Abstract
Life history theory emphasizes that finite resources result in allocation trade-offs among the competing interests of self-maintenance, growth, reproduction, and survival. Environmental conditions, particularly during development, can influence these life history trade-offs, leading to the coupling of physiological and behavioural traits with life history strategies. Thus, populations may vary in the pattern of trait covariation, clustering along a fast-slow continuum, termed the extended pace-of-life syndrome (POLS) theory. We aimed to test how variation in ecological conditions influence life history trade-offs and their association with behaviour and physiology by comparing captive bred and wild-collected southern rainforest sunskink (Lampropholis similis). The captive bred skinks were the offspring of the wild-caught skinks, and all tests were conducted in the laboratory. We found that the groups differed, on average, in growth rate, body condition, thermal preferences, sprint performance, and activity. Counter to our expectation, wild-caught skinks exhibited a faster pace of life relative to captive-bred skinks despite experiencing more challenging environmental conditions. Furthermore, life history trade-offs were not detected, nor were traits correlated to form the syndrome. Studies are needed to identify the proximate mechanisms causing life history trade-offs and how they lead to the coupling, or decoupling, of physiological and behavioural traits. Such information will provide vital insight into how ecological forces drive the evolution of traits.
Significance statement
We aimed to test how variation in ecological conditions influence life history trade-offs and their association with behaviour and physiology by comparing captive bred and wild-collected individuals. We found that wild-caught individuals exhibited a faster pace of life relative to captive-bred individuals despite experiencing more challenging environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms cannot simultaneously maximise all of the traits which define their life histories (Le Lann et al. 2011). Instead, the finite energetic resources available constrains the range of life history strategies that are able to evolve (Stearns 1989). Organisms are forced into making budgetary compromises as to how these limited resources are allocated towards the competing interests of self-maintenance, growth, reproduction, and survival, resulting in trade-offs (Stearns 1989; Montiglio et al. 2018). Selection shapes the manner under which these life history trade-offs are resolved. Extrinsic selective pressures (i.e., predation, temperature, parasites) as well as intrinsic factors, namely physiology (i.e., metabolism, immunity), act in dictating the rate at which energy can be acquired, processed, and assimilated (Kearney 2012; Londoño et al. 2014). In maximizing fitness, such selective pressures favour the functional integration of traits, where the combination and direction of correlations between life history and physiology are adaptively modulated by the distinct ecological conditions that are experienced (Réale et al. 2010; Dammhahn et al. 2018; Polverino et al. 2018). As a result, life histories and physiologies can vary markedly among species (Araya-Ajoy et al. 2018).
The extended pace-of-life syndrome (POLS) theory describes this pattern of trait covariation. Specifically, this theory states that individuals occurring across environmental gradients (i.e., temperature, predation, and resource availability) differ in suites of physiological and behavioural traits that have coevolved with life history strategies, all of which cluster along a fast-slow continuum (Stearns 1989; Ricklefs 2002; Biro and Stamps 2008; Réale et al. 2010; Le Galliard et al. 2012). By varying trait expression, and thus the pace of life, each individual can adjust to their particular ecological situation in a multifaceted way (Jablonszky et al. 2018). At the fast end of the continuum, it is possible to prioritize current reproduction at the expense of survival (Montiglio et al. 2018). Boldness can assist in increasing energy uptake, fuelling an elevation of of metabolic rate, leading to earlier reproduction and higher rates of fecundity (Dammhahn et al. 2018). However, as this approach physiologically expensive and may increase vulnerability to predation, it could come at a cost in terms of reduced longevity (Dammhahn et al. 2018). In contrast, individuals at the slow end may invest more towards future reproduction and thus survival (Dammhahn et al. 2018), with less risk behaviours and movement, which acts to slow the rate of energy acquisition and expenditure, and ultimately reducing metabolism and delaying reproduction (Réale et al. 2010). Yet, this reduction in rates among slow individuals is compensated for by higher survivorship (Wolf et al. 2007; Réale et al. 2010).
Much of the work investigating the extended POLS emphasizes that the trade-off between current and future reproduction underlies the integration of traits within the syndrome (Jablonszky et al. 2018; Polverino et al. 2018). However, alternative causal mechanisms have also been shown to lead to correlations among life history, physiology, and behaviour, and thus, may play an equally important role in the manifestation of the extended POLS (Monceau et al. 2017; Jablonszky et al. 2018; Montiglio et al. 2018). One such trade-off is that between growth rate and mortality (Stamps 2007). This is due to the fact that behavioural and physiological traits, which support high rates of growth through their mediating effects of resource acquisition and assimilation and predator avoidance, also increase the rate of mortality (Gotthard 2000; Biro et al. 2007; Stamps 2007). For example, fast growth places high demands on food uptake and processing, compelling individuals to increase their activity under all levels of risk (Sih et al. 2004; Biro and Stamps 2010). In addition, fast growth requires energy, which limits the energy available for locomotor performance and body repair (Arendt 2003). This growth-mortality trade-off was observed among anuran tadpoles (Bufo americanus and Scaphiopus hamondi), where individuals with rapid growth had slower burst speeds (Arendt 2003). Similarly, in three-spined stickleback (Gasterosteus aculeatus), Ward et al. (2004) found that faster growing fish sought out greater resource gains at the expense of increased predation risk. Fast-growing individuals not only resumed feeding more quickly following a simulated predator attack, but they also occupied the front positions within the shoal, both of which increased their vulnerability to being preyed upon.
