Abstract
This study systematically evaluated and ranked the efficacy of first- and second-line antibiotics antibiotic options for the clinical management of cellulitis and erysipelas through a network meta-analysis approach. From inception to July 04, 2024, a search for relevant randomized clinical trials (RCTs) was carried out using several databases. Antibiotics including azithromycin, cefaclor, cephalexin, cloxacillin, erythromycin, cephalexin plus trimethoprim-sulfamethoxazole, cephalexin plus placebo, flucloxacillin, clindamycin, ceftriaxone, penicillin, roxithromycin, and pristinamycin were assessed regarding cure rate, the eradication of baseline pathogens, diarrhea or vomiting, and rash. In total, 10 RCTs with 1,936 cellulitis or erysipelas patients were eligible for inclusion. There were no significant differences in the cure rates for cellulitis among the antibiotics analysed, with cefaclor demonstrating the most favorable profile for curative outcomes. In terms of side effects, ceftriaxone was identified as the least likely to induce diarrhea or vomiting. For erysipelas, pristinamycin showed the most promising results in achieving cure rates. Although a comparison of the three antibiotics revealed no significant differences in rash as a side effect in erysipelas, pristinamycin was observed to carry the highest risk for rash. Our findings indicate no significant differences in cure rates among antibiotics for cellulitis. However, ceftriaxone had the fewest gastrointestinal side effects. Pristinamycin showed the highest cure rates for erysipelas but with a higher risk of rash. Future research should focus on optimizing antibiotic selection for cellulitis and erysipelas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulitis and erysipelas are both skin infections caused by bacteria; erysipelas affect the upper layers of the skin, and cellulitis affects its deeper parts [1, 2]. Each year, more than 14 million instances of cellulitis are recorded in the United States, leading to approximately 3.7 billion dollars in outpatient medical costs and causing 650,000 hospital admissions annually [3]. Antibiotic therapy is essential for the treatment of cellulitis and erysipelas [2, 4]. Nonetheless, the treatment of cellulitis and erysipelas often involves the overuse of antibiotics [5]. Inappropriate and excessive use of antibiotics is linked to a heightened risk of adverse drug events, elevated treatment costs, and the emergence of antimicrobial resistance [6]. Investigations should concentrate on crafting personalized antibiotic prescription strategies for cellulitis, with the goal of reducing the unwarranted use of antibiotics.
Beta-lactam antibiotics are commonly recognized as first-line drugs of choice for treating cellulitis and erysipelas [4, 7]. Conversely, macrolides and lincosamides are typically considered as second-line agents [8]. However, a prior meta-analysis discovered that the effectiveness and side effect profile of treating cellulitis or erysipelas with a macrolide or lincosamide are comparable to those observed when using a beta-lactam antibiotic [8]. Optimal choice between first-line and second-line antibiotics for cellulitis and erysipelas is a critical consideration. Other antibiotics, such as trimethoprim-sulfamethoxazole, are used in combination with beta-lactam or macrolide antibiotics to treat cellulitis [9, 10]. After a seven-day course of treatment with cephalexin and trimethoprim-sulfamethoxazole, the cellulitis was successfully treated [10]. A study by Pallin and colleagues documented an 85% clinical cure rate when treating with a combination of cephalexin and trimethoprim-sulfamethoxazole [11]. A randomized clinical trial (RCT) found that the dual therapy of cephalexin and trimethoprim-sulfamethoxazole did not yield higher clinical cure rates than cephalexin administered alone [9]. However, the outcomes of the modified intention-to-treat-1 analysis did not rule out the potential for clinical superiority of the combination of cephalexin and trimethoprim-sulfamethoxazole [9]. Given the conflicting results and the optimum antibiotic regimen for cellulitis and erysipelas remains uncertain [12], a network meta-analysis is needed.
Objective
Herein, a network meta-analysis was conducted to evaluate and rank both the efficacy and the adverse events of primary and secondary antibiotic treatments for cellulitis.
