Abstract
A mass coral bleaching event occurred in the summer of 2022 in subtropical Hong Kong, driven by two marine heatwaves (MHWs) with high intensities of 1.56 and 0.86 °C above a mean climate condition, both MHWs 7 days with a short gap of 4 days during the strong La Niña year. A transect survey was conducted at nine study sites in three regions, which revealed widespread coral bleaching with bleached coral cover ranging from 2.4 to 70.3%. In situ environmental data revealed the presence of a thermocline and halocline. Local conditions, including depth and wave exposure, significantly influenced the bleaching response. Shallow-water (2–4 m) corals were primarily affected, particularly in sheltered and moderately sheltered sites that exhibited higher levels of bleached coral cover (42.97 ± 15.4% and 44.93 ± 29.4%, respectively) compared to the exposed sites (31.8 ± 5.2%). Bleaching in deep waters (4–6 m) was minimal, with only a few colonies of Goniopora at two of the three sheltered sites exhibiting bleaching (1.7 ± 1.5%). Heat stress resistance differed between coral genera. Recovery rate for four common coral genera is low for Acropora tumida. Additionally, a minor hypoxia event was found to cause mortality of non-coral benthos at a sheltered site (Sharp Island). These findings highlight the alarming impact of extreme heatwaves on subtropical coral communities and underscore the importance of monitoring coral bleaching.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine heatwaves (MHWs) refer to periods of abnormally high ocean temperatures relative to the average temperature in a specific region. MHWs can be categorized into four levels of intensity based on the multiples of the 90th percentile difference from the mean climatology value (i.e. 1–2 × , Category I, moderate; > 4 × , Category IV, extreme) (Hobday et al. 2018). During the period 1925–2016, the annual occurrence of MHWs increased globally, as measured by frequency (134%), duration (117%), and total MHW days (154%) (Oliver et al. 2018). Large-scale MHWs have been reported to cause mass mortality of the foundation species in coastal ecosystems, such as seagrasses, corals, and sponges (Garrabou et al. 2009; Arias-Ortiz et al., 2018; Leggat et al., 2019; Bell et al., 2023). Besides, MHWs can impact ecosystem functions and services, leading to lower productivity of both capture and culture fisheries and, eventually, massive economic losses (Smith et al. 2021). With the warming of the global ocean in the twenty-first century, the frequency, duration, and severity of MHWs will continue to increase, causing more severe degradation in marine ecosystems (Smale et al. 2019; Smith et al. 2022).
Warming events can cause coral bleaching and induce coral disease outbreaks, and extensive MHWs could make corals difficult to recover from bleaching, leading to coral mortality (Leggat et al. 2019; Roberts et al. 2019). Although corals are known to be susceptible to MHWs, local conditions such as eddies, wave exposure, and high sea urchin density can modulate the impact of MHWs on coral survival (Donovan et al. 2021; Wyatt et al. 2023). Furthermore, coral species usually exhibit differential sensitivity to MHWs (Fordyce et al. 2019). Monitoring the temporal changes in thermally tolerant and susceptible species can help predict the long-term fate of a coral ecosystem (Loya et al. 2001). Nevertheless, since most studies of MHWs have been conducted in tropical coral ecosystems (Pearce and Feng 2013; Fordyce et al. 2019; Donovan et al. 2021), there is relatively little information on the impacts of MHWs on subtropical coral ecosystems (Bridge et al. 2014; Couch et al. 2017; Mo et al. 2022).
Hong Kong is located 128 km south of the Tropic of Cancer on China’s southern coast (Fig. 1a). Its climate is monsoonal, with cool, dry winters and hot, wet summers. Precipitation is highly seasonal in Hong Kong, with a monthly mean of 453.2 mm in the wet season to 28.8 mm in the dry season (HKO 2022a). Due to the influence of the Pearl River, Hong Kong waters can be divided into a western estuarine zone, a central transitional zone, and an eastern oceanic zone (Morton and Morton 1983). Seawater temperatures in Hong Kong exhibited a strong seasonality, with monthly mean readings varying from 25–29 °C in the wet season to 17–21 °C in the dry season (Goodkin et al. 2011; Dellisanti et al. 2023). Due to the subtropical climate and influence of the Pearl River discharge, scleractinian corals in Hong Kong do not form reefs; they instead develop into colonies on rocks, mainly in the eastern oceanic waters. As the turbidity of the water is usually high, corals in most locations grow along shores in shallow waters, seldom extending to more than 10 m depth (Morton and Morton 1983). Nevertheless, in several protected bays, coral communities are well-developed, with coral cover reaching more than 70% of the substrate (Yeung et al. 2021). In the subtropical Hong Kong waters, only three coral bleaching events have been reported, including (1) the summer 1997 event that affected the whole of Hong Kong waters (McCorry 2002), (2) the summer 2014 event that affected six sites in Port Shelter associated with high-temperature and low dissolved oxygen (Xie et al. 2017), whereas (3) the summer 2017 event that affected eight sites in the eastern waters (Xie et al. 2020). Due to the lack of in situ monitoring of environmental data or the use of environmental data with low spatial resolution only (one datum per month for each parameter), these previous studies were not able to determine the correlation between the formation of MHWs and coral bleaching or between the cessation of MHWs and coral recovery.