A number of ecological factors influence the existence and structure of the extended POLS (Dammhahn et al. 2018; Montiglio et al. 2018). Of particular importance are extrinsic forces experienced during development, namely resource availability (Montiglio et al. 2018). Developing individuals might vary in the amount of food they are able to acquire due to differences in their perception of threat, thermal environments, or behaviour (i.e., boldness or locomotor performance). The degree to which resources are obtained in early life not only serves in establishing an individual’s growth trajectory that persists into adulthood (Madsen and Shine 2000; Stamps 2007), but it also contributes to shaping and maintaining the among-individual trait correlations and trade-offs underpinning the extended POLS through pleiotropic effects (Han 2015). Food availability during this ontogenetic stage can, therefore, act as a permanent source of variation in growth rate and mortality and has long-lasting consequences on an individual’s phenotype and syndrome expression (Royauté et al. 2018). Ultimately, differences in energetic uptake among individuals should favour either a slow or fast paced life strategy. In some situations, individuals which are food-limited may exhibit a slower pace of life relative to those that having high acquisition rates, as they have fewer resources to dedicate to somatic growth and behavioural expression, causing a reduction in metabolic rate (Dammhahn et al. 2018). Low rates of food uptake would ultimately reduce metabolism and preclude the need to express high levels of activity and boldness. Nevertheless, the amount of resources available can also work in altering the strength and direction of correlations among POLS traits (Santostefano et al. 2017mäläinen et al. 2018). Theoretical and empirical work suggests that significant deficiencies or excesses of resources can prompt plastic changes in trait expression that can mask expected phenotypic correlations among life history, behaviour, and physiology (Han 2015) (Jablonszky et al. 2018). Subsequently, limited resources may cause individuals to increase activity and boldness in order to acquire food and avoid starvation. Whereas, when resource availability is high, energetic requirements are easily met, reducing the need to forage frequently or during times of elevated predatory threat, and as a result, enabling both growth and longevity to be simultaneously maximized. Such plastic responses to temporal or spatial variation in resource availability may have contributed to the inability to detect allocation trade-offs or predicted associations with behaviour in previous studies (Jablonszky et al. 2018; Piquet et al. 2018; Royauté et al. 2018; Salzman et al. 2018).
Captive and wild populations of animals differ in the key environmental conditions which are posited as influencing the extended POLS (Mathot and Frankenhuis 2018). Wild individuals are confronted with managing their energy budgets when faced with fluctuations in food availability, environmental temperatures, as well as predator density. Those bred in captivity, by contrast, have few challenges, as food is provided ad libitum and the threat of predation is eliminated, allowing food uptake to be increased with few costs. Captivity also provides predictable climatic conditions, allowing maximisation of the rate of digestion and growth. Given the clear distinction in environmental regimes between wild and captive populations, investigating the extended POLS within this system would provide a promising avenue in determining the ecological conditions that promote life history trade-offs and their correlations with behaviour to emerge. Surprisingly, unlike the use of climatic and predation gradients, this approach has rarely been considered (but see Zavorka et al. 2015).
Thus, our aim was to evaluate the extended POLS using captive-bred and wild-caught individuals of the southern rainforest sunskink (Lampropholis similis). The captive-bred individuals were the offspring of the wild-caught individuals. As the trials were conducted across to two different research laboratories there were some differences (as outlined below in the Methods) in the housing conditions, experimental protocols (including the age at which the individuals were tested), and time spent in captivity. However, such temporal differences are unlikely to have a substantial impact on our research, as our previous studies on a related species (the delicate skink, Lampropholis delicata) have indicated that both behaviour (Polverino et al. 2023) and thermal physiology and locomotor performance (Goulet et al. 2017) are consistent and predictable across time in Lampropholis skinks. We began by testing if captive-bred and wild-caught skinks differed in their investments towards growth and body condition (proxy for mortality), as well as physiology (thermal preferences), morphology (body size), locomotor performance, and behaviour (activity, exploration, and sociality). Finally, syndrome structure between treatment groups was compared to determine if divergent ecological conditions caused variation in the extended POLS. It was expected that the challenging conditions experienced by the wild-caught population would prompt life history trade-offs and trait correlations to emerge, whereas high food availability and low predation risk would cause the usual life history trade-offs and phenotypic correlations to be masked within the captive-bred population. Alternatively, if resource availability drives the POLS, we expected that captive animals would have a faster pace. However, if competition or predation pressure drives the POLS, we expected to see a faster pace in wild-caught individuals.
It should be noted that the traits included in this study, although not formally included in the restricted definition of the POLS, follows the suggestion put forth by Dammhahn et al. (2018) where any behavioural, morphological, or physiological character associated with life history trade-offs should be considered within this framework. Thus, we included traits which act in facilitating resource acquisition because this process relates directly to growth rate (and its trade-off with mortality). We also included thermal preferences as a physiological trait given that body temperature has a strong influence on these organism’s growth rate, energy assimilation rate, performance, and behavioural expression (Angilletta 2001). Higher temperatures would increase vital rates, thus prompting a fast lifestyle, with the opposite being true of cooler temperatures. Similarly, because sociality could also affect net energy gain and mortality through the processes of predator dilution and reduced investment in anti-predatory behaviour, this trait was also considered.
Methods and materials
Field collection and animal husbandry
Lampropholis similis is a small (adult snout-to-vent length [SVL]: 45 mm) terrestrial skink endemic to the Wet Tropics of northeastern Queensland, Australia. This species ranges from Mt Bellenden Ker Range in the north to Hervey Range and The Pinnacles in the south (~ 16,200 km2) (Singhal et al. 2018). Within its rainforest habitat, this species basks in loose aggregations within canopy openings and forest edges. It is insectivorous and actively searches for prey within the leaf litter. Adult skinks (our wild-caught cohort) of each sex were collected from the southern limit of this species’ distribution, within the Hervey Range, between June 2013 and January 2014 (Phillips et al. 2016; de Jong et al. 2022). This ecologically-isolated population occurs at a low elevation (182 m above sea level) where it experiences a relatively dry climate for a rainforest specialist (Martins et al. 2018).