Databases and methodology for literature search
A comprehensive search for relevant studies was undertaken in the PubMed, Embase, Cochrane Library, and Web of Science databases, following a pre-established search strategy. This search spanned from the earliest records available in these databases up to July 04, 2024. The PubMed search strategy was as follows: “Cellulitis” OR “Phlegmon” OR “erysipelas” AND “β-lactam” OR “Antibiotics, Monobactam” OR “Monocyclic beta-Lactams” OR “Monocyclic beta Lactams” OR “Monocyclic beta-Lactam” OR “Monocyclic beta Lactam” OR “beta-Lactam, Monocyclic” OR “Penicillin” OR “Antibiotics, Penicillin” OR “Penicillin Antibiotics) OR “Cephalosporin) OR “Acids, Cephalosporanic) OR “Acid, Cephalosporanic” OR “Macrolides” OR “Macrolide” OR “Erythromycin” OR (Azithromycin” OR “Azitrocin” OR (Roxithromycin” OR “MTW-Roxithromycin” OR “Clarithromycin” OR “Telithromycin” OR “Ketek” OR “Erythromycin” OR “Erymax” OR “Erycette” OR “Ilotycin” OR “Lincomycin” OR “Lincolnensin” OR “Epilincomycin” OR “Lincocin” OR “Clindamycin” OR “Chlolincocin” OR “7-Chloro-7-deoxylincomycin” OR “7 Chloro 7 deoxylincomycin” OR “Dalacin C” OR “Cleocin” OR “Lincomycin” OR “Lincolnensin” OR “Epilincomycin” OR “Hemihydrate Lincomycin Monohydrochloride” OR “Lincocin”.
Selection criteria
The eligibility criteria for study selection were formulated in accordance with the PICOS framework: (1) P (participants): patients with suspected or confirmed cellulitis or erysipelas excluding pregnant women; (2) I (intervention) and C (comparison): azithromycin, cefaclor, cephalexin, cloxacillin, erythromycin, cephalexin plus trimethoprim-sulfamethoxazole, cephalexin plus placebo, flucloxacillin, clindamycin, ceftriaxone, penicillin, roxithromycin, and pristinamycin; (3) O (outcomes): the outcomes were cure rate, the eradication of baseline pathogens, diarrhea or vomiting, and rash; (4) S (study design): RCTs; (5) publications in the English language.
The following criteria led to the exclusion of studies: (1) the related topics were recurrent cellulitis and erysipelas; (2) studies in which antibiotics were compared with no antibiotics, placebos, or no controls; (3) other antibiotics not used in combination with first- or second-lines antibiotics; (4) animal experiments; (5) case reports, meta-analyses, reviews, abstracts, letters, news, and peer reviews.
Data extraction
From each eligible study, we systematically extracted the following details: the investigator’s name, the year the study was published, the country of origin, the research design, the specific population, the specific interventions applied, the size of the study sample, demographic patient information including age, gender, and complications, main pathogen, and the features of the intervention methods (drug delivery route, dose administered, modes of administration, duration of treatment, and outcome. Two independent reviewers conducted screenings of all studies to assess their adherence to the inclusion and exclusion criteria. In cases of disagreement, a third author was consulted for resolution through discussion.
Risk of bias: assessment of study quality
The quality of the retrieved RCTs was appraised with a modified version of the Jadad scale [15]. Scores on this seven-point quality scale range from 0, denoting very poor quality, to 7, which represents very good quality. The scale encompasses various criteria: randomization (1 point for being described as randomized, 2 points for a detailed randomization method), concealment of randomization (1 point for mention of randomization concealment, 2 points for a detailed concealment method), blinding (1 point for being described as blind, 2 points for a detailed blinding method), and follow-up (1 point for describing withdrawals in each group). Low quality was rated on a scale of 1–3 and high quality on a scale of 4–7.