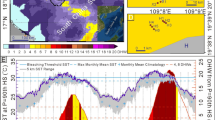
a Map of Hong Kong showing the selected regions for surveys, including northeastern (NE), hoi ha wan (HHW), and port shelter (PS). Triangles, circles, and squares indicate sheltered, moderately exposed, and exposed sites, respectively. The site names are as shown in the map. The asterisk sign represents the SST monitoring station. The MHW events detected in 2022 b The arrows indicate the MHW events during summer (highlighted area)
Here, we report a coral bleaching event that occurred in the summer of 2022 in Hong Kong after an extended period of MHW. A preliminary spot-check survey at several sites indicated that the spatial extent of the event was unprecedented. A quantitative field survey was conducted to determine the patterns of coral bleaching at nine sites spanning three regions and recovery at the severest bleaching site in the eastern waters of Hong Kong. We hypothesized that the impact of MHW and the associated coral bleaching in Hong Kong would be widespread across the territory and modulated by local environmental factors, including depth and wave exposure. Furthermore, we postulated that the bleaching response would exhibit variation based on the specific coral composition observed at each site. The data on sea surface temperature was obtained from the Hong Kong Government’s long-term monitoring programme and used to define the MHW threshold and the MHW events. Several environmental parameters, including temperature, dissolved oxygen, and salinity, were also measured in situ at different water depths during the surveys to determine the contributions of local conditions to coral bleaching (Donovan et al. 2021; Wyatt et al. 2023). Sites with different levels of wave exposure were chosen, and at each site, transect surveys were conducted in relatively shallow (2–4 m) and relatively deep (4–6 m) waters to quantify coral bleaching and recovery. Our study was one of the few empirical studies documenting how environmental gradient can modulate the effects of MHWs on subtropical coral communities. These results contribute to determining local drivers of coral bleaching and highlight the need to incorporate MHW hotspot identification in managing coral health.
Materials and methods
Coral bleaching survey
A coral bleaching survey was conducted on August 1st, 2nd, and 4th after recreational divers reported the first sighting of coral bleaching on July 25th on social media. The coral communities were surveyed in three regions, including the Northeastern (22°31′48″ N, 114°18′00″ E), Hoi Ha Wan (22°28′26″ N, 114°30′00″ E), and Port Shelter (22°20′27″ N, 114°18′10″ E). The regions shared similar characteristics of high salinity and low turbidity compared to western waters and covered a wide spatial range of coral communities in Hong Kong (Yeung et al. 2021). Each region randomly selected a sheltered, moderately sheltered, and exposed site (Fig. 1a). The wave exposure of each site was estimated based on its location within a bay. Sheltered sites are situated in the innermost part of the bay, surrounded by land, which minimizes the impact of waves. Moderately sheltered sites are positioned in the middle section of the bay. Exposed sites, on the other hand, are located near or in the outermost region of the bay, directly exposed to waves from the open water and lacking any natural shelter to mitigate the effects of wind. These sites are known to host a coral community with at least 18% coral cover in the shallow water (Yeung et al. 2021). A video-transect survey method was used to quantify coral bleaching at each site (Xie et al. 2020), following a quick spot-check to locate the area with the highest coral coverage. As a preliminary survey showed that the severity of bleaching was depth dependent, two 100-m transects were laid parallel to the shoreline, at 2–4 m depth chart datum (c.d.) (shallow water) and 4–6 m c.d. (deep water). A video clip of each transect was recorded at a resolution of 1920 × 1080 pixels using an Olympus Tough TG-6 camera. During the survey, a SCUBA diver swam above the transect at a speed of ~ 10 m per minute, with the camera pointing downward to capture a belt of about 0.75 m wide of the benthic substrate. In the laboratory, video clips were played and paused every 4 s to record the benthic types at 5 fixed points on each frame. The substrate was classified as living coral, recently dead coral, rock, rubble, sand, and others. Living corals were identified to at least the genus level, and the bleaching status was classified using the colour chart of CoralWatch (Siebeck et al. 2006; Montano et al. 2010; Nielsen et al. 2022), with a drop of at least two between the darkest and lightest parts of a coral colony, or the darkest and the lightest colonies of the same transect representing bleaching. On average, 841 points for each transect were analysed.
Sharp Island, the site with the most severe bleaching, was selected to conduct follow-up surveys to determine the consequence of the bleached coral colonies. A total of 25 completely bleached colonies, including five colonies of five common species (Acropora digitifera, Goniopora columna, Pavona decussata, Platygyra carnosa, and Porites lutea), were tagged using a steel stake with a unique number tag. The colonies were photographed biweekly in the field after the coral bleaching survey, and the recovery status scored in the laboratory: 0—no recovery, 1—< 1/3 surface area recovered, 2—1/3—2/3 surface area recovered, and 3—> 2/3 surface area recovered. Coral recovery was defined as an increase by 2 or more colour scores (Siebeck et al. 2006). The post-bleaching monitoring was completed at week 12 when all the tagged colonies either recovered or died (see examples in Fig. 5). The scores of all colonies for each species were averaged as a Recovery Index (RI) to represent the recovery capability of each species over time.
Environmental data
The sea surface temperature (SST) data measured by the Hong Kong Observatory (HKO 2022a) from 1974 to 2022 was used to define the climatological mean and identify and determine the severity of MHWs for 2005–2022. The data were taken at a monitoring station at North Point (Fig. 1a) daily in the morning and afternoon. Considering the warming effect of sunlight and air-sea heat flux on SST, the SST measured in the afternoon was used (De Szoeke et al. 2021). An MHW was defined as a period of the SST higher than the 90th percentile of the seasonally varying threshold that lasted for at least five days based on a 30 year historical baseline period (Hobday et al. 2016). Both climatological mean and MHWs were calculated using an 11 day window and smoothed by a 31 day moving average (Hobday et al. 2016). The heatwave intensity (HWI) and the duration (HWD) of each MHW event identified were calculated. The HWI is the average temperature deviation exceeding the MHW threshold throughout its duration. The characteristics of each MHW event were assessed with counting the number of days when the SST exceeded 30 °C.
To provide site-specific information on the environmental parameters, seawater temperature, salinity, and dissolved oxygen (DO) were measured in situ during the first coral bleaching survey using a multi-parameter sonde (YSI EXO2 Water Quality Sonde) calibrated in the laboratory before each sampling day. At each survey site, five measurements of each parameter were taken as replicates at each metre, from the surface to nine metres depth, to reveal the vertical profile of the water column and examine any stratification among or near the coral communities.
Data analysis
Utilizing the in situ environmental data, the physical profile of the water column at each site was depicted by averaging the five measurement replicates at each metre and examining the differences. The coral composition at each site and depth were also delineated by pooling the data points from the video transect.