Wild-caught skinks were transported to James Cook University in Townsville, Australia, and housed individually in plastic tubs (340 × 120 × 160 mm) within a temperature-controlled room (21 °C) with a 10 h light: 14 h dark cycle (0800–1800 h) for six months (Llewelyn et al. 2017; de Jong et al. 2022). Housing containers were lined with shredded paper and had dishes of moist vermiculite and Sphagnum moss. The housing containers were kept on heat racks that created a thermal gradient of 22–40 °C within each container, allowing natural thermoregulatory behaviour. Skinks were fed crickets (Acheta domesticus) three times weekly and provided water ad libitum. To produce offspring, each skink was then placed in a shared housing container with a randomly selected mating partner. Eggs were collected as they were produced by checking containers daily and placed individually in airtight containers (84 ml) that were two-thirds filled with moist vermiculite (50:50 ratio of vermiculite to water by weight). Egg containers were put in incubators set at one of two temperatures (23–26 °C). It should be noted that these incubation temperatures did not affect adult phenotype (Llewelyn et al. 2017). Offspring (our captive-bred cohort) hatched between January 2014 and 2015, and were housed individually as previously described for wild-caught skinks.
In 2015, both wild-caught and captive-bred skinks were transported to the temperature-controlled animal housing facility at Monash University, Melbourne, Australia (de Jong et al. 2022). Skinks were housed in same sex groups of six in plastic containers (300 × 230 × 370 mm) and maintained at 22 °C with a 14 h light: 10 h dark cycle (0600–2000 h). The housing other housing conditions were as per James Cook University, except that a thermal gradient of 20 to 35 °C was provided. Both wild-caught (n = 21) and captive-bred (n = 22) populations used in this study were individually marked with a unique toe clip sequence for identification during experiments.
Data collection
Body condition and growth rate
SVL and body mass were measured in captive bred skinks until they reached adult SVL. Measurements were then repeated in adulthood immediately before the onset of behavioural assays.
To assess life history trade-off between growth rate and mortality, body condition was used as a proxy for longevity: studies have indicated that an individual’s body condition is predictive of longevity and has also been previously considered within the POLS framework (Bowers et al. 2014; Piquet et al. 2018). It has also been suggested that longevity is not a good predictor of an individual’s POLS among short-lived species because there is little scope for variation (Araya-Ajoy et al. 2018). Body condition was therefore considered a better measure of the trade-off between growth rate and mortality (Araya-Ajoy et al. 2018). We used the ratio of mass to SVL as our measure of body condition (Bowers et al. 2014; Milenkaya et al. 2015).
Growth rate was calculated as follows:
where t1 and t2 denote successive days of measurement. For the wild-caught population, skinks were collected as adults. For the wild-caught, measurements of SVL were taken at the time of field collection and again prior to the onset of behavioural assays. Given the constraint of only having adult growth rate for the wild caught population, we only consider adult growth rate in our analyses. Lizards are known to exhibit indeterminate growth where body size increases throughout life (Warne and Charnov 2008) and juvenile growth rate is repeatable (adjusted repeatability = 0.54, CI = 0.25–0.80) as well as predictive of adult growth rate in this species (0.002 ± 0.001 mm, p = 0.01). Thus, assessing the POLS using growth during the adult stage in both groups, as opposed to throughout ontogeny, is expected to not affect the interpretation of the results.
Sprint performance
Sprint performance (Vmax) in the wild-caught population was measured immediately after field collection (2013 or 2014) and repeated approximately one year later. Before the test, skinks were placed in a 100 ml sealed plastic container and incubated at 30 °C for 20 min. Lizards were then raced down a 200 cm racetrack that was heated to 30 °C. Four 50 cm segments were marked on the racetrack. The time taken to cross each 50-cm segment was measured using a stopwatch with the fastest 50-cm segment being used to calculate its maximum sprint speed (cm/s).
For the captive-bred population, once they reached adult size, sprint speed was assessed twice in 2016 with five days between repeats. Skinks were acclimatized to the test temperature by being placed in a thermal chamber set to 30 °C for 20 min. Skinks were then raced three times down a 100-cm racetrack set to 30 °C. Between the three successive runs, skinks were placed back in the thermal chamber for at least 20 min to recover. Infrared sensors along the track recorded the time taken to traverse each 50-cm segment. The velocity for each segment was calculated and the maximum for all three runs of a given repeat was recorded as Vmax.
Selected body temperature
Thermal preferences of wild-caught skinks were calculated by measuring the body temperature of active skinks within a thermal gradient (∼ 15 °C to 36 °C) at multiple times each day during their normal activity period using a thermocouple probe. Selected body temperatures were assessed within 42 days of field collection with the total number of daily measurements per individual varying due to the measurements being taken opportunistically. Captive-bred skinks had their selected body temperatures calculated twice by placing individuals into a four-lane thigmo-thermal gradient (40 × 100 cm). A cold plate was placed beneath one end of the gradient and two 250 W infrared bulbs positioned over the other end to produce a temperature gradient ranging from 15 °C to 36 °C. A single skink was placed in the centre of each runway and allowed to acclimatise to the new environment for 30 min. Skinks were able to move about the gradient, selecting their preferred body temperatures after which body temperatures were measured every 15 min for four hours using an infrared thermometer (Fluke 566 Infrared thermometer). For each individual, the mean of all temperature measurements taken during a given repeat was calculated and assigned as the selected body temperature.
