with advanced cutaneous melanoma. Median overall survival in all treated patients and the prior PD-1 inhibitor subgroup was 21.3 months. In total, 5 patients (24%) had durable ongoing responses (> 30 months post‑TIL infusion) at data cutoff, and all patients who achieved CR remained alive and disease-free [46] (Supplementary Table 1).
The tumor microenvironment (TME) significantly influences the efficacy of immunotherapies, including TIL therapy. The TME comprises various components such as immune cells, cytokines, and stromal elements, which together create an environment that can either promote or inhibit antitumor responses. Immunosuppressive cells within the TME, including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), play a crucial role in dampening the immune response against tumors [47]. These cells secrete immunosuppressive cytokines and metabolites that inhibit the activity of cytotoxic T lymphocytes (CTLs) and other antitumor immune cells. This suppression leads to several resistance mechanisms that contribute to treatment failure, such as antigen loss, T cell dysfunction or exhaustion, and impaired T cell migration to the tumor site [48,49,50,51,52].
Moreover, the TME can prevent effective T cell infiltration and recognition of tumor cells, further complicating immunotherapy outcomes. Strategies to counteract these challenges include targeting immunosuppressive pathways, enhancing T cell functionality, and improving TIL trafficking and persistence within the tumor [52, 53]. By addressing these aspects, the effectiveness of TIL therapy and other immunotherapies can be significantly improved, offering better clinical outcomes for patients with various solid tumors [51, 52].
Combining TIL with immune checkpoint inhibitors (ICI)
One strategy to enhance efficacy and durability of response is to combine TIL with ICI. Mullinax JE et al. conducted the combination study of TIL and Ipilimumab (cytotoxic T lymphocyte-associated antigen 4 blockade: CTLA-4 antibody). They reported 38.5% ORR, PFS 7.3 months (95% CI 6.1–29.9 months) in metastatic melanoma, and one of patients became a complete response at 52 months [54]. Furthermore, earlier line treatment with lifileucel (LN-144) and LN-145 plus pembrolizumab (programmed death 1: PD-1 antibody) combination led by Iovance demonstrated 67% ORR and CR rate of 25% in immune checkpoint inhibitors (ICI)-naïve advance melanoma patients [55, 56]. In addition to melanoma, in combination with pembrolizumab, it has also shown promise in advanced ICI-naive head and neck squamous cell carcinoma (HNSCC; n = 18, ORR = 38.9%) and advanced untreated cervical carcinoma (n = 14, ORR = 57.1%), respectively [55] (Supplementary Table 1).
TIL therapy application for other cancer types
TIL therapy has exciting potential to overcome tumor heterogeneity and induce deep and durable remissions in treatment-refractory cancers other than melanoma [24].
A pilot study was conducted in patients who had advanced epithelial ovarian carcinoma with intraperitoneal (IP) TIL. There were no measurable responses due to possible manufacturing difficulties; however, ascites regression tumor and CA-125 reduction were observed [57].
Karbach J et al. reported that complete and durable tumor remission was observed after three TIL infusions for a patient with metastatic hormone-refractory prostate cancer (mHRPC) expressed New York esophageal squamous cell carcinoma 1 (NY-ESO-1) treated with in vitro expanded tumor-infiltrating lymphocytes (TILs) in conjunction with IL-2 and ICI [58].
In Phase I study of adjuvant immunotherapy with autologous TILs in locally advanced cervical cancer, 9 of 12 patients (75.0%) attained a complete response, with a disease control duration of 9–22 months [59] (Supplementary Table 1).
Stevanović et al. reported a phase II study of TIL therapy for human papillomavirus (HPV)-associated epithelial cancers (NCT01585428) in 2019. ORR in cervical cancer was observed 28% and 18% for non-cervical cancer patients. Two of the responses in cervical cancer were complete and lasted 67 and 53 months after treatment. The magnitude of HPV reactivity of the infused TILs was associated with clinical response. HPV-associated cancers also harbor somatic gene mutations (mutated neoantigens) and epigenetically dysregulated genes (cancer germline antigens) that may be targeted by the TILs. [30].
The phase I study of the combination nivolumab and TIL for the patients with advanced NSCLC was recently completed at H. Lee Moffitt Cancer Center (NCT03215810). Initial tumor regression occurred in 68.8% (11 of 16) of patients at first CT scan performed one month after TIL infusion, and the median best change was 35.5% (range + 20 to − 100). Two out of 16 patients achieved complete responses ongoing 1.5 years later. In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in responding patients. Neoantigen-reactive T cell clonotypes increased and persisted in the peripheral blood after treatment. [39].
Tran E et al. demonstrated that TILs from 9 out of 10 patients with metastatic gastrointestinal cancers contained CD4+ and/or CD8+ T cells that recognized one to three neo-epitopes derived from somatic mutations expressed by the patient’s own tumor. Interestingly, in one patient with metastatic colon cancer a human leukocyte antigen (HLA) –C*08:02–restricted T cell receptor from CD8+ TILs targeted the KRASG12D hotspot driver mutation. [60]
Colorectal, pancreatic, and ovarian cancers are traditionally known to be less responsive to immunotherapy [61]. Despite no objective responses being observed, 63% of patients exhibited stable disease [61] (Supplementary Table 1). Notably, one patient with pancreatic cancer experienced a reduction in tumor burden lasting over a year, with no new safety signals identified [61]. This suggests potential antitumor activity even in cancers typically resistant to such treatments.
In-depth immunological characterization paving the way of TIL therapy improvement
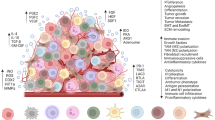
Tumor-reactive TIL selection (Fig. 1A)
Schematic overview of the next-generation TIL production. TILs are isolated from freshly resected tumor tissue and grown. Tumor-reactive cells are selected, based on expression markers, such as CD39+/CD103+, PD-1, CD39-/CD69-, and 4-1BB; B tumor tissue is cultured in the presence of antibodies, such as 4-1BB and OX40, C genetic modifications on TILs to enhance TIL functionality
TIL selection based on IFN-γ before rapid expansion procedure (REP)
In study by Rosenberg et al., tumor-reactive TILs which exhibited interferon-gamma (IFN-γ) in co-culture with autologous tumor cells or cancer cell lines in vitro were pre-selected for REP [29]. Adoptive cell transfer (ACT) using this selected TIL method showed ORR 49% (12% CR and 37% PR) in patients with metastatic melanoma. However, this method required extra time and materials for testing and TIL expansion [62].
Young TILs
Another key development is the so-called “young TILs” starting in 2008–2010. It reduces the initial expansion period by isolating TILs with enzymatic digestion of tumors and expands them instead of waiting for them to migrate out of tumor fragments in culture [63, 64]. The “young TIL” demonstrated that they have longer telomeres and higher levels of CD27 and CD28 compared to TIL expanded from fragments [65]. In one study using these “young TILs,” 10 out of 20 patients with metastatic melanoma (50%) achieved an objective clinical response with 2 complete remissions and eight partial responses (with progression-free survival ranging from 3 to 18 months).