Quality of evidence assessment
The evidence quality for each network estimate was evaluated based on the standards established by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group [16]. This method assesses the quality of evidence by considering factors such as study limitations, uncertainty in results, inconsistency across studies, relevance to the research question, and potential publication bias, with a focus on the primary outcomes. The evidence quality is then ranked into four tiers: high, moderate, low, or very low.
Outcomes assessment
Cure rate was defined as a general remission of one or more symptoms and signs after antibiotic treatment compared to baseline data, including T < 37.5 ° C/complete cure of symptoms and signs/erythema size or severity of swelling or improvement or remission of infected areas.
Statistical analysis
The network meta-analysis was conducted within a Bayesian framework, utilizing Markov Chain Monte Carlo (MCMC) methods to derive the estimates. The model was configured with four chains. The initial iterations were set at 20,000, followed by 50,000 continuous iterations, with a step size established at 1. Statistical analysis was performed using the R4.3.0 software Gemtc 1.0.1 package, except for the network diagram, which was analyzed by Stata17 software.
The statistical heterogeneity within the network meta-analysis was evaluated by scrutinizing the extent of variation present within each pairwise comparison [17]. The I2 statistic served as the primary measure for assessing statistical heterogeneity; values below 25% signified low heterogeneity, those between 25% and 50% denoted moderate heterogeneity, and values exceeding 50% were indicative of high heterogeneity. Discrepancies between direct and indirect estimations may indicate inconsistency within the network meta-analysis and can cast doubts on the suitability of the transitivity assumption [18]. The evaluation of inconsistency between the direct and indirect evidence within the treatment network was conducted through an analysis of deviance residuals and the deviance information criterion (DIC) statistics within the constructed models adhering to both consistency and inconsistency [19]. If the difference is within 5, the data are basically consistent with the premise of consistency.
The risk ratios (RRs) complete with their 95% confidence intervals (CIs) from the direct meta-analysis were presented, alongside the 95% credible intervals (CrIs) derived from the network meta-analysis. Forest plots were created to illustrate the RR estimates alongside their 95% CIs. The league Table estimated interventions according to their RR in the network analysis. The network diagram offered a visual synthesis of both direct and indirect evidence gleaned from the studies. The heaviness of the lines was reflective of the quantity of studies within each comparative analysis. Additionally, the magnitude of the circles corresponded to the study’s sample size, with larger circles denoting larger cohorts. The probability of each treatment achieving a specific rank for each intervention was calculated and presented in rank probability tables.
Results
Basic characteristics of included studies
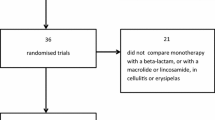
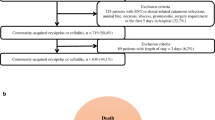
Initially, the database search yielded 3,274 studies. An additional 3 studies were also uncovered through other avenues, including direct communication with investigators in the field. Following the removal of duplicates, 2,035 studies remained. Subsequent to the application of inclusion and exclusion criteria during the screening process, 34 articles were selected for further consideration. Finally, 10 studies [9, 20,21,22,23,24,25,26,27,28] were included. The diagram of study selections is depicted in Fig. 1. In total, 1,936 patients diagnosed with cellulitis or erysipelas were incorporated into the analysis. All of 10 RCTs, 8 were rates as high quality. Table 1 presents the fundamental features of the studies included in this analysis.
A network meta-analysis of cure rate in different antibiotics for cellulitis
Seven studies involving 1,305 patients were included to assess the cure rate of different antibiotics for cellulitis. Cephalexin, cephalexin plus placebo, cephalexin plus trimethoprim-sulfamethoxazole, erythromycin, azithromycin, and cefaclor were assessed. Azithromycin was directly compared with cefaclor, cephalexin, and erythromycin. Cephalexin plus trimethoprim-sulfamethoxazole was directly compared with cephalexin, and cephalexin plus placebo. The large circle of cephalexin plus trimethoprim-sulfamethoxazole indicated that the sample sizes of studies about cephalexin plus trimethoprim-sulfamethoxazole were larger than those of the other five antibiotics. The network diagrams providing a summary of direct and indirect evidence of the cure rate of different antibiotics for cellulitis shown in Fig. 2a. Forest plots depict comparisons of cure rate between the six antibiotics (Fig. 3). There were no significant differences among the six antibiotics in cure rate for cellulitis (Table 2). According to the rank probability table (Table 3), cefaclor had the highest priority of cure rate (45.1%), followed by cephalexin plus placebo.