Behavioural assays
In 2016, a series of three assays were conducted to measure activity, exploration, and sociability behaviours of wild-caught and captive-bred skinks, using the methodology outlined in Michelangeli et al. (2016). Briefly, skinks were tested individually and exposed to each behavioural assay twice with four days between repeats. Assays were conducted in opaque plastic containers (∼ 55 × 32 × 24 cm) in a temperature-controlled room (∼ 22 °C). Video cameras (Panasonic HC-V130) were suspended above each test arena to record behavioural responses during the experiments. Videos were analysed using the program JWatcher (Blumstein et al. 2006). To minimise potential observer bias, when these video recordings were scored, the researcher was blind to the treatment (i.e. wild-caught or captive-bred). Each experiment included a 10-min acclimation period where the skink was placed under a clear plastic container in the centre of the arena, with the trial beginning after this container was removed. Assays were conducted over 30 min. Following each test, the equipment was washed with scentless detergent and thoroughly dried to prevent scent contamination between trials. Activity was measured by placing skinks individually into a test arena marked with 20 equal grid squares on the base. The level of activity was scored based on the number of transitions between squares. Exploration was measured by presenting skinks with a 10-cm high, opaque trapezium barrier dividing the arena into two equally sized compartments: starting compartment and goal compartment. Skinks had to squeeze between the barrier and arena wall to move from one compartment to the other. Time to reach the goal compartment was used as a measure of exploratory behaviour. Lizards not reaching the goal compartment were assigned 1800 s as their time. Finally, sociability was measured by placing skinks in a test arena divided into three equally sized zones: social zone, asocial zone, and an intermediate neutral zone. The social zone was comprised of a basking site that was divided in half by a clear Perspex™ partition that ran the length of the test arena. Three stimulus skinks that were not part of the study were placed behind the partition. The asocial zone located at the opposite end of the arena was identical however it contained no stimulus skinks. The amount of time spent basking with conspecifics was used as measures of sociability.
Statistical analysis
Data analysis was undertaken in R 3.50 (R Development Core Team 2016), with statistical significance set to α = 0.05. Prior to analyses, assumptions were tested by examining diagnostic plots. The exploratory measure of Time to Goal was square-root transformed and SVL and growth rates were log transformed to improve normality.
We have previously shown that there are no relevant sexual differences in behaviour in L. similis (Goulet et al. 2021), and therefore we did not include sex as a factor in our analyses. Mean differences in growth rates between the wild-caught and captive-bred populations was assessed using linear models with population and mass included as fixed effects. Separate linear mixed-effects models (‘lme4’ package) were used to determine if wild-caught vs. captive bred lizards differed on average in morphology, sprint performance, thermal physiology, and behavioural traits with treatment group as a fixed effect and skink ID included as a random effect to account for repeated measures. Selected body temperature also included SVL and mass as fixed factors. Sprint performance included the fixed effects of SVL and number of days between repeats to account for body size and temporal effects, respectively, on Vmax. Mass was considered to be a more appropriate fixed effect for investigations of growth rate and selected body temperature, whereas SVL often influences sprint speed (Young et al. 2022). Likelihood ratio tests with and without population as a fixed effect were then performed for all models to calculate significance in mean differences between populations.
Repeatability of body condition, sprint performance, thermal preferences, and behavioural traits was tested for wild-caught and captive lizards separately using linear mixed-effects models with a Gaussian error distribution. SVL was specified as a fixed effect and skink ID as a random effect. The number of days between repeats was included as an additional fixed effect in the model for sprint performance. For the captive-bred group, mother ID had no effect on phenotypic traits so was excluded from analyses. Adjusted repeatability was calculated using the variance components from the mixed-effects models (ratio among-individual variation to total phenotypic variation). Parametric bootstrapping provided 95% confidence intervals and statistical significance was evaluated with likelihood ratio tests.
The presence of the extended POLS was tested within wild-collected and captive-bred lizards separately by estimating patterns of covariance using multivariate Gaussian models fitted with Monte Carlo Markov Chain sampling (‘MCMCglmm’, Hadfield 2010). Models were run for 1,500,000 iterations and, after a burn in of 500,000 iterations, were thinned by 100 iterations. No evidence of autocorrelation between posterior samples was found. These models used the same structure as those used in the previously described univariate models and were fitted with all repeatable traits as the response variable. The multivariate models included two unstructured covariance matrices in order to partition phenotypic correlations into two levels: among-individual correlations (Donihue et al. 2015) and within-individual (residual) correlations (re). The rind represents consistent association between individual mean values for a series of traits over the time period within which measurements were taken, whereas re indicates whether an individual’s change in one trait between time period t and t + 1 is correlated with its change in another trait over the same time period (Dingemanse and Dochtermann 2013). Correlation estimates whose 95% CI excluded zero were considered significant. Finally, the relationship between an individual’s average value of repeatable traits and growth rate was examined using Pearson correlation tests.
Results
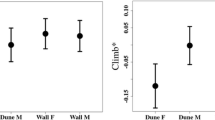
Wild-caught and captive-bred populations of L. similis were found to differ in terms of growth rate, body length, mass, thermal physiology, and behaviour (Table 1). Wild-caught skinks, on average grew faster, attained larger body sizes, had higher sprint performance, selected lower body temperatures, and were more active than captive-bred skinks. Body condition and exploratory and social behaviour did not vary between wild-caught and captive-bred animals. Within populations, the number of transitions made was found to be repeatable over time in both wild-caught and captive-bred populations (Table 2).
Repeatability in this trait was similar between populations as indicated by substantial overlap in confidence intervals. The level of activity did not vary according to a skink’s body size (Table 2). Sprint performance was also repeatable, though only within the captive-bred population. Maximal sprint speed was less repeatable than activity and was not influenced by an individual’s SVL (Table 2). Body condition, thermal preferences, the time to cross the barrier (exploratory behaviour), and the amount of time being social were not repeatable and thus were excluded from further analyses.
No evidence of the POLS was detected, in either population (Table 3). For the captive-bred population, correlations between activity and sprint performance at the among- or within-individual level were not found. Neither of these traits were associated with growth rate (r = -0.02, P = 0.94; r = 0.31, P = 0.17; respectively). Similarly, no relationship between activity and growth rate was found within the wild-caught population (r = 0.21, P = 0.56).