Neoantigen-reactive TILs
Technological advancement over the past decades enabled the development of neoantigen-reactive and high potent TIL, which is considered as the “next-generation TIL therapies” [66]. By mapping 55 neoantigen-specific TCR clonotypes (NeoTCRs) from 10 metastatic human tumors, including breast, melanoma, and colon, to their single-cell transcriptomes, Lowery et al. identified signatures of CD8+ and CD4+ neoantigen-reactive TILs. Neoantigen-specific TILs exhibited tumor-specific clonal expansion [67]. In phase I/II feasibility study with TIL therapy for metastatic melanoma, immune monitoring using exome sequencing demonstrated that neoantigen-specific T cells were detectable in TILs. In the two CR patients in whom neoantigen-specific T cell responses were analyzed, persistence of neoantigen-specific T cell was detectable for up to 3 years after TIL infusion [35]. Achilles Therapeutics (https://achillestx.com) developed VELOSTM manufacturing process to selectively expand clonal neoantigen-specific TILs (cNeT) by co-coculturing TILs with autologous dendritic cells-loaded peptides corresponding to the patient’s identified neoantigens.
The products are currently under clinical studies with patients with NSCLC (NCT04032847) and melanoma (NCT03997474) [68].
Phenotypic markers
Various technological developments in recent years enabled investigators to identify and sort tumor-reactive TILs based on phenotypic markers, such as programmed cell death 1 (PD-1), CD39-/CD69-, and CD39+/CD103+. These enrichment strategies would improve clinical efficacy, and further development for clinical manufacturing and clinical study are needed.
PD-1
PD-1 is highly expressed on tumor-reactive T cells; Inozume et al. showed that sorted and expanded CD8+PD-1+ T cells in tumor digests showed much higher tumor-specific IFN-γ production compared with CD8+PD-1- T cells and PD-1 receptor can be a useful biomarker for enriching tumor-specific T cells from fresh melanomas [69]. Similarly, Fernandez-Poma SM et al., reported that PD-1+CD8 TILs maintain the tumor-specific TCRs repertoires and their ability to recognize tumor cells after in vitro expansion compared to PD-1- or bulk CD8 TILs. In tumor mice model, PD-1+CD8 TILs improved the survival compared to PD-1- CD8 TILs (p < 0.05), and the combination with PDL-1 blockade enhanced tumor regression (p < 0.001) compared to PDL-1 blockade alone, although the fold expansion of PD-1+CD8 TILs was 10 times lower than that of PD-1- cells, which suggested outgrowth of PD-1- cells was the limiting factor in the tumor specificity of cells derived from bulk CD8 TILs. The combined administration of anti-PDL-1 mAb with PD-1+TILs would be critical in the treatment of large established tumor [70]. PD-1-selected TIL therapy was withdrawn unfortunately due to funding issue (NCT04223648).
CD39-/CD69-
Single-cell analysis of TILs from 7 CRs and 9 NRs by mass cytometry (CyTOF) revealed heterogeneous expression of 34 T cell surface markers (CD2, CD3, CD4, CD5, CD7, CD8a, CD9, CD11a, CD16, CD25, CD27, CD28, CD39, CD44, CD45, CD45RA, CD45RO, CD49D, CD57, CD69, CD95, IL7R, OX40, 41BB, CTLA4, KLRB1, CXCR3, CCR4, CCR5, CCR7, LAG3, ICOS, PD-1, and TIM3). Machine learning-based unsupervised clustering to define T cell clusters with activation/exhaustion states to classify patients according to their clinical outcome revealed CD69 and CD39 expression as two crucial features of most clinical relevance and associated with complete cancer regression. Manual visualization of TIL CyTOF profiles identified a cluster, which was fourfold more abundant in complete responder relative to non-responder (corrected p = 0.0264), corresponded to CD8+ T cells with abundant CD44, CD27, CD28, and low expression of TIM3, characterized in prior studies as memory-like and stem-like T cells. Higher numbers of CD8+CD39-CD69- cells in the infused TIL products were significantly associated with improved PFS (p < 0.0001, HR = 0.255, 95% CI 0.1257–0.5186) and melanoma-specific survival (MSS, p < 0.0001, HR = 0.217, 95% CI 0.101–0.463) in metastatic melanoma who enrolled and underwent TIL infusion. Furthermore, in tumor mice model, isolated CD39-CD69- CD8+ T cells let to substantial tumor regression and improved survival in a dose-dependent manner compared to CD39+CD69+ CD8+ T cells.
These findings suggest the strategies to isolate and expand stem-like neoantigen-specific T cells, or the engineering of T cells to have stem-like attributes, might provide opportunities for the development of more effective T cell-based immunotherapies [71] (CD39-CD69-).
CD103+/CD39+
Duhen et al. identified a unique population of co-expression of CD39 and CD103 in CD8 TILs, which have a tissue-resident memory T cells (TRM) phenotype and express elevated levels of exhaustion markers. CD103+CD39+ double-positive (DP) TILs exhibited over 2 times greater tumor reactivity against autologous tumor compared to the double negative (DN) or single positive (SP) CD8 TILs. High frequency (above the mean frequency; 32.9%) of DP CD8 TILs is associated with better OS compared to low frequency (below 32.9%) of DP TILs in a cohort of HNSCC (3-year survival: 89% vs. 56%; p = 0.0191). In addition, sequencing of the CDR3 region of the TRB genes revealed that DP CD8 TILs were more oligoclonal compared to memory CD8 T cells in the peripheral blood and to DN and SP CD8 TILs. These indicate that using CD39 and CD103 to identify tumor-reactive CD8 TILs in solid tumors will lead to important mechanistic insights, and selection of those population possibly enhances clinical outcome of TIL therapy [72].
CD137+(4-1BB)
A member of the TNF-receptor (TNFR) superfamily, CD137 (4-1BB; TNFRS9) is known as inducible costimulatory receptor and expresses on activated T and natural killer (NK) cells [73]. CD137+ cells positively isolated by flow sorting or magnetic beads were enriched populations as functional and antigen-specific T cells [74, 75]. Seliktar-Ofir et al. successfully isolated CD137+ cells by magnetic bead separation, and these cells demonstrated significantly increased a cytotoxicity against autologous tumor cells compared to unselected cells (% cytotoxicity of CD137+ fraction 52.3 ± 10.6%; % cytotoxicity of unseparated 6.6 ± 6.6%; p ≤ 0.007). The CD137 + fraction was examined for large-scale expansion. The CD137+ fraction reached a similar fold expansion as unseparated TIL (p = 0.78) or CD137− TIL (p = 0.12) on day 14. The CD137+ fraction of TIL recognized the mutated peptide increase by 3.2-fold compared to unseparated TIL. The direct implementation of the CD137 separation method may improve the clinical outcome of TIL therapy [76]. The phase II trial with 4-1BB selected TIL for metastatic melanoma patients was terminated unfortunately due to low accrual and change in research focus (NCT02111863).
High-potency TIL induction (Fig. 1B)
Efficient expansion of TILs is essential for manufacturing and successful clinical effects. Addition of agonistic 4-1BB antibody improved TIL expansion from primary bladder tumors regardless of pre-treatment with chemotherapy, and they exhibited tumor reactivity against autologous tumor cells [77, 78]. Notably, adding 4-1BB co-stimulation promoted the functional enhancement of CD8 TILs as well as enhancement of anti-PD-1-mediated reinvigoration of exhausted CD8 TILs from both the primary tumor sites and the metastatic sites of patients with ovarian cancer [79]. Targeting 4-1BB together with anti-PD-1 blocking antibodies in TIL culture could be a promising strategy for improving the poor responses to immunotherapy in metastatic ovarian cancer.
OX 40 (CD134, TNFRSF4) is a potent costimulatory receptor that can potentiate T cell receptor signaling on the surface of T cells [80]. Administration of OX40 promotes proliferation and survival of T cells via the NF-kB pathway and has been shown to enhance CD8 infiltration and decreases immune suppression in mouse model [81]. Anti-OX40 agonistic antibody on the ex vivo expansion significantly increased CD8+ T cells and maintained TCR Vβ repertoire [82].