A comparison of environmental parameters between depth and wave exposures was conducted. The environmental variables in in situ measurements were extracted. The measurements in different metres were pooled into two different depth ranges: Shallow (2–4 m) and deep (4–6 m) water. The measurements were averaged for each depth and site and comparisons using ANOVA with post hoc Tukey HSD tests were conducted to detect statistically significant differences.
To indicate the recovery status of each selected coral species over time, the initial RI of each species was compared with the RI recorded during the recovery period. Paired t-tests were conducted to indicate the recovery when the statistical significance was detected.
All statistical tests were performed using RStudio v2023.09.0 software (RStudio Team 2020). The LMM analysis was conducted using the package ‘lmerTest’ (Kuznetsova et al. 2017).
Results
The 2022 summer MHWs
The daily sea surface temperatures (SSTs) recorded by the Hong Kong Observatory (HKO) in 2022 were compared to the MHW threshold and the climatological mean, which were calculated based on the baseline period of the last 30 years. Two MHW events were identified during the summer of 2022 (Fig. 1b). These MHWs occurred from July 10th to July 16th and from July 21st to July 27th, which coincided with or preceded the first sighting report of coral bleaching on July 25th, as reported by recreational divers (personal communication).
In the available SST data from 2005 to 2021, there were 1 to 4 MHW events per year; however, there was no MHW in 2011. The intensity of these MHW events, as measured by the heatwave magnitude index (HWI), ranged from 0.03 to 1.18 °C above the threshold (Supplementary Table S1). During the two MHWs in 2022, the daily mean SSTs were 0.06 to 2.08 °C and 0.99 to 2.00 °C higher than the MHW threshold, respectively. Compared to previous MHWs, the first MHW in 2022 ranked 1st in intensity (1.56 °C above the threshold) and 9th in duration (7 days). The second MHW ranked 8th in intensity (0.86 °C above the threshold) and 9th in duration (7 days). The fact that there was only a 5 day gap between the two MHWs raises concerns about the capacity of local corals to withstand repeated thermal stress.
The characteristics of the water column during the MHW
The environmental data collected during the survey (Fig. 2) revealed apparent declines in water temperature and DO with depth, with the surface layer at around 3–4 m having temperatures around 30 °C and DO levels between 6 and 8 mg/L. In the layers below 6 m temperatures dropped to about 23.5–27.5 °C or lower, while DO levels decreased to around 1.40–5.36 mg/L or lower. These parameters varied significantly between depths (temperature: F1 = 65.961, p < 0.05, DO: F1 = 18.541 p < 0.05; Table 1) and among sites with wave exposures (temperature: F2 = 5.554, p < 0.05, DO; F2 = 11.932 p < 0.05; Table 1). The Tukey HSD test for both parameters revealed differences between sheltered and exposed sites but no significant differences between moderately exposed sites and sheltered or exposed sites. At Sharp Island (PS), a sheltered site, the surface water temperature was exceptionally high, at 32.2 °C, while the dissolved oxygen levels were low, at 1.40 mg/L. These suggested that these areas experienced different water temperature levels due to varying levels of wave exposure and stratification.
The water temperature (first column), dissolved oxygen (DO, second column), and salinity (third column) changes along the water column in the sheltered (red triangle), moderate (blue circle), and exposed (green square) sites in the NE (first row), HHW (second row), and PS (third row). The red dotted line for water temperature represents the threshold of coral bleaching (30 °C); the red dotted line for DO represents the threshold of hypoxia (2 mg/L)
The salinity showed an opposite trend to temperature, which varied from 31.64 to 33.5 PSU in the surface water to 33.35–35.67 PSU in the deep water (Fig. 2). Since the full-strength seawater should have a salinity of ~ 35 PSU, surface water in some sites, with a salinity of only 31 to 33 PSU indicated minor levels of freshwater dilution. Significant differences in salinity were observed between depths (F1 = 46.115, p < 0.05; Table 1), but no significant differences were found between wave exposures (F2 = 1.529, p > 0.05; Table 1), and the interaction between these factors was also not significant (F2 = 0.828, p > 0.05; Table 1). In the NE and HHW waters, the salinity differences between the surface and bottom layers were minimal, typically increasing by approximately 2 PSU across the entire water column. At PS, the salinity exceptionally increased by 3–4 PSU, accompanied by a more contrasting halocline.
Coral communities and the bleaching response
The coral communities at the study sites were predominantly composed of one to three coral genera: Pavona, Platygyra, and Porites (Fig. 3). Each site exhibited a different dominance pattern and proportion of these genera. Coral Beach in HHW was exclusively dominated by Pavona, accounting for 82.7% of the coral cover. Crescent Island and Shelter Island were solely dominated by Platygyra, with percentages ranging from 46% to 93.9%. Port Island exhibited a co-dominance of Platygyra (59.8%) and Porites (20.5%). Tung Ping Chau showed a co-dominance of Pavona (50.5%) and Platygyra (34.8%). The Pier of Hoi Ha Wan, Au Yue Tsui, and Sharp Island displayed a co-dominance of Pavona (26% to 58.3%) and Porites (16.8% to 34.8%). Finally, Bluff Island exhibited a co-dominance of Porites (27.3%), Pavona (23.2%), and Platygyra (16.6%) (Fig. 3).