Discussion
We tested the predictions of the extended POLS within wild-caught L. similis and their captive-bred offspring to identify a growth-body condition trade-off and evaluate its covariation with behaviour and physiology. As the two groups were related, we could control for genetic differences when investigating differences between wild-caught and captive-bred individuals. However, our predictions were not supported in that we failed to find strong evidence for the extended POLS in the captive-bred population. In addition, there was no apparent life history trade-offs or associated correlations among traits in the wild-caught skinks, despite them experiencing more challenging environmental conditions. Furthermore, our expectation that the wild-caught population would exhibit a slower pace of life relative to captive-bred skinks was also not supported. Instead, wild-caught skinks grew rapidly and had higher rates of activity and locomotor performance, but surprisingly, selected lower body temperatures. However, as the wild caught skinks were kept in captivity prior to our studies, this period of captivity could have influenced our results. Similarly, differences in methodology between wild-caught and captive-bred skinks might have influenced our locomotor performance results.
Population variation in POLS traits
Ecological conditions, such as resource availability, temperature, and predation, are thought to influence resource allocation trade-offs and result in the functional integration of traits. Indeed, much research has demonstrated that populations experiencing divergent selective regimes differ in life history strategies along with their associated physiological and behavioural traits (Debecker et al. 2016; Gangloff et al. 2017; Segev et al. 2017; Auer et al. 2018; Polverino et al. 2018). These studies show that individuals from populations which are less restricted in terms of resource availability or are exposed to reduced rates of predation follow a fast-paced life strategy, having rapid growth, elevated physiologies, and higher expression of behaviours whereas the opposite pattern emerges among populations enduring harsher conditions. This was not the case in our study. Rather, our wild-caught population, which was assumed to have lower food acquisition rates, followed a faster pace of life compared to the captive skinks experiencing relatively benign conditions. Similar findings were demonstrated by Polverino et al. (2016), where mosquitofish (Gambusia holbrooki) that exhibited a fast-paced lifestyle had shorter travel distances, longer freezing times, and greater latencies to emerge from a refuge relative to fish from the slow population indicating that rapid growth was not mediated by the expression of high levels of behaviour.
These contrary results may have arisen as a response to the effects of predation pressure in the natural environment. It has been suggested that larger body sizes may confer a safety advantage as predators may be gape limited (Kok et al. 2019). Thus, in our study, wild-caught skinks would benefit from growing faster, attaining larger body sizes, and running faster, as it would reduce their vulnerability to predation. It would follow then, to fuel a rapid rate of growth, an equivalently high level of activity and locomotor performance would also be required to acquire adequate level of food resources, which was observed. Furthermore, because wild-caught skinks must cope with higher rates of extrinsic mortality, fast growth would also be advantageous in terms of fecundity, as reproductive maturity is often size-dependent in lizards (Tomašević-Kolarov et al. 2010). Greater investment towards growth would, therefore, act to optimize a wild-caught skink’s probability of surviving long enough to reproduce. Additional studies considering the trade-off between current and future reproduction should, therefore, be performed in order to fully elucidate the role that reproductive investment plays in this system.
Unintuitively, wild-caught skinks preferred lower, not higher, body temperatures despite exhibiting a fast pace of life. It was expected that fast individuals would select hotter temperatures to maximize energy acquisition and assimilation as well as to facilitate rapid movement. These results are, thus, in stark contrast to the extensive evidence indicating that ectotherms perform better and express higher levels of behaviour at hotter body temperatures (Angilletta 2001; Angilletta et al. 2010; Qu et al. 2011; Cerqueira et al. 2016; Goulet et al. 2017). It is therefore puzzling that low thermal preference is associated with fast traits in our skinks. One explanation proposed by Killen (2014), is that in food deprived situations individuals engage in compensatory growth where lower temperature preferences allows for a reduction in maintenance costs, thereby, enabling more energy to be diverted towards growth. This makes sense given that wild-caught skinks may have been subjected to low resource levels during their development and adulthood in the wild, but once in captivity, experienced a higher availability of food, prompting individuals to undergo compensatory growth later in life. However, selecting low body temperatures to facilitate higher growth is costly in terms of disease risk and body condition (Stamps 2007), contributed to wild-caught skinks having slightly lower body condition in this study. It is also possible that captive bred lizards having higher thermal preferences could simply reflect that they were more accustomed to the higher temperatures in the laboratory. Alternatively, it may be that plasticity in thermal preferences enables skinks to behaviourally shift their body temperatures upwards after feeding or prior to activity by selecting warmer microhabitats. Indeed, it was found that thermal preferences were not repeatable among both wild-caught and captive-bred skinks in this study, suggesting that this trait is context specific. Moreover, if the wild-caught skinks adjusted their body temperatures to their activities more carefully than did the captive-born skinks, this could explain the lower mean body temperature in this group.
Absence of the extended POLS
Analyses revealed no evidence of life history trade-offs in either the wild-caught or captive-bred population. Unlike growth rate, body condition was found not to differ between captive and wild skinks, suggesting the animals did not allocate more resources to one life history trait at the expense of the other. An absence of a trade-off was unexpected among wild-caught skinks, because trade-offs are thought to arise when resources are limited (De Gasperin et al. 2018). Several reasons may have caused the trade-off to be absent or go undetected. Firstly, resources may not actually be as limited in the wild-caught population as assumed, which would lead to similar levels of body conditioning between captive and wild skinks. Prey abundance or predator density, which acts to limit access to food, was not measured in the field site, so it is unclear if this is indeed the case. More work needs to be performed to directly quantify this. However, this still does not fully explain why a trade-off was not detected. A more convincing explanation may be that our index of body condition (size-adjusted body mass) was not sensitive enough to capture the physiological cost of increased levels of growth and activity. Condition is meant to reflect an individual’s level of energy reserves so perhaps alternative indices, such as packed cell volume, hemoglobin, or muscle and fat scores, would be a more effective measurement (Milenkaya et al. 2015). Or, the absence of a trade-off may instead be due to the fact that wild-caught skinks were kept in captivity for a period of time prior to the second measure of body condition was measured. This stint in captivity prior to our experiments also had the potential to influence our measurements and the behavioural and physiological traits. Despite much evidence suggesting that behavioural and physiological traits measured in the lab is reflective of those performed in the field (Herborn et al. 2010; Mathot and Frankenhuis 2018), acquisition rates, thus body condition, are likely to be more plastic and may quickly be adjusted as a function of resource availability. Skinks may have responded plasticly by increasing the level of food intake, and ultimately body condition, once they entered the laboratory setting. Not only would this affect the degree of variation between populations in this trait, but greater resource availability may have also served to mask the negative relationship between growth rate and body condition (proxy for mortality) occurring at the genetic level (Dammhahn et al. 2018).