Engineering TIL (Fig. 1C)
There are several directions to engineering TIL using genetic engineering techniques to (1) enhance antitumor activity, (2) improve ex vivo expansion efficiency, (3) decrease TIL suppression and exhaustion, (4) reduce IL-2 toxicity.
Genetical engineering to enhance antitumor activity
CRISPR
Recent studies have explored gene editing approaches, such as “clustered regularly interspaced short palindromic repeats-Cas (CRISPR-Cas9),” to enhance TILs’ tumor-killing potential. By editing specific genes involved in T cell suppression and exhaustion, researchers try to create TILs with improved cytotoxicity and resistance to immunosuppression [83]. Fix SM et al. implemented CRISPR/Cas9-mediated knockout of transforming growth factor beta receptor 2 (TGFBR2), which is TGF-β-resistant TILs from ovarian cancer patients before undergoing a REP. TGFBR2-knockout TILs are protected from the immunosuppressive effects of TGF-β (Fig. 2A) on effector cytokine production, proliferation, and cytotoxicity, when compared to non-transfected control and Cas9 mock transfected TIL (p < 0.001). However, it is worth noting that these approaches of CRISPR modified TILs did not alter the ex vivo expansion efficiency, immunophenotype, nor the TCR clonal diversity of TIL, and may not improve the treatment efficacy, especially for “cold tumors” [84]. Similarly, Arthofer et al. reported that genetic editing of the cytokine-induced SH2 protein (CISH) enhanced T cell effector function. CISH is a novel intra-cellular immune checkpoint molecule and an important negative regulator of T cell signaling and antigen-specific effector function [85]. In a pre-clinical mice model, CISH knockout results in enhanced tumor regression when combined with PD-1 mAb blockade [86]. Intima Bioscience, Inc. (https://www.intimabioscience.com) is currently conducting a clinical trial initially at the University of Minnesota Masonic Cancer Center for patients with metastatic gastrointestinal epithelial cancer administering TIL which CISH has been inhibited using CRISPR (NCT04426669).
Mechanistic overview of the next-generation TIL strategy. A CRISPR/Cas9-mediated knockout of TGFBR2: knockout of the TGF-β receptor II (TGFBR2) gene would render T cells resistant to TGF-β-mediated immunosuppression, consequently, increase effector function and persistence. B IL-15/IL-15Rα complex named IL-15 superagonist (sIL-15) greatly enhances IL-15 bioactivity. The Fc domain of αPD-1 antibody to conceal sIL-15 binding to the IL-15Rβ. Anti-PD-1 antibody not only anchored the concealed sIL-15 on PD-1+CD8+ T cells directly but also exposed sIL-15 activity to these cells. This would enable to decrease toxicity while maintaining efficacy
Transduce TIL using a retrovirus platform
The chemokine receptor CXCR2 and its ligands are intimately involved in tumor regulation and growth, and its functional inhibition shows promising results in several cancer types [87]. By using CXCR2, it facilitated TIL migration into the tumor. This modification displayed an improved in vivo antitumor activity [88, 89]. Forget MA et al. optimized procedure to genetically modify TIL. The transduction levels of CXCR2 at infusion were ranging from 31.28 to 57.82% and expanded raged from 465- to 3096-fold expansion [4]. CXCR2 and nerve growth factor receptor (NGFR) transduced TIL for treating metastatic malignant melanoma had been conducted and completed at M.D. Anderson Cancer Center (NCT01740557) as of April 2023.
Interleukin 12 (IL-12) enhances activity of CD4 and CD8 T cells, and infusion of IL-12 can mediate antitumor immunity in animal models [90]. However, systemic administration of IL-12 to cancer patients resulted in minimal efficacy and severe toxicity [91]. To improve TIL therapy, genetic engineering of TIL with a gene encoding IL12 was developed to deliver the potent cytokine selectively to the tumor site. In its first-in-man trial, 10 of 16 (63%) achieved an objective response; however, significant toxicities were seen such as liver dysfunction, high fevers (exceeding 40 °C), and sporadic life-threatening hemodynamic instability. Further scientific research and technology development are required before this approach can be safely used in the treatment of cancer patients [92].
Reprograming TIL
Another unique attempt is to reprogram exhausted TIL that possess T cell receptors (TCR) specific for tumor antigens into induced pluripotent stem cells (iPSC) to rejuvenate them for more potent ACT [93]. Islam et al. optimized the method to selectively reprogram tumor antigen-specific T cells from heterogeneous TIL populations by coculturing with autologous tumor cells and sorting the PD1+4-1BB+ CD8+ T cell population before reprogramming ACT [93]. Lyell (https://lyell.com) has developed “Epi-R reprogramming technology” to enrich CD8+T cells that express costimulatory markers (CD27, CD62L, and CD127) and stem-like T cells (CD39-CD69-), to enhance stemness (gene expression of CD28, TCF7, KLF3, SELL, BACH2, and LEF1), to reduce exhaustion associated genes (ENTPD1, TIGIT, CTLA4, LAYN, and LAG3), potential durable expansion, maintain polyclonality of TILs during production, and to enrich the TIL product with tumor-reactive T cells (LYL845) [94, 95]. The phase I clinical study using LYL845 for patients with relapsed and/or refractory metastatic or locally advanced melanoma, with expansion cohorts for patients with melanoma, NSCLC, and CRC is currently ongoing (NCT05573035), and initial data are expected in 2024.
Double trigger approach
A CD28/CD40-based chimeric CoStimulatory Antigen Receptor (CoStAR)-transduced TIL is distinctive therapeutic strategy that is led by Instil Bio. CoStAR-transduced TILs are expected to be elevated cytokine secretion, such as IFN-g, TNF-a and IL-2, enhanced tumor killing in vitro and in vivo, increased proliferative response, and reduced activation-induced cell death (AICD) [96]. Instil Bio is currently evaluating the product as ITIL-306 in their clinical trials with first patient.
Reduce IL-2 toxicity
Current TIL treatment regimens require high-dose IL-2 administration to support TIL survival in vivo, which limits their clinical applications due to IL-2-related toxicity. Obsidian Therapeutics (https://obsidiantx.com) is engineering TIL with membrane-bound IL-15 (mbIL15) to eliminate the dependence of TIL on exogenous IL-2, and they successfully expanded mbIL15-engineered TIL from both CRC and sarcoma ex vivo. A Phase I clinical trial of OBX-115 (engineered TIL product armed with mbIL15), a cytokine that is designed to remove the need for concomitant IL2 therapy, is currently evaluated in patients with metastatic melanoma (NCT05470283).
Another approach is an engineered an anti–PD-1 fusion with IL-15-IL-15Rα, whose activity was geographically concealed by immunoglobulin Fc region with an engineered linker (αPD-1-IL-15-R) to bypass systemic NK cells. Systematic administration of αPD-1-IL-15-R elicited extraordinary antitumor efficacy with undetectable toxicity. Mechanistically, cis-delivery of αPD-1-IL-15-R vastly expands tumor-specific CD8+T cells for tumor eradication. Additionally, αPD-1-IL-15-R upregulated PD-1 and IL-15Rβ on T cells to create a feedforward activation loop, thus rejuvenating TILs resulted in reducing tumor burden, improving survival, and also suppressing tumor metastasis compared to control (p < 0.0001). Collectively, renavigating IL-15 to tumor-specific PD-1+CD8+T cells, αPD-1-IL-15-R elicits effective systemic antitumor immunity [97] (Fig. 2B).