The coral generic composition in shallow (upper box) and deep waters (lower box) quantified by per cent healthy and bleached cover in each location (AYT: au yue tsui, CI: crescent island, TPC: tung ping chau, Pier: the pier in HHW, CB: coral beach, PI: port island, Sharp: sharp island, Shelter: shelter island, Bluff: bluff island). The number in brackets in each graph represents the total bleached coral cover
Coral bleaching was observed across all study sites, indicating a territory-wide event (Fig. 3). The severity of bleaching varied among sites and water depths, ranging from 2.4 to 70.3%. In shallow water, the bleached coral cover in the sheltered and moderately sheltered sites was generally higher, with a mean cover of 42.97 ± 15.4% and 44.93 ± 29.4%, respectively. The exposed sites had a lower mean coral cover of 31.8 ± 5.2%. In deep water, coral bleaching was found only at two of the three sheltered sites, with only Goniopora observed as the bleached genus, but the affected colonies were few, with only 1 affected colony corresponding to 2.4% coral cover at AYT and 2 affected colonies corresponding to 2.7% coral cover at the Pier. Goniopora was mainly distributed in the deep water; only a small percentage of this genus (0.08% at AYT and 0.06% at the Pier) had undergone bleaching. In shallow water, this genus was uncommon, with a 2.4% coral cover at AYT and absence at the Pier, but its bleaching incidence was high, which indicates its susceptibility to bleaching. There is no coral bleaching at the moderately sheltered and exposed sites. Platygyra and Pavona exhibited more considerable differences in bleached coral cover between sheltered, moderately sheltered, and exposed sites, with 62.7 ± 1.2–75.2 ± 0% at the sheltered and moderately sheltered sites, and 31.1 ± 6.2%–47.8 ± 19.7% at the exposed sites (Table 2). Pavona exhibited fewer differences, with a mean bleached coral cover of 23.2 ± 17.5% at the sheltered sites and 19.0 ± 10% in the exposed sites (Table 2). The differential responses of these coral genera to bleaching events likely also influenced the observed patterns of bleaching severity across the study sites.
Post-bleaching recovery
Coral colonies revisited after the bleaching event was categorized into three groups: Fully recovered, partially recovered, or completely dead, within a timeframe of 12 weeks (Fig. 4). The final recovery index (RI) in the last week was significantly different among the species (H4 = 13.68, p < 0.05; Fig. 4). A. digitifera had a notably lower value of 1.2 compared to the other species, which had values ranging from 2.6 to 3.0 (Fig. 4). It is worth noting that two colonies of A. tumida did not survive, contributing significantly to the low recovery index observed for this species.
On the other hand, the other species generally exhibited a high capability for recovery, with a mean recovery index of 2.6, 2.6, 2.8, and 3.0 for P. decussata, P. lutea, P. carnosa, and G. columna, respectively. This result indicates that most colonies of these species either fully or partially recovered. The recovery progress, as indicated by the significant enhancement of the RI throughout the survey period, varied among the different species. G. columna quickly recovered, with all colonies fully recovered within four weeks after bleaching. In contrast, P. carnosa exhibited the slowest recovery, with one of the colonies taking 12 weeks to recover. The colonies of P. decussata and P. lutea experienced either full or partial recovery within 2 to 8 weeks after bleaching.
Discussion
The potential cause of MHWs and hypoxia in 2022
The MHWs occurred in 2022 were associated with an apparent water column stratification, with a surface layer temperature ranging from 30 to 32 °C, dissolved oxygen (DO) levels of 6 to 8 mg/L, and a salinity of 31 to 33 PSU. The bottom layer had a temperature of 22 to 26°C, DO levels of 2 to 4 mg/L, and a salinity of 33 to 35 PSU. The warm surface water layer likely caused the widespread coral bleaching, while the low-oxygen layer only caused minor hypoxia in the sheltered site of PS (Sharp Island), resulting in mass benthos mortality at that location (Fig.S2, Appendix I). Similar stratification of the water column has been previously well-documented during the warm season in Hong Kong (Morton and Morton 1983; Xu et al. 2012), but how such a stratification affected benthos is largely unknown, except that stratification-associated hypoxia had been reported to cause widespread death of benthos during the summer in Tolo Harbour (Fleddum et al. 2011) and Mirs Bay (Binnie Consultants Ltd1995). The strong stratification in the north-eastern waters of Hong Kong was attributed to the high temperature in the surface water coupled with a low upwelling of cold water from the South China Sea (Li et al. 2014). Here, we recorded a small-scale mass mortality event at the hypoxia-affected site at Sharp Island in the summer of 2022 (Appendix I in Supplementary Information), supporting this explanation.
Like the MHW events in the South China Sea (SCS) surrounding Hong Kong (Yao and Wang 2021; Zhao et al. 2023), high sea surface temperatures (SST) in Hong Kong are usually induced by air-sea heat flux and solar radiation. During each wet season, the dominant currents (CCC and SCSWC) in the northern SCS, including Hong Kong waters, are warm but seldom exceed 30 °C (Kang et al. 2021). The warming effect becomes more severe when a La Niña event forms in the summer while El Niño decays, as the robust western North Pacific subtropical high is formed simultaneously (Chen et al. 2012). This reduces cloud cover and increases solar radiation reaching the sea surface (Yao and Wang 2021). Meanwhile, the southwest monsoon weakens or disappears, preventing the cold upwelling current from reaching the coast. In July 2022, La Niña developed while El Niño decayed, according to the World Meteorological Organization’s monitoring (https://community.wmo.int/en/activity-areas/climate/wmo-el-ninola-nina-updates). An extreme subtropical high formed on July 8th, inducing abnormal weather conditions with reduced cloud cover (11% below normal), prolonged sunshine period (28.9 h above normal), and higher daily solar radiation (2.58 mJ m−3 above normal). These conditions caused July 2022 to become the hottest month since 1884, breaking many high-temperature records in Hong Kong, including the highest mean temperature for all months (30.3 °C), the highest number of days with a daily maximum temperature equal to or higher than 35.0 °C for all months (10 days), and the highest number of very hot days for all months (21 days) (HKO 2022b). Therefore, the scorching weather was likely the triggering factor for the MHWs in the summer of 2022.
Based on the above findings, the water column stratification in 2022 began with the annual intrusion of the cold, hypersaline, and low-oxygen bottom water in the early wet season. On July 8, 2022, the subtropical high induced scorching weather, greatly enhanced the air-sea heat flux and raised the SST above the coral bleaching threshold. Concurrently, with the developed La Niña event and decaying El Niño, the southwest summer monsoon weakened significantly, unable to transfer the cold upwelling current to the coastal waters of Hong Kong. Without the mixing effect of storms and under extremely hot weather conditions, a thermohalocline was formed, triggering the MHW associated with a minor hypoxia event, as reported in this study.