Adding to this lack of support for the extended POLS, correlations were also not found among any of the repeatable traits. Within the wild-caught population, activity was not associated with the rate of growth and correlations between activity, locomotor performance, and growth rate were not found at either the among-individual, within-individual, or phenotypic levels. These findings are similar to those of Segev et al. (2017) where the majority of proactive behaviours were uncorrelated within ant (Temnothorax longispinosus) colonies. It is often assumed that high activity levels and performance would serve to increase food acquisition and ultimately growth. Perhaps, then, these behaviours are not as important in determining an individual’s energy budget as previously thought and, therefore, do not play a direct role in the speed at which an individual grows. Foraging strategies, resource distribution (ephemeral versus abundant), and encounter rates with predators are all known to dictate the level of energy expenditure and risk involved in acquiring food (Chen et al. 2019). Thus, it may be where other behaviours not measured in this study may be more influential to the acquisition of energetic resources.
Conclusions
Weak or absent support for the extended POLS, as demonstrated here, is common in studies conducted at the within-population level (Zavorka et al. 2015; Binder et al. 2016; Santostefano et al. 2017; Segev et al. 2017; Montiglio et al. 2018; Royauté et al. 2018). It seems quite possible that POLS may be a phenomenon that manifests at higher organizational levels than the population: a result that has been shown true for hypothesized trade-offs in thermal biology, for instance (Phillips et al. 2014). It has also been suggested that POLS traits can evolve independently from one another depending upon the population’s specific ecological conditions (Polverino et al. 2018). As a result, the extended POLS could be absent from some systems, which may be the case in these populations of L. similis. Much work is therefore required to identify the proximate mechanisms causing life history trade-offs and how they lead to the coupling or decoupling of physiological and behavioural traits. Such information could provide vital information of how ecological forces drive the evolution of traits.
References
Angilletta MJ (2001) Thermal and physiological constraints on energy assimilation in a widespread lizard (Sceloporus undulatus). Ecology 82:3044–3056. https://doi.org/10.1890/0012-9658(2001)082[3044:TAPCOE]2.0.CO;2
Angilletta MJ, Huey RB, Frazier MR (2010) Thermodynamic effects on organismal performance: is hotter better? Physiol Biochem Zool 83:197–206. https://doi.org/10.1086/648567
Araya-Ajoy YG, Bolstad GH, Brommer J, Careau V, Dingemanse NJ, Wright J (2018) Demographic measures of an individual’s pace of life: fecundity rate, lifespan, generation time, or a composite variable? Behav Ecol Sociobiol 72:75. https://doi.org/10.1007/s00265-018-2477-7
Arendt JD (2003) Reduced burst speed is a cost of rapid growth in anuran tadpoles: problems of autocorrelation and inferences about growth rates. Func Ecol :328–334
Auer SK, Dick CA, Metcalfe NB, Reznick DN (2018) Metabolic rate evolves rapidly and in parallel with the pace of life history. Nat Commun 9:14. https://doi.org/10.1038/s41467-017-02514-z
Binder TR, Wilson ADM, Wilson SM, Suski CD, Godin J-GJ, Cooke SJ (2016) Is there a pace-of-life syndrome linking boldness and metabolic capacity for locomotion in bluegill sunfish? Anim Behav 121:175–183. https://doi.org/10.1016/j.anbehav.2016.09.006
Biro PA, Stamps JA (2008) Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368. https://doi.org/10.1016/j.tree.2008.04.003
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent indivdual differences in behavior? Trends Ecol Evol 25:653–659
Biro PA, Post JR, Booth DJ (2007) Mechanisms for climate-induced mortality of fish populations in whole-lake experiments. P Natl Acad Sci USA 104:9715–9719. https://doi.org/10.1073/pnas.0701638104
Blumstein DT, Daniel JC, Evans CS (2006) JWatcherTM 1.0 an introductory User’s guide. https://www.jwatcher.ucla.edu/
Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BG, Johnson LS, Thompson CF, Sakaluk SK (2014) Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95:3027–3034. https://doi.org/10.1890/14-0418.1
Cerqueira M, Rey S, Silva T, Featherstone Z, Crumlish M, MacKenzie S (2016) Thermal preference predicts animal personality in Nile tilapia Oreochromis Niloticus. J Anim Ecol 85:1389–1400. https://doi.org/10.1111/1365-2656.12555
Chen J, Qi Y, Wu Y, Wang X, Tang Y (2019) Covariations between personality behaviors and metabolic/performance traits in an Asian agamid lizard (Phrynocephalus vlangalii). PeerJ 7:e7205. https://doi.org/10.7717/peerj.7205
Dammhahn M, Dingemanse NJ, Niemelä PT, Réale D (2018) Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behav Ecol Sociobiol 72:62–70. https://doi.org/10.1007/s00265-018-2473-y
De Gasperin O, Duarte A, English S, Attisano A, Kilner RM (2018) The early-life environment and individual plasticity in life-history traits. Ecol Evol 9:339–351. https://doi.org/10.1002/ece3.4749
de Jong M, Phillips BL, Llewelyn J, Chapple DG, Wong BBM (2022) Effects of development environment on animal personality in a tropical skink. Behav Ecol Sociobiol 76:137. https://doi.org/10.1007/s00265-022-03240-3