Three studies with a total of 203 patients evaluated the cure rate of oral administration of different antibiotics for cellulitis. Cefaclor, cephalexin, erythromycin, and azithromycin were included (Fig. 2b). In subgroup analysis of route of administration, there was no statistically significant difference in cure rates between the four oral antibiotics for cellulitis (Table 2). The priority of cure rate of 2 antibiotics was cefaclor > azithromycin (Table 3).
A network meta-analysis of the eradication of baseline pathogens in different antibiotics for cellulitis
One study involving 4 groups of data assessed the eradication of baseline pathogens in different antibiotics for cellulitis, including three antibiotics (Fig. 4). According to the league table (Table 2), azithromycin had a higher likelihood to eradicate the baseline pathogensthan than cloxacillin (RR: 2.23, 95% CrI: 1.14 to 5.06). The priority of cure rate of the 3 antibiotics was azithromycin (94.5%) > erythromycin > cloxacillin (Table 3).
A network meta-analysis of diarrhea or vomiting in different antibiotics for cellulitis
Diarrhea or vomiting after different antibiotics for cellulitis was examined in 2 studies. Flucloxacillin was directly compared with clindamycin and ceftriaxone (Fig. 5). Clindamycin (RR: 15.39, 95% CrI: 1.05 to 698.87) and flucloxacillin (RR: 9.42, 95% CrI: 1.45 to 281.36) had higher rates of diarrhea or vomiting than ceftriaxone (Table 2). Ceftriaxone had the lowest probability of diarrhea or vomiting (Table 3).
A network meta-analysis of cure rate in different antibiotics for erysipelas
The cure rate in different antibiotics for erysipelas was investigated in 2 studies including 286 patients. Penicillin was directly compared with roxithromycin and pristinamycin (Fig. 6). Pristinamycin had a higher cure rate compared with penicillin (RR: 1.20, 95% CrI: 1.05 to 1.37) (Table 4). Based on the rank table (Table 5), pristinamycin had the greatest likelihood of a cure rate for erysipelas.
A network meta-analysis of rash in different antibiotics for erysipelas
Two studies assessed rash in different antibiotics for erysipelas. Penicillin, roxithromycin, and pristinamycin were evaluated (Fig. 7). There were no significant differences among the three antibiotics in rash for erysipelas (Table 4). Pristinamycin was most likely to have rash in erysipelas, followed by penicillin (Table 5).
Discussion
In this study, we assessed and ranked the efficacy and safety profiles of both first-line and second-line antibiotic treatments for cellulitis and erysipelas. Based on the findings, there were no significant differences in the cure rate for cellulitis among the six antibiotics evaluated, with cefaclor showing the highest priority for achieving cure. Ceftriaxone was associated with the lowest likelihood of causing diarrhea or vomiting as a side effect. Additionally, for erysipelas, pristinamycin exhibited the greatest likelihood of achieving a cure rate. Although no significant differences were observed among the three antibiotics in terms of causing rash in erysipelas, pristinamycin was noted to have the highest potential for this side effect.