Coral bleaching and recovery
The summer 2022 MHWs had a significant impact on coral bleaching, while the local conditions, including depth and wave exposure, primarily modulated the spatial distribution of heat stress and, consequently, coral bleaching in Hong Kong waters. Coral bleaching predominantly occurred in shallow water, typically within a depth range of 1 to 3 m below the surface. The extent of bleached coral cover exhibited an apparent variation corresponding to the wave exposure gradient, whereby areas with lower wave exposure demonstrated a higher degree of bleaching. The coral composition had a relatively lower overall influence, with only the common and sensitive genus, Platygyra, demonstrating a significant impact. Additionally, two other common coral genera, Porites and Pavona, exhibited varying levels of bleaching among locations. Still, their impact on the bleaching response of the entire coral communities was found to be insignificant. In the deep water of two of the three studied sheltered, 3 colonies of Goniopora also suffered from bleaching, indicating this genus is particularly susceptible to heat stress, which may explain its rare occurrence in shallow water.
Previous reports showed that hyposalinity stress might be a causal factor for coral bleaching in local waters. For instance, in the summer of 1997, McCorry (2002) reported coral bleaching in nine sites, with the most severe bleaching occurring at Sham Wan in the southern waters, resulting in the complete death of the coral community. Moderate-to-mild bleaching and mortality levels were observed in north-eastern to eastern waters, where 10.9% to 12.3% of coral cover was bleached, and 3% to 25.4% of coral cover was dead. Because Sham Wan is located in the transitional zone, her results are consistent with the greater influence of freshwater insurgents in the southern waters closer to the Peral River Estuary. In the summer of 2014, Xie et al. (2017) reported localized bleaching only in the Port Shelter area, with moderate bleaching levels (30.6%) recorded in Sharp Island and minor bleaching recorded in seven other sites, where 0.8% to 10% of coral cover was bleached. The 2017 bleaching event documented by Xie et al. (2020) revealed more extensive but scattered bleaching in locations along the north-eastern to eastern waters, with 5.9% to 57.6% of coral cover affected. These studies demonstrated that hyposalinity could also trigger bleaching in protected bays without adequate tidal flushing. Here, our study confirmed a spatial pattern of coral bleaching response associated with the wave exposure gradient. This is because heat from air-sea heat flux evenly spreads to different locations, while wave advection driven by tidal changes or ocean swells reduces heat accumulation in more exposed sites (Wyatt et al. 2020), resulting in a predictable impact on local coral communities. Although previous reports of coral bleaching occurred during the summer, it was difficult to disentangle the influences of heat and low salinity. With the in situ measurements of environmental factors, our study showed that the 2022 summer bleaching events were caused by heat stress, not hyposalinity.
Twelve weeks after the initial coral bleaching event, a follow-up survey was conducted to assess the consequence of bleached coral at Sharp Island. No further bleaching was observed on the tagged coral colonies, and the recovery status varied among five locally common species. A. tumida showed the worst performance, with a recovery index of only 1.2 and a relatively high mortality rate (Fig. 4). P. carnosa took the longest time to recover but eventually showed a good recovery. G. columna, P. decussata, and P. lutea generally exhibited good recovery, with only some colonies experiencing partial mortality. Although the environmental stress in this bleaching event differed from the previous event (heat stress versus hyposalinity stress), our results also indicated a relatively higher mortality rate for A. digitifera, consistent with the observation of Xie et al. (2020). Since branching-form Acropora corals contribute significantly to habitat topography, providing vital support for high biodiversity in coral habitats (Ferrari et al. 2016), the loss of Acropora could lead to a dramatic reduction in the value of the coral community as a habitat for other species. In addition, contrary to the general understanding that massive corals are thermally tolerant (Van Woesik et al. 2011), our study showed that the massive coral Platygyra was highly susceptible and had a high bleached cover under thermal stress. Furthermore, the minor decline observed in other genera during each bleaching event should not be overlooked, as coral recruitment and larval survival rates are low in Hong Kong (Chui and Ang 2017).
The implication of the 2022 MHWs
The 2022 MHWs and their implications are significant in the context of global coral bleaching events. Previous MHWs associated with El Niño, such as the 1997–1998 event, caused widespread and severe coral bleaching, resulting in significant mortality in reefs across various regions, including Kenya, Vietnam, and Japan (Wilkinson 1998). The 2014–2017 MHW is considered the most damaging event on record, affecting reefs worldwide for an extended period (Eakin et al. 2019). However, Hughes et al. (2018) discovered that the warming effect during the La Niña years has been intensifying, comparable to the El Niño events. From 1985 to 2006, the sea surface temperature (SST) in the Coral Triangle increased at 0.2 °C per decade, and thermal stress in the northern and eastern parts was linked to La Niña (Peñaflor et al. 2009). In Hong Kong, the annual average of HWI also increased by 0.05 per year (Appendix II, Fig. S3). Since 2000, more instances of mass coral bleaching associated with La Niña have been reported, particularly in subtropical reefs worldwide. Examples include the mass coral bleaching in the Fiji Islands in 2000 (Cumming et al. 2000), the extensive bleaching along 1200 km of coastline in Western Australia from 2010 to 2011 (Moore et al. 2012), and the record-high SST-induced mass coral bleaching in the Beibu Gulf of the South China Sea in 2020 (Chen et al. 2022; Feng et al. 2022). These subtropical reefs are considered refuges for coral reefs under climate change, and certain subtropical corals have been reported to exhibit heat stress tolerance (Begar et al. 2014; Dellisanti et al. 2020, 2023). However, prolonged and intense MHWs can still lead to mass coral bleaching and threaten these ecosystems. Therefore, it is crucial to investigate the effects of La Niña on different tropical and subtropical regions and explore the potential interactions between MHWs and other stressors, such as hypoxia, as examined in this study (Fig. 5).