Recruiting and accumulating intravenously administered TIL within solid tumors
The challenge of recruiting and accumulating intravenously administered cells within solid tumors, such as in TIL therapy, is a critical issue in current adoptive cell therapies. This process is vital for enhancing the effectiveness of these therapies. Several strategies are explored to address this challenge. One approach involves enhancing the homing capabilities of TILs to the tumor site. Research indicates that tumor blood vessels play a crucial role in this process. High endothelial venules (HEVs), which are specialized blood vessels found in some tumors, are particularly effective at recruiting lymphocytes from the bloodstream into cancerous tissues [98,99,100,101,102]. Targeting these HEVs or engineering TILs to better recognize and home to these structures could improve cell accumulation within tumors.
Another strategy is the use of combination therapies [103]. For instance, combining TIL therapy with immune checkpoint inhibitors like anti-PD-L1 has shown promise. This combination can enhance the recruitment and persistence of TILs in the TME by overcoming local immunosuppression and promoting T cell infiltration into the tumor [104, 105]. Moreover, optimizing the pre-conditioning regimens for patients before TIL infusion can sensitize tumor vasculature, making it more receptive to T cell infiltration [106, 107]. This involves using treatments that induce a more favorable TME for T cell homing and survival. However, determining the predictive value of PD-L1 expression in the context of combination therapy presents significant challenges. These limitations include therapy-induced changes in the immune microenvironment and the inherent heterogeneity of PD-L1 expression within tumors [108, 109]. Therapy can alter the local immune landscape, potentially affecting PD-L1 levels and their interpretability. Additionally, the variability of PD-L1 expression within different tumor regions complicates its use as a consistent predictive biomarker.
Overall, addressing the recruitment and accumulation of intravenously administered cells within tumors is multifaceted, involving improvements in cell engineering, combination therapies, and patient pre-conditioning. Further research and clinical trials are crucial to refine these strategies and enhance the efficacy of adoptive cell therapies against solid tumors.
In-depth understanding of TIL clonality through novel technologies
Single-cell sequencing on TIL to define active pharmaceutical ingredient
Tumor-reactive T cells have been demonstrated to bear a unique gene signature profile that can be captured by a single-cell sequencing analysis. Lowery et al. identified gene expression signatures of CD8+ and CD4+ neoantigen-reactive TILs, which can be considered as the “active pharmaceutical ingredient” in conventional pharmaceutical considerations. Specifically, this study [67] utilized single-cell RNA and T cell receptor sequencing to establish a training dataset, comprising 45,676 tumor-infiltrating lymphocytes (TILs) from 10 metastatic human tumors, and experimentally determined 55 neoantigen-specific T cell receptor clonotypes (NeoTCRs) as true positive. Gene expression signatures were subsequently identified for CD8+ and CD4+ neoantigen-reactive TILs, encompassing 243 and 40 genes, respectively. To assess the robustness of these gene signatures, an independent test set was employed, revealing experimental confirmation for 37 out of 73 predicted T cell receptor clonotypes (positive predictive value = 50.7%). It is worthy to note that only a specific subset of NeoTCRs, characterized by reduced differentiation and a more stem-like phenotype, demonstrated associations with both checkpoint inhibitor responses and adoptive cell therapy [71, 110,111,112].
Ongoing challenges transforming laboratory therapy into industrial product
Robust CMC
Routinely manufacturing of TIL presents several challenges if it becomes a therapeutic option. As a pharmaceutic product, the first priority in manufacturing is robustness. TIL shares many challenges with other autologous therapies.
-
1.
Variability in patient samples: each patient is unique with different clinical manifestation, and the intrinsic characteristics of tumor tissue as the starting material for TIL manufacturing can vary significantly in terms of quality and quantity.
-
2.
Scaling up the manufacturing of TILs from small batch to a larger clinically relevant scale can be complex.
-
3.
Quality control on materials, such as human AB serum and feeder cells, is critical for TIL expansion and activation. Commercially available human AB serum varies significantly based on source and lot [113, 114].
-
4.
Maintaining the TILs in optimal culture conditions, including appropriate temperature, oxygen levels, and nutrient supply, is critical for TIL expansion and function.
-
5.
Contamination with bacteria, fungi, or other microorganisms can compromise the quality and safety of product. Robust procedures for aseptic processing, ideally closed system, and quality control are essential.
-
6.
Managing the supply chain for reagents, culture media, and equipment is vital to ensure consistent manufacturing since variations in these materials can affect the final product.
-
7.
Robust analytical methods are needed to characterize the final product, including assessments of cell viability, phenotype, potency, and purity.
Overall, achieving robust CMC for TIL therapy manufacturing involves addressing these challenges to ensure that final product is safe, effective, and consistently reproducible for patient treatment.
Health economy considerations
Recent years, as the pharmaceutical development of cell and gene therapies is becoming more exciting, a number of therapies have been introduced into the clinical applications and an exceptionally substantial number of candidates are in the pipelines. We all need to be mindful with their soaring prices and affordability of patients and even the affluent society like the USA.
The excessive cost of immunotherapy treatments is an additional and significant challenge. For example, immune checkpoint inhibitor therapies typically range in cost from $103,400 to $168,948, with an average cost of $148,431 [115, 116]. Newer treatments like ACT can be even more expensive than traditional methods.
While health insurance can reduce much of the financial burden for patients and their families, out-of-pocket costs continue to rise. This substantial expense can limit access for many patients and imposes a considerable financial burden on healthcare systems.
Scaling up TIL therapy production to treat a larger number of patients efficiently may help reduce costs per patient. Economies of scale can make the therapy more cost effective over time. Evaluating the effectiveness of TIL therapy compared to other cancer treatments, such as chemotherapy, radiation therapy, or immunotherapies like CAR-T cell therapy, is essential. This involves considering factors like response rates, survival outcomes, and quality of life.
Cost and manufacturing challenges
TIL therapy's manufacturing process is indeed complex and expensive, involving the isolation, expansion, and reinfusion of TILs into patients. The costs stem from the need for specialized facilities, extensive labor, and stringent quality control measures. Additionally, the personalized nature of the therapy means that each batch is patient specific, further driving up costs.
Strategies for improving scalability:
-
1.
Process automation: Automating various stages of TIL production, such as cell isolation and expansion, can significantly reduce labor costs and increase efficiency [117]. Advances in bioreactor technologies and automated culture systems hold promise in this area [2, 118, 119].
-
2.
Standardization and optimization: Developing standardized protocols for TIL expansion can improve consistency and reduce variability. Optimizing culture conditions to maximize TIL yield and potency can also enhance the overall efficiency of the process [2].
-
3.
Partnerships and infrastructure investments: Collaborations between academic institutions, industry, and regulatory bodies can foster innovation and share the burden of high initial costs. Investing in dedicated TIL manufacturing facilities and shared infrastructure can also help achieve economies of scale.
-
4.
Regulatory and reimbursement: Clear regulatory guidelines and supportive reimbursement frameworks are crucial for the widespread adoption of TIL therapy. Engaging with regulatory agencies early in the development process can facilitate smoother approval and market access.
TIL therapy can be expensive due to its autologous nature, which limits economies of scale when producing it lot by lot. The cost includes the entire process, from biopsy and cell isolation to cell expansion and infusion, as well as post-treatment monitoring and care. By addressing these cost and scalability challenges through innovative strategies and collaborative efforts, and by assessing the long-term cost-effectiveness of TIL therapy, we can pave the way for broader accessibility and adoption, ultimately benefiting more patients in need.