The study’s results indicate that among the six antibiotics assessed for treating cellulitis, there were no statistically significant differences in terms of cure rates. Cefaclor was identified as having the most favorable ranking for cure achievement. In addition, ceftriaxone was associated with the lowest likelihood of causing diarrhea or vomiting as a side effect compared with flucloxacillin or clindamycin. Cefaclor and ceftriaxone are antibiotics of the cephalosporin [29, 30]. An evidence-based review has shown that the standard treatment for cellulitis usually involves the use of cephalosporin antibiotics [7]. A cost-effectiveness analysis found that home treatment with intravenous ceftriaxone was not only more cost-effective but also more efficient than hospital-based treatment with intravenous flucloxacillin for children suffering from moderate or severe cellulitis [31]. A prior study also demonstrated that home treatment using intravenous ceftriaxone is not less effective than hospital-based treatment with intravenous flucloxacillin for children with cellulitis [24]. In light of the findings, it is imperative to conduct further research to identify the most efficacious antibiotics for the treatment of cellulitis in future studies.
In this analysis, pristinamycin exhibited the greatest likelihood of achieving a cure rate for erysipelas. An open-label study involving 42 patients has demonstrated the efficacy of pristinamycin in the treatment of non-necrotizing bacterial cellulitis in adults, particularly erysipelas [32]. In a study conducted across 22 French hospitals, pristinamycin was identified as a viable alternative to the standard intravenous-to-oral penicillin regimen for the treatment of erysipelas in adult hospital patients, offering the advantage of being a first-line oral treatment option [21]. Another study corroborated the findings, suggesting that pristinamycin could serve as an alternative to the conventional penicillin regimen in the management of erysipelas, with the added benefit of oral administration [33]. The adverse event is another consideration of antibiotics for cellulitis or erysipelas. In this study, the rash risk of pristinamycin in erysipelas was the highest. In a previous study, the rash was secondary to pristamycin treatment [34]. I In a study examining a patient with right hemiplegia who predominantly experienced a skin reaction on the left side after taking pristinamycin, it was found that maculopapular exanthema-like reactions represent the most common type of delayed-type hypersensitivity skin reaction to antibiotics in general and, specifically, to pristinamycin [35]. More adverse events, primarily gastrointestinal symptoms like nausea, vomiting, and diarrhea, were observed in the pristinamycin group compared to the penicillin group, but they were typically minor and seldom led to treatment discontinuation [21]. The adverse events of currently used first-line and second-line antibiotics for the treatment of erysipelas should also be investigated in the future.
A pilot RCT that compared high-dose cephalexin with standard-dose cephalexin for patients with cellulitis in emergency departments found that the high-dose cephalexin group experienced fewer treatment failure, however, the proportion of minor adverse reactions was higher [12]. Research conducted by Trottier et al. indicated that high-dose oral cephalexin appears to be an effective and safe treatment option for children with moderate cellulitis, achieving a success rate of 89.7% [36]. A multicenter trial has identified that the prophylactic use of low-dose penicillin following the first episode or recurrence of lower limb cellulitis is an intervention with exceedingly low cost [37]. A trial by Cranendonket al. highlight that more patients with severe cellulitis can achieve good long-term outcomes with more prolonged therapy [38]. In a systematic review and meta-analysis evaluating the impact of antibiotics on the clinical response of uncomplicated cellulitis over time, it was determined that the optimal timing for clinical reassessment is between 2 and 4 days post-treatment initiation [39]. However, the author suggested that due to significant heterogeneity and a limited number of studies included, it is essential to interpret these findings with caution [39]. A systematic review and meta-analysis, which assessed the durations of antibiotic treatment for acute cellulitis, found no evidence of differences in clinical response rates attributable to t the duration of therapy [5]. A systematic review assessing clinical responses to antibiotic treatment regimens for lower limb cellulitis has concluded that there are no significant differences in clinical responses among various types of antibiotics, routes of administration, durations of treatment, or dosages [40]. Due to the heterogeneity in treatment regimens, we were unable to conduct a subgroup meta-analysis. This limitation restricts our ability to draw definitive conclusions about the optimal dosing and duration of antibiotic therapy. This study underscore the need for future research to focus on well-defined treatment regimens, including specific antibiotics at precise doses and durations, to provide more conclusive evidence for clinical practice.