Before this study, there had been no reported cases of MHW-associated mass coral bleaching or benthos mortality in Hong Kong. Although MHW events have occurred almost yearly since 2009 (except for 2011), their severity did not lead to coral bleaching (Supplementary information, Table S1). In the past, coral bleaching events were considered to be triggered by hyposalinity resulting from heavy rainfall (Xie et al. 2017, 2020), although they did not have high-resolution environmental data to disentangle the effects of high temperature or low salinity. The occurrence of mass coral bleaching induced by MHWs, as observed in this study, highlights the issue of understanding the differential heat stress tolerance among local corals. Duprey et al. (2016) investigated the sensitivity of various local coral genera to water quality associated with eutrophication and identified the coral's tolerance to different physical water parameters. The results provided valuable insights for predicting potential variations in coral composition under future eutrophication conditions. Given the escalating threat of global warming, similar studies, whether conducted in laboratories or observed in the field, are essential to gather relevant information for predicting the future impact on coral communities. Moreover, understanding heat stress tolerance is crucial for restoration programmes aiming at reestablishing local coral communities.
This study also revealed depth-dependent bleaching due to the presence of thermocline, which was not extensively discussed in previous bleaching reports. The transect surveys conducted in shallow and deep waters documented distinct coral communities and varying levels of bleaching across different sites. For instance, the coral community in Bluff Island was co-dominated by Porites, Pavona, and Platygyra, with moderate bleaching observed in shallow water, while only Goniopora showed minor bleaching in deep water. The variation in coral community composition with depth has been previously established in Hong Kong, even within a few metres below the water surface (Tam and Ang 2008). It contributes to the differences in bleaching levels.
References
Arias-Ortiz A, Serrano O, Masqué P, Lavery PS, Mueller U, Kendrick GA, Rozaimi M, Esteban A, Fourqurean JW, Marbà N, Mateo MA, Murray K, Rule MJ, Duarte CM (2018) A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat Clim Change 8(4):338–344. https://doi.org/10.1038/s41558-018-0096-y
Beger M, Sommer B, Harrison PL, Smith SDA, Pandolfi JM (2014) Conserving potential coral reef refuges at high latitudes. Divers Distrib 20(3):245–257. https://doi.org/10.1111/ddi.12140
Bell JJ, Smith RO, Micaroni V, Strano F, Balemi CA, Caiger PE, Miller KI, Spyksma AJP, Shears NT (2023) Marine heat waves drive bleaching and necrosis of temperate sponges. Curr Biol 33(1):158–163 e2. https://doi.org/10.1016/j.cub.2022.11.013
Binnie Consultants Ltd (1995) 1994 hypoxia and mass mortality event in Mirs Bay final report. for geotechnical engineering office, Civil Enginering Department, HKSAR Government Hong Kong
Chen W, Park JK, Dong B, Lu R, Jung WS (2012) The relationship between El Niño and the western north pacific summer climate in a coupled GCM: role of the transition of El Niño decaying phases: impacts of El Niño decaying on western north pacific. J Geophys 117:D12111. https://doi.org/10.1029/2011JD017385
Chen Y, Zhai F, Li P, Gu Y, Wu K (2022) Extreme 2020 summer SSTs in the northern south China sea: implications for the beibu gulf coral bleaching. J Clim 35(13):4177–4190. https://doi.org/10.1175/JCLI-D-21-0649.1
Chui APY, Ang P (2017) Recruitment failure of scleractinian corals in a subtropical marginal environment: three-year monitoring in a Hong Kong marine park. Mar Pollut Bull 124(2):668–677. https://doi.org/10.1016/j.marpolbul.2017.06.005
Couch CS, Burns JHR, Liu G, Steward K, Gutlay TN, Kenyon J, Eakin CM, Kosaki RK (2017) Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (northwestern Hawaiian Islands). PLoS ONE 12(9):e0185121. https://doi.org/10.1371/journal.pone.0185121
Cumming RL, Toscano MA, Lovell ER, Carlson BA, Dulvy NK, Hughes A, Koven JF, Quinn NJ, Sykes HR, Taylor OJS, Vaughan D (2000) Mass coral bleaching in the Fiji Islands. Ninth Int Coral Reef Symp Bali 2:1161–1167
Dellisanti W, Tsang RHL, Ang P, Wu J, Wells ML, Chan LL (2020) Metabolic performance and thermal and salinity tolerance of the coral Platygyra carnosa in Hong Kong waters. Mar Pollut Bull 153:111005. https://doi.org/10.1016/j.marpolbul.2020.111005
Dellisanti W, Chung JTH, Yiu SKF, Tsang RHL, Ang P, Yeung YH, Qiu JW, McIlroy SE, Wells ML, Wu J, Chan LL (2023) Seasonal drivers of productivity and calcification in the coral Platygyra carnosa in a subtropical reef. Front Mar Sci 10:994591. https://doi.org/10.3389/fmars.2023.994591
Donovan MK, Burkepile DE, Kratochwill C, Shlesinger T, Sully S, Oliver TA, Hodgson G, Freiwald J, Van Woesik R (2021) Local conditions magnify coral loss after marine heatwaves. Science 372(6545):977–980. https://doi.org/10.1126/science.abd9464
Duprey NN, Yasuhara M, Baker DM (2016) Reefs of tomorrow: eutrophication reduces coral biodiversity in an urbanized seascape. Glob Change Biol 22(11):3550–3565. https://doi.org/10.1111/gcb.13432
Eakin CM, Sweatman HPA, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral reefs 38(4):539–545. https://doi.org/10.1007/s00338-019-01844-2