Debecker S, Sanmartin-Villar I, de Guinea-Luengo M, Cordero-Rivera A, Stoks R (2016) Integrating the pace-of-life syndrome across species, sexes and individuals: covariation of life history and personality under pesticide exposure. J Anim Ecol 85:726–738. https://doi.org/10.1111/1365-2656.12499
Dingemanse NJ, Dochtermann NA (2013) Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol 82:39–54. https://doi.org/10.1111/1365-2656.12013
Donihue CM, Brock KM, Foufopoulos J, Herrel A, Grindstaff J (2015) Feed or fight: testing the impact of food availability and intraspecific aggression on the functional ecology of an island lizard. Funct Ecol 30:366–375. https://doi.org/10.1111/1365-2435.12550
Gangloff EJ, Chow M, Leos-Barajas V, Hynes S, Hobbs B, Sparkman AM (2017) Integrating behaviour into the pace-of-life continuum: divergent levels of activity and information gathering in fast- and slow-living snakes. Behav Process 142:156–163. https://doi.org/10.1016/j.beproc.2017.06.006
Gotthard K (2000) Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J Anim Ecol 69:896–902. https://doi.org/10.1046/j.1365-2656.2000.00432.x
Goulet CT, Thompson MB, Chapple DG (2017) Repeatability and correlation of physiological traits: do ectotherms have a thermal type? Ecol Evol 7:710–719. https://doi.org/10.1002/ece3.2632
Goulet CT, Hart W, Phillips BL, Llewelyn J, Wong BBM, Chapple DG (2021) No behavioral syndromes or sex-specific personality differences in the southern rainforest sunskink (Lampropholis Similis). Ethology 127:102–108. https://doi.org/10.1111/eth.13103
Hämäläinen A, Immonen E, Tarka M, Schuett W (2018) Evolution of sex-specific pace-of-life syndromes: causes and consequences. Behav Ecol Sociobiol 72:50. https://doi.org/10.1007/s00265-018-2466-x
Han CS (2015) Effect of diet on the structure of animal personality. Front Zool 12:S5. https://doi.org/10.1186/1742-9994-12-S1-S5
Herborn KA, Macleod R, Miles WTS, Schofield ANB, Alexander L, Arnold KE (2010) Personality in captivity reflects personality in the wild. Anim Behav 79:835–843. https://doi.org/10.1016/j.anbehav.2009.12.026
Jablonszky M, Szász E, Krenhardt K, Markó G, Hegyi G, Herényi M, Laczi M, Nagy G, Rosivall B, Szöllősi E (2018) Unravelling the relationships between life history, behaviour and condition under the pace-of-life syndromes hypothesis using long-term data from a wild bird. Behav Ecol Sociobiol 72:52. https://doi.org/10.1007/s00265-018-2461-2
Kearney M (2012) Metabolic theory, life history and the distribution of a terrestrial ectotherm. Funct Ecol 26:167–179. https://doi.org/10.1111/j.1365-2435.2011.01917.x
Killen SS (2014) Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J Anim Ecol 83:1513–1522. https://doi.org/10.1111/1365-2656.12244
Kok EMA, Burant JB, Dekinga A, Manche P, Saintonge D, Piersma T, Mathot KJ (2019) Within-individual canalization contributes to age-related increases in trait repeatability: a longitudinal experiment in red knots. Am Nat 194:455–469. https://doi.org/10.1086/704593
Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L, Franklin C (2012) Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Funct Ecol 27:136–144. https://doi.org/10.1111/1365-2435.12017
Le Lann C, Wardziak T, Van Baaren J, van Alphen JJ (2011) Thermal plasticity of metabolic rates linked to life-history traits and foraging behaviour in a parasitic wasp. Funct Ecol 25:641–651. https://doi.org/10.1111/j.1365-2435.2010.01813.x
Llewelyn J, Macdonald S, Hatcher A, Moritz C, Phillips BL (2017) Thermoregulatory behaviour explains countergradient variation in the upper thermal limit of a rainforest skink. Oikos 126:748–757. https://doi.org/10.1111/oik.03933
Londoño GA, Chappell MA, Castañeda MR, Jankowski JE, Robinson SK (2014) Basal metabolism in tropical birds: latitude, altitude, and the ‘pace of life’. Funct Ecol 29:338–346. https://doi.org/10.1111/1365-2435.12348
Madsen T, Shine R (2000) Silver spoons and snake body sizes: prey availability early in life influences long-term growth rates of free‐ranging pythons. J Anim Ecol 69:952–958. https://doi.org/10.1111/j.1365-2656.2000.00477.x
Martins F, Kruuk L, Llewelyn J, Moritz C, Phillips B (2018) Heritability of climate-relevant traits in a rainforest skink. Heredity 122:41–52. https://doi.org/10.1038/s41437-018-0085-y
Mathot KJ, Frankenhuis WE (2018) Models of pace-of-life syndromes (POLS): a systematic review. Behav Ecol Sociobiol 72:41. https://doi.org/10.1007/s00265-018-2459-9
Michelangeli M, Wong BBM, Chapple DG (2016) It’s a trap: sampling bias due to animal personality is not always inevitable. Behav Ecol 27:62–67. https://doi.org/10.1093/beheco/arv123
Milenkaya O, Catlin DH, Legge S, Walters JR (2015) Body condition indices predict reproductive success but not survival in a sedentary, tropical bird. PLoS ONE 10:e0136582. https://doi.org/10.1371/journal.pone.0136582
Monceau K, Dechaume-Moncharmont FX, Moreau J, Lucas C, Capoduro R, Motreuil S, Moret Y (2017) Personality, immune response and reproductive success: an appraisal of the pace-of-life syndrome hypothesis. J Anim Ecol 86:932–942. https://doi.org/10.1111/1365-2656.12684
Montiglio PO, Dammhahn M, Messier GD, Réale D (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behav Ecol Sociobiol 72:116. https://doi.org/10.1007/s00265-018-2526-2