The primary strength of this meta-analysis lies in its ability to provide a quantitative assessment of various interventions for the treatment of cellulitis and erysipelas. The findings were organized based on specific outcome measures, allowing for the identification of the most effective treatment strategies. Furthermore, all the studies included in the analysis were RCTs. This systematic review and network meta-analysis may potentially assist in customizing antibiotic regimens for patients with cellulitis, ultimately leading to a reduction in unnecessary antibiotic utilization.
Limitations
Several limitations should be noted. Firstly, the use of language restrictions, such as only accepting articles in English, could introduce bias by omitting studies published in other languages. Secondly, the inability to perform subgroup analyses based on treatment duration and dose are a significant limitation. The variability in dosing and treatment duration across the included studies precluded a precise evaluation of the impact of specific doses and treatment durations on treatment outcomes. This limitation restricts our ability to draw definitive conclusions about the optimal dosing and duration of antibiotic therapy. Additionally, the diversity in dosing and treatment durations may have introduced potential sources of bias or confounding factors that could affect the generalizability of our findings. Thirdly, one such limitation pertains to the potential impact of comorbidities on treatment outcomes. While we aimed to provide a comprehensive analysis, the reporting of comorbid conditions in the included studies was not uniform, and detailed data on individual patient comorbidities were often lacking. This lack of information limits our ability to fully assess how the presence of comorbidities may influence the efficacy and safety of antibiotic treatments. Fourthly, a notable limitation of our study is the absence of detailed pathogen-specific data in the majority of the included trials. This lack of information on the causative organisms restricts our ability to assess the potential impact of specific pathogens on the efficacy of various antibiotic regimens. Fifthly, another of the key limitations is the variability in the time of result measurement across the included studies. The timing of outcome assessment can significantly influence the determination of cure rates and other treatment outcomes. Different studies reported outcomes at various time points’ post-treatment initiation, which could introduce heterogeneity in the interpretation of the results. This inconsistency in follow-up duration may affect the comparability of cure rates and the assessment of treatment efficacy over time. Sixthly, the scarcity of published studies for certain outcomes constrains the robustness of the conclusions drawn for those specific endpoints. The paucity of data limits the statistical power of the analysis and may introduce uncertainties in the estimated treatment effects, thereby affecting the generalizability of the results to broader clinical contexts.
Further research is needed to gain a more thorough insight into the efficacy and safety of antibiotics in treating cellulitis and erysipelas.
Conclusion
Our meta-analysis revealed no significant differences in cellulitis cure rates across antibiotics, highlighting cefaclor for its curative potential and ceftriaxone for its low side effect profile. Pristinamycin was most effective for erysipelas but with a higher rash risk. Our study suggests that future research should focus on prescribing antibiotics appropriately for patients with cellulitis and erysipelas.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Michael Y, Shaukat NM (2024) Erysipelas. In: StatPearls edn. Treasure Island (FL) ineligible companies. Disclosure: Nadia Shaukat declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC
Bystritsky RJ (2021) Cellulitis. Infect Dis Clin N Am 35(1):49–60