More importantly, continued research and clinical trials are necessary to refine TIL therapy protocols, improve its effectiveness, and reduce costs. Moore’s law might be realized in cell and gene therapy space like many other technological achievements over the past 5–6 decades. Health economies may need to allocate resources to support such research efforts. Identifying right patients who are most likely to benefit from TIL therapy is important to ensure that resources are directed toward those who are most likely to derive clinical benefits.
Ethical and regulatory aspects
Ethical and regulatory issues are critical components that must be thoroughly addressed in the development and implementation of TIL therapy.
-
1.
Patient selection criteria: The criteria for selecting patients for TIL therapy can raise ethical concerns, especially regarding fairness and equity. It is essential to ensure that these criteria are transparent, evidence-based, and designed to maximize benefits while minimizing potential biases [120]. Additionally, it is important to address how these criteria might exclude certain populations and to explore ways to make the therapy accessible to a broader range of patients.
-
2.
Access disparities: Access to TIL therapy may vary significantly based on geographic, socioeconomic, and institutional factors. Addressing these disparities is vital to ensure that all patients who could benefit from this treatment have the opportunity to receive it, regardless of their background or location.
-
3.
Regulatory hurdles: Navigating the regulatory landscape for new therapies can be complex and time-consuming. Ensuring that TIL therapy complies with all necessary regulations while maintaining a focus on patient safety and treatment efficacy is a challenging but essential task. Streamlining these processes without compromising ethical standards could facilitate faster and broader access to TIL therapy.
-
4.
Ethical landscape: Ethical considerations must be central to the development of TIL therapy. This includes ensuring informed consent, balancing risks and benefits, and safeguarding patient autonomy. Continuous ethical review and active stakeholder engagement are essential to address emerging ethical dilemmas and to ensure that patient welfare remains the highest priority.
Incorporating these considerations into the discussion can provide a more comprehensive understanding of the ethical landscape and future development of TIL therapy.
Conclusion
Undoubtedly, TIL therapy, like any groundbreaking medical advancement, presents its share of challenges. The complexity of the treatment process, human immune system, substantial costs involved, and the potential for adverse effects necessitate ongoing scrutiny and refinement. However, the medical community's commitment to overcoming these hurdles is inspiring, instilling confidence that TIL therapy will continue to evolve into a cornerstone of modern cancer care.
In conclusion, TIL therapy stands as the path toward more effective, personalized, and compassionate cancer treatments. Its potential to revolutionize the lives of patients and families cannot be overstated. As someone who values progress in medicine, I wholeheartedly commend the scientists, clinicians, and researchers who have brought TIL therapy to the forefront of oncology. This therapy is a testament to the indomitable spirit of human ingenuity and compassion in the face of one of the most challenging adversaries—cancer.
Recent research in TIL therapy has brought promising advancements that address key challenges in the field. Through enhancements in TIL selection, overcoming the suppressive TME, and the strategic used of genetic engineering, researchers are significantly enhancing the therapeutic efficacy and durability of TIL therapy. While TIL therapy still faces hurdles on the path to clinical implementation, the rapid progress in this field holds great promise for the future of cancer immunotherapy. Continued research efforts and collaborations between scientists, clinicians, and biotech companies are absolutely imperative to refine TIL therapy and expedite its translation into clinical practice, ultimately benefiting cancer patients worldwide.
References
Rosenberg SA, Spiess P, Lafreniere R (1986) A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233(4770):1318–1321
Donia M, Larsen SM, Met O, Svane IM (2014) Simplified protocol for clinical-grade tumor-infiltrating lymphocyte manufacturing with use of the Wave bioreactor. Cytotherapy 16(8):1117–1120
Besser MJ, Schallmach E, Oved K, Treves AJ, Markel G, Reiter Y, Schachter J (2009) Modifying interleukin-2 concentrations during culture improves function of T cells for adoptive immunotherapy. Cytotherapy 11(2):206–217
Forget MA, Tavera RJ, Haymaker C, Ramachandran R, Malu S, Zhang M, Wardell S, Fulbright OJ, Toth CL, Gonzalez AM, Thorsen ST, Flores E, Wahl A, Peng W, Amaria RN, Hwu P (2017) A novel method to generate and expand clinical-grade, genetically modified. Tumor-Infiltrating Lymph Front Immunol 8:908
Bianchi V, Harari A, Coukos G (2020) Neoantigen-specific adoptive cell therapies for cancer: making T-cell products more personal. Front Immunol 11:1215
Fouad A (2016) Cell-based therapies formulations: unintended components. AAPS J 18(4):844–848
Baust JM, Campbell LH, Harbell JW (2017) Best practices for cryopreserving, thawing, recovering, and assessing cells. In Vitro Cell Dev Biol Anim 53(10):855–871
Pellegrino S, Saunders J (2023) Iovance biotherapeutics announces US food and drug administration acceptance of the biologics license application of lifileucel for the treatment of advanced melanoma. IOVANCE
Rohaan MW, Wilgenhof S, Haanen JBAG (2019) Adoptive cellular therapies: the current landscape. Virchows Arch 474(4):449–461
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL et al (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P et al (2018) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20(1):31–42
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J et al (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396(10254):839–852
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS et al (2020) KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382(14):1331–1342
Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, Moreau P, Yakoub-Agha I, Delforge M, Cavo M, Einsele H, Goldschmidt H, Weisel K et al (2021) Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384(8):705–716
Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, Avigan D, Deol A, Htut M, Lesokhin A, Munshi NC, O’Donnell E, Stewart AK, Schecter JM, Goldberg JD, Goldberg JD et al (2023) Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 41(6):1265–1274
Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B (2023) From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol 14:1188049
Schendel DJ (2023) Evolution by innovation as a driving force to improve TCR-T therapies. Front Oncol 13:1216829
Zhang B, Ren Z, Zhao J, Zhu Y, Huang B, Xiao C, Zhang Y, Deng J, Mao L, Tang L, Lan D, Gao L, Zhang H, Chen G, Luo OJ (2023) Global analysis of HLA-A2 restricted MAGE-A3 tumor antigen epitopes and corresponding TCRs in non-small cell lung cancer. Theranostics 13(13):4449–4468
Al-Marayaty R, Pollack SM (2023) Pushing forward in sarcoma with a new TCR targeting NY-ESO-1. Cell Rep Med 4(8):101159