Feng Y, Bethel BJ, Dong C, Zhao H, Yao Y, Yu Y (2022) Marine heatwave events near Weizhou Island, Beibu Gulf in 2020 and their possible relations to coral bleaching. Sci Total Environ 823:153141. https://doi.org/10.1016/j.scitotenv.2022.153414
Ferrari R, Bryson M, Bridge T, Hustache J, Williams SB, Byrne M, Figueira W (2016) Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob Change Biol 22(5):1965–1975. https://doi.org/10.1111/gcb.13197
Fleddum A, Cheung SG, Hodgson P, Shin PKS (2011) Impact of hypoxia on the structure and function of benthic epifauna in Tolo Harbour. Hong Kong Marine Pollut Bull 63(5–12):221–229. https://doi.org/10.1016/j.marpolbul.2011.03.019
Fordyce AJ, Ainsworth TD, Heron SF, Leggat W (2019) Marine heatwave hotspots in coral reef environments: physical drivers, ecophysiological outcomes, and impact upon structural complexity. Front Mar Sci 6:498. https://doi.org/10.3389/fmars.2019.00498
Garrabou J, Coma R, Bensoussan N, Bally M, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido N, Torrents O, Zabala M, Zuberer F, Cerrano C (2009) Mass mortality in Northwestern mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol 15(5):1090–1103. https://doi.org/10.1111/j.1365-2486.2008.01823.x
Goodkin N, Switzer A, McCorry D, DeVantier L, True J, Hughen K, Angeline N, Yang T (2011) Coral communities of Hong Kong: long-lived corals in a marginal reef environment. Mar Ecol Prog Ser 426:185–196. https://doi.org/10.3354/meps09019
HKO (Hong Kong Observatory) (2022b) Meteorological Observations in Hong Kong for July 2022. Hong Kong Observatory. http://www.weather.gov.hk/wxinfo/pastwx/metob202207.htm. Accessed 31 August 2022
HKO (Hong Kong Observatory) (2022a) Daily Mean Sea Temperature (°C) (p.m.) at North Point, 2022. Hong Kong Observatory. https://www.hko.gov.hk/en/cis/dailyElement.htm?ele=SEATEMP_NP_PM&y=2022. Accessed 1 Oct 2022
Hobday AJ, Alexander LV, Perkins SE, Smale DA, Straub SC, Oliver ECJ, Benthuysen JA, Burrows MT, Donat MG, Feng M, Holbrook NJ, Moore PJ, Scannell HA, Sen Gupta A, Wernberg T (2016) A hierarchical approach to defining marine heatwaves. Progr Oceanogr 141:227–238. https://doi.org/10.1016/j.pocean.2015.12.014
Hobday A, Oliver E, Sen Gupta A, Benthuysen J, Burrows M, Donat M, Holbrook N, Moore P, Thomsen M, Wernberg T, Smale D (2018) Categorizing and Naming Marine Heatwaves. Oceanog 31(2):162–173. https://doi.org/10.5670/oceanog.2018.205
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371):80–83. https://doi.org/10.1126/science.aan8048
Kang B, Pecl GT, Lin L, Sun P, Zhang P, Li Y, Zhao L, Peng X, Yan Y, Shen C, Niu W (2021) Climate change impacts on China’s marine ecosystems. Rev Fish Biol Fisheries 31(3):599–629. https://doi.org/10.1007/s11160-021-09668-6
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) Lmertest package: tests in linear mixed effects models. J Stat Soft 82(13):1–26. https://doi.org/10.18637/jss.v082.i13
Leggat WP, Camp EF, Suggett DJ, Heron SF, Fordyce AJ, Gardner S, Deakin L, Turner M, Beeching LJ, Kuzhiumparambil U, Eakin CM, Ainsworth TD (2019) Rapid coral decay is associated with marine heatwave mortality events on reefs. Curr Biol 29(16):2723-2730 e4. https://doi.org/10.1016/j.cub.2019.06.077
Li XL, Shi HM, Xia HY, Zhou YP, Qiu YW (2014) Seasonal hypoxia and its potential forming mechanisms in the Mirs Bay, the northern south China sea. Cont Shelf Res 80:1–7. https://doi.org/10.1016/j.csr.2014.03.003
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, Van Woesik, R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4(2):122–131. https://doi.org/10.1046/j.1461-0248.2001.00203.x
McCorry D (2002) Hong Kong’s scleractinian coral communities: status, threats and proposals for management. Univ Hong Kong. https://doi.org/10.5353/th_b3124347
Mo S, Chen T, Chen Z, Zhang W, Li S (2022) Marine heatwaves impair the thermal refugia potential of marginal reefs in the northern South China Sea. Sci. Total Environ. 825:154100. https://doi.org/10.1016/j.scitotenv.2022.154100
Montano S, Seveso D, Galli P, Obura DO (2010) Assessing coral bleaching and recovery with a colour reference card in Watamu Marine park Kenya. Hydrobiol 655(1):99–108. https://doi.org/10.1007/s10750-010-0407-4
Moore JAY, Bellchambers LM, Depczynski MR, Evans RD, Evans SN, Field SN, Friedman KJ, Gilmour JP, Holmes TH, Middlebrook R, Radford BT, Ridgway T, Shedrawi G, Taylor H, Thomson DP, Wilson SK (2012) Unprecedented mass bleaching and loss of coral across 12° of latitude in Western Australia in 2010–11. PLoS ONE 7(12):e51807. https://doi.org/10.1371/journal.pone.0051807
Morton B, Morton J (1983) The sea shore ecology of Hong Kong. Hong Kong University Press, Hong Kong, Hong Kong
Nielsen JJV, Matthews G, Frith KR, Harrison HB, Marzonie MR, Slaughter KL, Suggett DJ, Bay LK (2022) Experimental considerations of acute heat stress assays to quantify coral thermal tolerance. Sci Rep 12(1):16831. https://doi.org/10.1038/s41598-022-20138-2
Oliver ECJ, Donat MG, Burrows MT, Moore PJ, Smale DA, Alexander LV, Benthuysen JA, Feng M, Sen Gupta A, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Straub SC, Wernberg T (2018) Longer and more frequent marine heatwaves over the past century. Nat Commun 9(1):1324. https://doi.org/10.1038/s41467-018-03732-9
Pearce AF, Feng M (2013) The rise and fall of the marine heat wave off western Australia during the summer of 2010/2011. J Mar Syst 111–112:139–156. https://doi.org/10.1016/j.jmarsys.2012.10.009