Brown BD, Hood Watson KL (2024) Cellulitis. In: StatPearls edn. Treasure Island (FL) ineligible companies. Disclosure: Kristen Hood Watson declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC
Boettler MA, Kaffenberger BH, Chung CG (2022) Cellulitis: a review of current practice guidelines and differentiation from Pseudocellulitis. Am J Clin Dermatol 23(2):153–165
Cross ELA, Jordan H, Godfrey R, Onakpoya IJ, Shears A, Fidler K, Peto TEA, Walker AS, Llewelyn MJ (2020) Route and duration of antibiotic therapy in acute cellulitis: a systematic review and meta-analysis of the effectiveness and harms of antibiotic treatment. J Infect 81(4):521–531
Gunderson CG (2016) Overtreatment of nonpurulent cellulitis. J Hosp Med 11(8):587–590
Long B, Gottlieb M (2022) Diagnosis and management of Cellulitis and Abscess in the Emergency Department setting: an evidence-based review. J Emerg Med 62(1):16–27
Ferreira A, Bolland MJ, Thomas MG (2016) Meta-analysis of randomised trials comparing a penicillin or cephalosporin with a macrolide or lincosamide in the treatment of cellulitis or erysipelas. Infection 44(5):607–615
Moran GJ, Krishnadasan A, Mower WR, Abrahamian FM, LoVecchio F, Steele MT, Rothman RE, Karras DJ, Hoagland R, Pettibone S et al (2017) Effect of Cephalexin Plus Trimethoprim-Sulfamethoxazole vs Cephalexin alone on clinical cure of uncomplicated cellulitis: a Randomized Clinical Trial. JAMA 317(20):2088–2096
Weesner E, Ghassemi H, Salapenka I, Konakanchi JS, Maggio G, Sethi R (2022) Injection site reaction to extended-release buprenorphine (Sublocade(®)) for opioid use disorder fourteen days after Administration. Kans J Med 15:302–304
Pallin DJ, Binder WD, Allen MB, Lederman M, Parmar S, Filbin MR, Hooper DC, Camargo CA Jr. (2013) Clinical trial: comparative effectiveness of cephalexin plus trimethoprim-sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Diseases: Official Publication Infect Dis Soc Am 56(12):1754–1762
Yadav K, Eagles D, Perry JJ, Taljaard M, Sandino-Gold G, Nemnom MJ, Corrales-Medina V, Suh KN, Stiell IG (2023) High-dose cephalexin for cellulitis: a pilot randomized controlled trial. Cjem 25(1):22–30
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y (2001) Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord 12(3):232–236
Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH (2014) A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical Res ed) 349:g5630
Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH, Valero V, Shen Y, Ueno NT (2015) Effectiveness of an adjuvant chemotherapy regimen for early-stage breast Cancer: a systematic review and network Meta-analysis. JAMA Oncol 1(9):1311–1318
Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking consistency in mixed treatment comparison meta-analysis. Stat Med 29(7–8):932–944
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE (2013) Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making: Int J Soc Med Decis Mak 33(5):641–656
Bernard P, Plantin P, Roger H, Sassolas B, Villaret E, Legrain V, Roujeau JC, Rezvani Y, Scheimberg A (1992) Roxithromycin versus penicillin in the treatment of erysipelas in adults: a comparative study. Br J Dermatol 127(2):155–159
Bernard P, Chosidow O, Vaillant L (2002) Oral pristinamycin versus standard penicillin regimen to treat erysipelas in adults: randomised, non-inferiority, open trial. BMJ (Clinical Res ed) 325(7369):864
Daniel R (1991) Azithromycin, erythromycin and cloxacillin in the treatment of infections of skin and associated soft tissues. European azithromycin Study Group. J Int Med Res 19(6):433–445
Griffith ME, Ellis MW (2013) Antimicrobial activity against CA-MRSA and treatment of uncomplicated nonpurulent cellulitis. Expert Rev anti-infective Therapy 11(8):777–780
Ibrahim LF, Hopper SM, Orsini F, Daley AJ, Babl FE, Bryant PA (2019) Efficacy and safety of intravenous ceftriaxone at home versus intravenous flucloxacillin in hospital for children with cellulitis (CHOICE): a single-centre, open-label, randomised, controlled, non-inferiority trial. Lancet Infect Dis 19(5):477–486