Pan Q, Weng D, Liu J, Han Z, Ou Y, Xu B, Peng R, Que Y, Wen X, Yang J, Zhong S, Zeng L, Chen A, Gong H, Lin Y, Chen J, Ma K, Lau JYN, Li Y, Fan Z, Zhang X (2023) Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A∗02:01 patients with advanced soft tissue sarcoma. Cell Rep Med 4(8):101133
Wolf B, Zimmermann S, Arber C, Irving M, Trueb L, Coukos G (2019) Safety and tolerability of adoptive cell therapy in cancer. Drug Saf 42(2):315–334
Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG (2018) Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer 6(1):102
Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ (2019) The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book 39:433–444
Warner AB, Corrie PG, Hamid O (2023) Tumor-infiltrating lymphocyte therapy in melanoma: facts to the future. Clin Cancer Res 29(10):1835–1854
Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, Thomas SS, Domingo-Musibay E, Pavlick AC, Whitman ED, Martin-Algarra S, Corrie P, Curti BD, Oláh J, Lutzky J et al (2021) Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol 39(24):2656–2666
Borch TH, Andersen R, Ellebaek E, Met Ö, Donia M, Svane IM (2020) Future role for adoptive T-cell therapy in checkpoint inhibitor-resistant metastatic melanoma. J Immunother Cancer 8(2):e000668
Yang JC (2015) Toxicities associated with adoptive T-cell transfer for cancer. Cancer J 21(6):506–509
Weber JS, Yang JC, Atkins MB, Disis ML (2015) Toxicities of immunotherapy for the practitioner. J Clin Oncol 33(18):2092–2099
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17(13):4550–4557
Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, Restifo NP, Rosenberg SA, Hinrichs CS (2015) Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 33(14):1543–1550
Chesney J, Lewis KD, Kluger H, Hamid O, Whitman E, Thomas S, Wermke M, Cusnir M, Domingo-Musibay E, Phan GQ, Kirkwood JM, Hassel JC, Orloff M, Larkin J, Weber J, Furness AJS et al (2022) Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts. J Immunother Cancer 10(12):e005755
Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Hölmich LR, Hendel HW, Met Ö, Andersen MH, thor Straten P, Svane IM (2016) Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 22(15):3734–3745
Ratto GB, Zino P, Mirabelli S, Minuti P, Aquilina R, Fantino G, Spessa E, Ponte M, Bruzzi P, Melioli G (1996) A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected nonsmall cell lung carcinoma. Cancer 78(2):244–251
Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM et al (2016) Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol 34(20):2389–2397
van den Berg JH, Heemskerk B, van Rooij N, Gomez-Eerland R, Michels S, van Zon M, de Boer R, Bakker NAM, Jorritsma-Smit A, van Buuren MM, Kvistborg P, Spits H, Schotte R, Mallo H, Karger M et al (2020) Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer 8(2):e000848
Aoki Y, Takakuwa K, Kodama S, Tanaka K, Takahashi M, Tokunaga A, Takahashi T (1991) Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res 51(7):1934–1939
Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, Gartner J, Jia L, Trebska-McGowan K, Somerville RP, Robbins PF, Rosenberg SA et al (2018) Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 24(6):724–730
Ben-Avi R, Farhi R, Ben-Nun A, Gorodner M, Greenberg E, Markel G, Schachter J, Itzhaki O, Besser MJ (2018) Establishment of adoptive cell therapy with tumor infiltrating lymphocytes for non-small cell lung cancer patients. Cancer Immunol Immunother 67(8):1221–1230
Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, Landin AM, Mullinax JE, Saller JJ, Saltos AN, Noyes DR, Montoya LB, Curry W, Pilon-Thomas SA, Chiappori AA, Tanvetyanon T et al (2021) Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med 27(8):1410–1418
Aydin AM, Hall M, Bunch BL, Branthoover H, Sannasardo Z, Mackay A, Beatty M, Sarnaik AA, Mullinax JE, Spiess PE, Pilon-Thomas S (2021) Expansion of tumor-infiltrating lymphocytes (TIL) from penile cancer patients. Int Immunopharmacol 94:107481
Balzeau J, Ravindran A, Wang X, Maisuria J, Lucchesi A, Yao H, Matsueda S (2023) Successful ex vivo expansion of tumor infiltrating lymphocytes with systemic chemotherapy prior to surgical resection. Cancer Immunol Immunother 72(10):3377–3385
Jia W, Guo H, Wang M, Li J, Yu J, Zhu H, Wu G (2023) High post-chemotherapy TIL and increased CD4+TIL are independent prognostic factors of surgically resected NSCLC following neoadjuvant chemotherapy. MedComm 4(1):e213
Rohaan MW, Borch TH, van den Berg JH, Met Ö, Kessels R, Foppen MHG, Granhøj JS, Nuijen B, Nijenhuis C, Jedema I, van Zon M, Scheij S, Beijnen JH, Hansen M, Voermans C et al (2022) Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med 387(23):2113–2125
Seitter SJ, Sherry RM, Yang JC, Robbins PF, Shindorf ML, Copeland AR, McGowan CT, Epstein M, Shelton TE, Langhan MM, Franco Z, Danforth DN, White DE, Rosenberg SA, Goff SL (2021) Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clin Cancer Res 27(19):5289–5298
Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Hölmich LR, Hendel HW, Met Ö, Andersen MH, Straten PT, Svane IM (2016) Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 22(15):3734–3745
Pillai M, Jiang Y, Lorigan PC, Thistlethwaite FC, Thomas M, Kirillova N, Bridgeman JS, Kueberuwa G, Biswas S, Velazquez P, Chonzi D, Guest RD, Roberts ZJ, Hawkins RE (2022) Clinical feasibility and treatment outcomes with nonselected autologous tumor-infiltrating lymphocyte therapy in patients with advanced cutaneous melanoma. Am J Cancer Res 12(8):3967–3984
Wargo JA, Reddy SM, Reuben A, Sharma P (2016) Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol 41:23–31
Qin SS, Melucci AD, Chacon AC, Prieto PA (2021) Adoptive T cell therapy for solid tumors: pathway to personalized standard of care. Cells 10(4):808
Zhao Y, Deng J, Rao S, Guo S, Shen J, Du F, Wu X, Chen Y, Li M, Chen M, Li X, Li W, Gu L, Sun Y, Zhang Z, Wen Q, Xiao Z, Li J (2022) Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: progressions and challenges. Cancers (Basel) 14(17):4160
Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T, Chen H, Wang C (2022) Immunotherapy: reshape the tumor immune microenvironment. Front Immunol 13:844142
Czajka-Francuz P, Prendes MJ, Mankan A, Quintana Á, Pabla S, Ramkissoon S, Jensen TJ, Peiró S, Severson EA, Achyut BR, Vidal L, Poelman M, Saini KS (2023) Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Front Oncol 13:1200646
Faraj JA, Al-Athari AJH, Mohie SED, Kadhim IK, Jawad NM, Abbas WJ, Jalil AT (2022) Reprogramming the tumor microenvironment to improve the efficacy of cancer immunotherapies. Med Oncol 39(12):239
Frankel T, Lanfranca MP, Zou W (2017) The role of tumor microenvironment in cancer immunotherapy. Adv Exp Med Biol 1036:51–64
Mullinax JE, Hall M, Prabhakaran S, Weber J, Khushalani N, Eroglu Z, Brohl AS, Markowitz J, Royster E, Richards A, Stark V, Zager JS, Kelley L, Cox C, Sondak VK et al (2018) Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Front Oncol 8:44
O’Malley D, Lee SM, Psyrri A, Sukari A, Thomas S, Wenham RM, Gogas H, Jazaeri A, Monk BJ, Rose PG, Rueda A, Finckenstein FG, Jagasia M, Fiaz R, Garelik B, Shi W, Desai A, et al (2021) Phase 2 efficacy and safety of autologous tumor-infiltrating lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor-naïve patients with advanced cancers. SITC, Washington