Peñaflor EL, Skirving WJ, Strong AE, Heron SF, David LT (2009) Sea-surface temperature and thermal stress in the coral triangle over the past two decades. Coral Reefs 28(4):841–850. https://doi.org/10.1007/s00338-009-0522-8
Roberts SD, Van Ruth PD, Wilkinson C, Bastianello SS, Bansemer M S (2019) Marine heatwave, harmful algae blooms and an extensive fish kill event during 2013 in South Australia. Front Mar Sci 6:610. https://doi.org/10.3389/fmars.2019.00610
RStudio Team (2020) RStudio: Integrated Development Environment for R. http://www.rstudio.com/. Assessed 17 October 2023
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25(3):453–460. https://doi.org/10.1007/s00338-006-0123-8
Smale DA, Wernberg T, Oliver ECJ, Thomsen M, Harvey BP, Straub SC, Burrows MT, Alexander LV, Benthuysen JA, Donat MG, Feng M, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Sen Gupta A, Payne BL, Moore PJ (2019) Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat Clim Chang 9(4):306–312. https://doi.org/10.1038/s41558-019-0412-1
Smith KE, Burrows MT, Hobday AJ, Sen Gupta A, Moore PJ, Thomsen M, Wernberg T, Smale DA (2021) Socioeconomic impacts of marine heatwaves: global issues and opportunities. Science 374(6566):eabj3593. https://doi.org/10.1126/science.abj3593
Smith KE, Burrows MT, Hobday AJ, King NG, Moore PJ, Sen Gupta A, Thomsen MS, Wernberg T, Smale DA (2022) Biological impacts of marine heatwaves. Annu Rev Mar Sci 15(1):119–145. https://doi.org/10.1146/annurev-marine-032122-121437
De Szoeke SP, Marke T, Brewer WA (2021) Diurnal Ocean Surface Warming Drives Convective Turbulence and Clouds in the Atmosphere. Geophys Res Lett 48(4): 2020GL091299
Tam TW, Ang P (2008) Repeated physical disturbances and the stability of sub-tropical coral communities in Hong Kong. China Aquatic Conserv Mar Freshw Ecosyst 18(6):1005–1024. https://doi.org/10.1002/aqc.922
Van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76. https://doi.org/10.3354/meps09203
Wilkinson CR (1998) The 1997–1998 Mass bleaching event around the world. In: Status of coral reefs of the world: 1998, Australian Institute of Marine Science and Global Coral Reef Monitoring Network: Townsville, Queensland, Australia, pp 15–38
Wyatt ASJ, Leichter JJ, Toth LT, Miyajima T, Aronson RB, Nagata T (2020) Heat accumulation on coral reefs mitigated by internal waves. Nat Geosci 13(1):28–34. https://doi.org/10.1038/s41561-019-0486-4
Wyatt ASJ, Leichter JJ, Washburn L, Kui L, Edmunds PJ, Burgess SC (2023) Hidden heatwaves and severe coral bleaching linked to mesoscale eddies and thermocline dynamics. Nat Commun 14(1):25. https://doi.org/10.1038/s41467-022-35550-5
Xie JY, Lau DCC, Kei K, Yu VPF, Chow WK, Qiu JW (2017) The 2014 summer coral bleaching event in subtropical Hong Kong. Mar Pollut Bull 124(2):653–659. https://doi.org/10.1016/j.marpolbul.2017.03.061
Xie JY, Yeung YH, Kwok CK, Kei K, Ang P, Chan LL, Cheang CC, Chow W, Qiu JW (2020) Localized bleaching and quick recovery in Hong Kong’s coral communities. Mar Pollut Bull 15:110950. https://doi.org/10.1016/j.marpolbul.2020.110950
Xu J, Yin K, Lee JH, Anderson DM, Jiang Y, Yuan X, Ho AYT, Harrison PJ (2012) Resistance of Hong Kong waters to nutrient enrichment: assessment of the role of physical processes in reducing eutrophication. J Oceanogr 68:545–560. https://doi.org/10.1007/s10872-012-0118-8
Yao Y, Wang C (2021) Variations in summer marine heatwaves in the south China sea. JGR Oceans. https://doi.org/10.1029/2021JC017792
Yeung YH, Xie JY, Kwok CK, Kei K, Ang P, Chan LL, Dellisanti W, Cheang CC, Chow WK, Qiu JW (2021) Hong Kong’s subtropical scleractinian coral communities: baseline, environmental drivers and management implications. Mar Pollut Bull 167:112289. https://doi.org/10.1016/j.marpolbul.2021.112289
Zhao Y, Chen M, Chung TH, Chan LL, Qiu JW (2023) The 2022 summer marine heatwaves and coral bleaching in China’s greater bay area. Mar Environ Res 189:106044. https://doi.org/10.1016/j.marenvres.2023.106044
Acknowledgements
The authors thank Mr. Parco Ng, Mr. Gavin Chow, and Mr. Ho Nam Leung for assistance in field surveys. We also extend our gratitude to Dr. Xiaowan Liu, Dr. Jingyi Zhu, and Dr. Xian Qin for their help in proofreading the manuscript.
Funding
Open access publishing enabled by City University of Hong Kong Library's agreement with Springer Nature. This study is supported by the Environmental and Conservation Fund (Project No.: 2020–08) of the Government of the Hong Kong Special Administrative Region, China.
Author information
Authors and Affiliations
Contributions
Jeffery Tzu Hao Chung presented conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and visualization. Walter Dellisanti performed methodology, formal analysis, validation, and writing—review. Ball Keng Po Lai revised writing—review & editing. Jiajun Wu carried out writing—review & editing, project administration, and funding acquisition. Jian-Wen Qiu conducted supervision and writing—review & editing. Leo L. Chan provided supervision, writing—review & editing, project administration, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, T.H., Dellisanti, W., Lai, K.P. et al. Local conditions modulated the effects of marine heatwaves on coral bleaching in subtropical Hong Kong waters. Coral Reefs 43, 1235–1247 (2024). https://doi.org/10.1007/s00338-024-02533-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02533-5