Phillips BL, Llewelyn J, Hatcher A, Macdonald S, Moritz C (2014) Do evolutionary constraints on thermal performance manifest at different organizational scales? J Evol Biol 27:2687–2694. https://doi.org/10.1111/jeb.12526
Phillips BL, Muñoz MM, Hatcher A, Macdonald SL, Llewelyn J, Lucy V, Moritz C (2016) Heat hardening in a tropical lizard: geographic variation explained by the predictability and variance in environmental temperatures. Funct Ecol 30:1161–1168. https://doi.org/10.1111/1365-2435.12609
Piquet JC, López-Darias M, van der Marel A, Nogales M, Waterman J (2018) Unraveling behavioral and pace-of-life syndromes in a reduced parasite and predation pressure context: personality and survival of the barbary ground squirrel. Behav Ecol Sociobiol 72:147. https://doi.org/10.1007/s00265-018-2549-8
Polverino G, Cigliano C, Nakayama S, Mehner T (2016) Emergence and development of personality over the ontogeny of fish in absence of environmental stress factors. Behav Ecol Sociobiol 70:2027–2037. https://doi.org/10.1007/s00265-016-2206-z
Polverino G, Santostefano F, Diaz-Gil C, Mehner T (2018) Ecological conditions drive pace-of-life syndromes by shaping relationships between life history, physiology and behaviour in two populations of Eastern mosquitofish. Sci Rep 8:14673. https://doi.org/10.1038/s41598-018-33047-0
Polverino G, Buchholz KM, Goulet CT, Michelangeli M, Chapple DG (2023) Temporal repeatability of behaviour in a lizard: implications for behaviour syndrome studies. Evol Ecol 37:401–418. https://doi.org/10.1007/s10682-023-10232-w
Qu Y, Li H, Gao J, Xu X, Ji X (2011) Thermal preference, thermal tolerance and the thermal de-pendence of digestive performance in two Phrynocephalus lizards (Agamidae), with a review of species studied. Curr Zool 57:684–700. https://doi.org/10.1093/czoolo/57.6.684
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063. https://doi.org/10.1098/rstb.2010.0208
Ricklefs (2002) The physiology/life history nexus. Trends Ecol Evol 17:462–468. https://doi.org/10.1016/S0169-5347(02)02578-8
Royauté R, Berdal MA, Garrison CR, Dochtermann NA (2018) Paceless life? A meta-analysis of the pace-of-life syndrome hypothesis. Behav Ecol Sociobiol 72:64. https://doi.org/10.1007/s00265-018-2472-z
Salzman TC, McLaughlin AL, Westneat DF, Crowley PH (2018) Energetic trade-offs and feedbacks between behavior and metabolism influence correlations between pace-of-life attributes. Behav Ecol Sociobiol 72:54. https://doi.org/10.1007/s00265-018-2460-3
Santostefano F, Wilson AJ, Niemela PT, Dingemanse N (2017) Behavioural mediators of genetic life-history trade-offs: a test of the pace-of-life syndrome hypothesis in field crickets. Proc R Soc B 284:20171567. https://doi.org/10.1098/rspb.2017.1567
Segev U, Burkert L, Feldmeyer B, Foitzik S (2017) Pace-of-life in a social insect: behavioral syndromes in ants shift along a climatic gradient. Behav Ecol 28:1149–1159. https://doi.org/10.1093/beheco/arx079
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Singhal S, Hoskin CJ, Couper P, Potter S, Moritz C (2018) A framework for resolving cryptic species: a case study from the lizards of the Australian wet tropics. Syst Biol 67:1061–1075. https://doi.org/10.1093/sysbio/syy026
Stamps JA (2007) Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol Lett 10:355–363. https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1111/j.1461-0248.2007.01034.x
Stearns SC (1989) Trade-Offs in Life-History evolution. Funct Ecol 3:259–268. https://doi.org/10.2307/2389364
Tomašević-Kolarov N, Ljubisavljević K, Polović L, Džukić G, Kalezić M (2010) The body size, age structure and growth pattern of the endemic balkan mosor rock lizard (Dinarolacerta Mosorensis Kolombatović, 1886). Acta Zool Acad Sci H 56:55–71
Ward AJW, Thomas P, Hart PJB, Krause J (2004) Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 55:561–568. https://doi.org/10.1007/s00265-003-0751-8
Warne RW, Charnov EL (2008) Reproductive allometry and the size-number trade-off for lizards. Am Nat 172:E80–98. https://doi.org/10.1086/589880
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life-history trade-offs favour the evolution of animal personalities. Nature 447:581–584. https://doi.org/10.1038/nature05835
Young A, Anderson RO, Naimo A, Alton LA, Goulet CT, Chapple DG (2022) How do the physiological traits of a lizard change during its invasion of an oceanic island? Oecologia 198:567–578. https://doi.org/10.1007/s00442-021-05054-y
Zavorka L, Aldven D, Naslund J, Hojesjo J, Johnsson JI (2015) Linking lab activity with growth and movement in the wild: explaining pace-of-life in a trout stream. Behav Ecol 26:877–884. https://doi.org/10.1093/beheco/arv029
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by an Australian Research Council Discovery Project Grant (to DGC; DP170100684).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Monash University Animal Ethics Committee (BSCI/2013/19 and BSCI/2016/02).
Data accessibility
The data and R code is included in the online supplementary material.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Communicated by T. Madsen.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buchholz, K.M., Goulet, C.T., de Jong, M. et al. Does the development environment cause the pace of life to change in a rainforest lizard?. Behav Ecol Sociobiol 78, 86 (2024). https://doi.org/10.1007/s00265-024-03502-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03502-2