Olson D, Hong Y, Thomas S, Martín-Liberal J, Finckenstein FG, Wu X, Sulur G, Shi W, Larkin J (2023) Trial in progress: a phase 3 study (TILVANCE-301) to assess the efficacy and safety of lifileucel, an autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in combination with pembrolizumab compared with pembrolizumab alone in patients with untreat. ASCO, Chicago
Freedman RS, Edwards CL, Kavanagh JJ, Kudelka AP, Katz RL, Carrasco CH, Atkinson EN, Scott W, Tomasovic B, Templin S et al (1994) Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J Immunother Emphasis Tumor Immunol 16(3):198–210
Karbach J, Kiselicki D, Brand K, Wahle C, Sinelnikov E, Gustavus D, Hoffmeister H, Prisack HB, Atmaca A, Jäger E (2023) Tumor-infiltrating lymphocytes mediate complete and durable remission in a patient with NY-ESO-1 expressing prostate cancer. J Immunother Cancer 11(1):e005847
Huang H, Nie CP, Liu XF, Song B, Yue JH, Xu JX, He J, Li K, Feng YL, Wan T, Zheng M, Zhang YN, Ye WJ, Li JD, Li YF, Li JY, Cao XP, Liu ZM, Zhang XS, Liu Q, Zhang X, Liu JH, Li J (2022) Phase I study of adjuvant immunotherapy with autologous tumor-infiltrating lymphocytes in locally advanced cervical cancer. J Clin Invest 132(15):w157726
Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, Wunderlich JR, Somerville RP, Rosenberg SA (2015) Immunogenicity of somaticmutations in humangastrointestinal cancers. Science 350(6266):1387–1390
Amaria R, Knisely A, Vining D, Kopetz S, Overman MJ, Javle M, Antonoff MB, Tzeng CWD, Wolff RA, Pant S, Lito K, Rangel K, Fellman B, Yuan Y, Lu KH et al (2024) Efficacy and safety of autologous tumor-infiltrating lymphocytes in recurrent or refractory ovarian cancer, colorectal cancer, and pancreatic ductal adenocarcinoma. J Immunother Cancer 12(2):e006822
Zhao Y, Deng J, Rao S, Guo S, Shen J, Du F, Wu X, Chen Y, Li M, Chen M, Li X, Li W, Gu L, Sun Y, Zhang Z, Wen Q, Xiao Z, Li J (2022) Tumor infiltrating lymphocyte (TIL) therapy for solid tumor. Cancers 14(17):4160
Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi L (2012) Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J 18(2):160–175
Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, Restifo NP, Wunderlich J, Prieto PA, Hong JJ, Langan RC (2010) CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 16(24):6122–6131
Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME (2008) Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother 31(8):742–751
Robertson J, Salm M, Dangl M (2019) Adoptive cell therapy with tumour-infiltrating lymphocytes: the emerging importance of clonal neoantigen targets for next-generation products in non-small cell lung cancer. Immunooncol Technol 3:1–7
Lowery FJ, Krishna S, Yossef R, Parikh NB, Chatani PD, Zacharakis N, Parkhurst MR, Levin N, Sindiri S, Sachs A, Hitscherich KJ, Yu Z, Vale NR, Lu YC, Zheng Z, Jia L, Gartner JJ et al (2022) Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 375(6583):877–884
Kotsiou E, Hou TZ, Robinson J, Varsani S, Oakes T, Becker PD, Patel S, Mootien J, Craig A, Robertson J, Samuel E, Reading J, Rosario LD, Haynes A, Turajlic S, Islam F et al (2020) Next generation clonal neoantigen targeting T cells, generated using the PELEUSTM bioinformatics platform and the VELOSTM manufacturing method show superior reactivity and phenotypic characteristics than classical TIL products. Cancer Res 80:875
Inozume T, Hanada KI, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC (2010) Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T-cells. J Immunother 33(9):956–964
Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheño U, Elizalde E, Alignani D, Zubeldia N, Otano I, Conde E, Sarobe P, Lasarte JJ, Hervas-Stubbs S (2017) Expansion of tumor-infiltrating CD8+ T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy. Cancer Res 77(13):3672–3684
Krishna S, Lowery FJ, Copeland AR, Bahadiroglu E, Mukherjee R, Jia L, Anibal JT, Sachs A, Adebola SO, Gurusamy D, Yu Z, Hill V, Gartner JJ, Li YF, Parkhurst M, Paria B (2020) Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370(6522):1328–1334
Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang SC, Grunkemeier G, Leidner R, Bell RB, Weinberg AD (2018) Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun 9(1):2724
Chester C, Sanmamed MF, Wang J, Melero I (2018) Immunotherapy targeting 4–1BB: mechanistic rationale, clinical results, and future strategies. Blood 131(1):49–57
Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD (2007) Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110(1):201–210
Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ Jr (2014) CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res 20(1):44–55
Seliktar-Ofir S, Merhavi-Shoham E, Itzhaki O, Yunger S, Markel G, Schachter J, Besser MJ (2017) Selection of shared and neoantigen-reactive T cells for adoptive cell therapy based on CD137 separation. Front Immunol 8:1211
Poch M, Hall M, Joerger A, Kodumudi K, Beatty M, Innamarato PP, Bunch BL, Fishman MN, Zhang J, Sexton WJ, Pow-Sang JM, Gilbert SM, Spiess PE, Dhillon J, Kelley L, Mullininax J (2018) Expansion of tumor infiltrating lymphocytes (TIL) from bladder cancer. Oncoimmunology 7(9):e1476816
Innamarato P, Asby S, Morse J, Mackay A, Hall M, Kidd S, Nagle L, Sarnaik AA, Pilon-Thomas S (2021) Intratumoral activation of 41BB co-stimulatory signals enhances CD8 T cell expansion and modulates tumor-infiltrating myeloid cells. J Immunol 205(10):2893–2904
Leem G, Park J, Jeon M, Kim ES, Kim SW, Lee YJ, Choi SJ, Choi B, Park S, Ju YS, Jung I, Kim S, Shin EC, Lee JY, Park SH (2020) 4–1BB co-stimulation further enhances anti-PD-1-mediated reinvigoration of exhausted CD39+ CD8 T cells from primary and metastatic sites of epithelial ovarian cancers. J Immunother Cancer 8(2):e001650
Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, Moudgil T, Miller W, Haley D, Coffey T, Fisher B (2013) OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 73(24):7189–7198
Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD (2008) OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res 68(13):5206–5215
Ritthipichai K, Machin M, Fardis M, Chartier C (2018) Anti-OX40 agonistic antibody enhances ex vivo CD8+ TIL expansion with increased T-cell effector function. Am Assoc Cancer Res 78:LB-110
Tas L, Jedema I, Haanen JBAG (2023) Novel strategies to improve efficacy of treatment with tumor-infiltrating lymphocytes (TILs) for patients with solid cancers. Curr Opin Oncol 35(2):107–113
Fix SM, Forget MA, Sakellariou-Thompson D, Wang Y, Griffiths TM, Lee M, Haymaker CL, Dominguez AL, Basar R, Reyes C, Kumar S, Meyer LA, Hwu P, Bernatchez C, Jazaeri AA (2022) CRISPR-mediated TGFBR2 knockout renders human ovarian cancer tumor-infiltrating lymphocytes resistant to TGF-β signaling. J Immunother Cancer 10(7):e003750
Arthofer E, Chakraborty K, Viney L, Johnson MJ, Webber BR, Moriarity BS, Lou E, Choudhry M, Klebanoff CA, Henley T (2021) Genetic editing of CISH enhances T cell effector programs independently of 1 immune checkpoint cell surface ligand expression. biRxiv
Palmer DC, Webber BR, Patel Y, Johnson MJ, Kariya CM, Lahr WS, Parkhurst MR, Gartner JJ, Prickett TD, Lowery FJ, Kishton RJ, Gurusamy D, Franco Z, Vodnala SK, Diers MD et al (2022) Internal checkpoint regulates T cell neoantigen reactivity and 701 susceptibility to PD-1 blockade. Med 3(10):682-704.e8
Jaffer T, Ma D (2016) The emerging role of chemokine receptor CXCR2 in cancer progression. Translational Cancer Research
Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM, Hwu P (2002) Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther 13(16):1971–1980
Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, Whittington M, Yang Y, Overwijk WW, Lizée G, Hwu P (2010) Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res 16(22):5458–5468
Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK (1993) Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 178(4):1223–1230
Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL (1997) Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90(7):2541–2548
Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ, Hughes MS, Kammula US, Feldman SA, Toomey MA, Kerkar SP, Restifo NP, Yang JC, Steven A (2015) Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 21(10):2278–2288
Islam SMR, Maeda T, Tamaoki N, Good ML, Kishton RJ, Paria BC, Yu Z, Bosch-Marce M, Bedanova NM, Liu C, Kruhlak MJ, Restifo NP, Vizcardo R (2023) Reprogramming of tumor-reactive tumor-infiltrating lymphocytes to human-induced pluripotent stem cells. Cancer Res Commun 3(5):917–932
Harris BD, Patel Y, Ha NH, Kritikou J, Zhao L, Lou S, Siebert S, Fu-Sum E, Kishton R, Sundar P, Vodnala SK, Potluri S (2022) Epi-R™ technology produces a polyclonal TIL product (LYL845) with diverse tumor-reactive clones that have stem-like qualities and anti-tumor function
Patel Y, Harris BD, Bedard M, Kritikou J, Casas MG, Harms C, De Francesco M, Sundar P, Restifo NP, Lee G, Potluri S, Vodnala SK (2022) Epi-R™ technology produces a polyclonal TIL product (LYL845) with a greater expansion success rate across hot and cold tumors, improved product phenotype, and maintenance of TCR diversity. SITC
Bridgeman J (2023) A CD28/CD40 based chimeric costimulatory antigen receptor (CoStAR™) targeting folate receptor alpha enhances anti-tumour activity of tumour infiltrating lymphocytes. BSGCT annual conference
Shen J, Zou Z, Guo J, Cai Y, Xue D, Liang Y, Wang W, Fu YX, Peng H (2022) An engineered concealed IL-15-R elicits tumor-specific CD8+T cell responses through PD-1-cis delivery. Skip Nav Destination 219(12):e20220745
Hong SA, Hwang HW, Kim MK, Lee TJ, Yim K, Won HS, Sun DS, Kim EY, Ko YH (2020) High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. J Clin Med 9(8):2628
Park HS, Kim YM, Kim S, Lee WS, Kong SJ, Yang H, Kang B, Cheon J, Shin SJ, Kim C, Chon HJ (2021) High endothelial venule is a surrogate biomarker for T-cell inflamed tumor microenvironment and prognosis in gastric cancer. J Immunother Cancer 9(10):e003353
Colbeck EJ, Jones E, Hindley JP, Smart K, Schulz R, Browne M, Cutting S, Williams A, Parry L, Godkin A, Ware CF, Ager A, Gallimore A (2017) Treg depletion licenses T cell-driven HEV neogenesis and promotes tumor destruction. Cancer Immunol Res 5(11):1005–1015
Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, Cutting S, Ladell K, Wynn KK, Withers D, Price DA, Ager A, Godkin AJ, Gallimore AM (2012) T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res 72(21):5473–5482
Milutinovic S, Abe J, Godkin A, Stein JV, Gallimore A (2021) The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer 7(3):214–225
Wang C, Li M, Wei R, Wu J (2020) Adoptive transfer of TILs plus anti-PD1 therapy: an alternative combination therapy for treating metastatic osteosarcoma. J Bone Oncol 25:100332
Hall MS, Mullinax JE, Cox CA, Hall AM, Beatty MS, Blauvelt J, Innamarato P, Nagle L, Branthoover H, Wiener D, Schachner B, Martinez AJ, Richards AD, Rich CJ et al (2022) Combination nivolumab, CD137 agonism, and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Clin Cancer Res 28(24):5317–5329
Kverneland AH, Chamberlain CA, Borch TH, Nielsen M, Mørk SK, Kjeldsen JW, Lorentzen CL, Jørgensen LP, Riis LB, Yde CW, Met Ö, Donia M et al (2021) Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types. J Immunother Cancer 9(10):e003499
Nissani A, Lev-Ari S, Meirson T, Jacoby E, Asher N, Ben-Betzalel G, Itzhaki O, Shapira-Frommer R, Schachter J, Markel G, Besser MJ (2021) Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy. J Immunother Cancer 9(5):e001743
Hirai I, Funakoshi T, Kamijuku H, Fukuda K, Mori M, Sakurai M, Koda Y, Kato J, Mori T, Watanabe N, Noji S, Yaguchi T, Iwata T, Ohta S, Fujita T, Tanosaki R et al (2021) Adoptive cell therapy using tumor-infiltrating lymphocytes for melanoma refractory to immune-checkpoint inhibitors. Cancer Sci 112(8):3163–3172
Barbari C, Fontaine T, Parajuli P, Lamichhane N, Jakubski S, Lamichhane P, Deshmukh RR (2020) Immunotherapies and combination strategies for immuno-oncology. Int J Mol Sci 21(14):5009
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K (2018) Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 17(1):129
Miller BC, Sen DR, Abosy RA, Bi K, Virkud YV, Fleur MWL, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S et al (2019) Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20(3):326–336
Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, Freeman SS, Reuben A, Hoover PJ, Villani AC, Ivanova E et al (2018) Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175(4):998-1013.e20
Siddiqui I, Schaeuble K, Chennupati V, Marraco SAF, Calderon-Copete S, Ferreira DP, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, Luther SA, Speiser DE, Held W (2019) Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50(1):195-211.e10
Hopewell EL, Cox C, Pilon-Thomas S, Kelley LL (2019) Tumor-infiltrating lymphocytes: Streamlining a complex manufacturing process. Cytotherapy 21(3):307–314
Dwarshuis NJ, Parratt K, Santiago-Miranda A, Roy K (2017) Cells as advanced therapeutics: state-of-the-art, challenges, and opportunities in large scale biomanufacturing of high-quality cells for adoptive immunotherapies. Adv Drug Deliv Rev 114:222–239
Guirgis HM (2021) Costs of extended use of the immune checkpoint inhibitors in first-line non-small cell lung cancer. J Clin Pathways 7(10):32–36
Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Burke T (2018) Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ 21(12):1191–1205
Li A, Kusuma GD, Driscoll D, Smith N, Wall DM, Levine BL, James D, Lim R (2021) Advances in automated cell washing and concentration. Cytotherapy 23(9):774–786
Nikita S, Mishra S, Gupta K, Runkana V, Gomes J, Rathore AS (2023) Advances in bioreactor control for production of biotherapeutic products. Biotechnol Bioeng 120(5):1189–1214
Wu YY, Liu D, Naing MW (2020) Development of a closed and automated bioreactor technology for cell therapy manufacturing—a sharing of our journey. Regen Med 15(12):2335–2340
Wu M, Zhou S (2023) Harnessing tumor immunogenomics: tumor neoantigens in ovarian cancer and beyond. Biochim Biophys Acta Rev Cancer 1878(6):189017
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
SM developed the concept, coordinated author activities, revised the article, and provided final approval of the version to be submitted. LC, HL, HY, and FY contributed the revised the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsueda, S., Chen, L., Li, H. et al. Recent clinical researches and technological development in TIL therapy. Cancer Immunol Immunother 73, 232 (2024). https://doi.org/10.1007/s00262-024-03793-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03793-4