Kiani R (1991) Double-blind, double-dummy comparison of azithromycin and cephalexin in the treatment of skin and skin structure infections. Eur J Clin Microbiol Infect Diseases: Official Publication Eur Soc Clin Microbiol 10(10):880–884
Montero L (1996) A comparative study of the efficacy, safety and tolerability of azithromycin and cefaclor in the treatment of children with acute skin and/or soft tissue infections. J Antimicrob Chemother 37(Suppl C):125–131
Thomas MGJIDCP (2014) Oral clindamycin compared with sequential intravenous and oral Flucloxacillin in the treatment of Cellulitis in adults: a Randomized. Double-Blind Trial 22(6):1
Zar FA (2017) Adding trimethoprim-sulfamethoxazole to cephalexin did not increase clinical cure in uncomplicated cellulitis. Ann Intern Med 167(8):Jc40
Jeong SH, Jang JH, Cho HY, Lee YB (2021) Population Pharmacokinetic Analysis of Cefaclor in healthy Korean subjects. Pharmaceutics 13(5)
Alsowaida YS, Benitez G, Bin Saleh K, Almangour TA, Shehadeh F, Mylonakis E (2022) Effectiveness and safety of Ceftriaxone compared to Standard of Care for Treatment of Bloodstream Infections due to Methicillin-Susceptible Staphylococcus aureus: a systematic review and Meta-analysis. Antibiotics 11(3):375
Ibrahim LF, Huang L, Hopper SM, Dalziel K, Babl FE, Bryant PA (2019) Intravenous ceftriaxone at home versus intravenous flucloxacillin in hospital for children with cellulitis: a cost-effectiveness analysis. Lancet Infect Dis 19(10):1101–1108
Bernard P, Risse L, Bonnetblanc JM (1996) [Pristinamycin in the treatment of acute bacterial dermohypodermitis in adults. An open study of 42 patients]. Ann Dermatol Venereol 123(1):16–20
Oral alternative to (2002) Penicillin for adult erysipelas. Nurs Stand 17(13):10
Schmutz JL, Trechot P (2014) [Skin rash mimicking pityriasis rosea Gibert secondary to pristinamycin therapy]. Ann Dermatol Venereol 141(4):325–326
Delcroix F, Arnault JP, Chaby G, Gras-Champel V, Lok C (2016) A predominantly left-sided skin reaction to pristinamycin in a patient with right hemiplegia. JAAD case Rep 2(1):84–86
Trottier ED, Farley St-Amand B, Vincent M, Chevalier I, Autmizguine J, Tremblay S, Gouin S (2022) Outpatient management of moderate cellulitis in children using high-dose oral cephalexin. Paediatr Child Health 27(4):213–219
Mason JM, Thomas KS, Crook AM, Foster KA, Chalmers JR, Nunn AJ, Williams HC (2014) Prophylactic antibiotics to prevent cellulitis of the leg: economic analysis of the PATCH I & II trials. PLoS ONE 9(2):e82694
Cranendonk DR, Opmeer BC, van Agtmael MA, Branger J, Brinkman K, Hoepelman AIM, Lauw FN, Oosterheert JJ, Pijlman AH, Sankatsing SUC et al (2020) Antibiotic treatment for 6 days versus 12 days in patients with severe cellulitis: a multicentre randomized, double-blind, placebo-controlled, non-inferiority trial. Clin Microbiol Infect 26(5):606–612
Yadav K, Krzyzaniak N, Alexander C, Scott AM, Clark J, Glasziou P, Keijzers G (2022) The impact of antibiotics on clinical response over time in uncomplicated cellulitis: a systematic review and meta-analysis. Infection 50(4):859–871
Mistry K, Sharma S, Patel M, Grindlay D, Janjuha R, Smart P, Levell NJ (2021) Clinical response to antibiotic regimens in lower limb cellulitis: a systematic review. Clin Exp Dermatol 46(1):42–49
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
(1) Zhou Shu, Peishan Cai, conceiving and designing the study; (2) Zhou Shu, Jie Cao, He Li, Ping Chen, collecting the data; (3) Zhou Shu, Jie Cao, He Li, Ping Chen, analyzing and interpreting the data; (4) Zhou Shu, writing the manuscript; (5) Peishan Cai, Zhou Shu, providing critical revisions that are important for the intellectual content; (6) Zhou Shu, Jie Cao, He Li, Ping Chen, Peishan Cai, approving the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, because PubMed, Embase, Cochrane Library, and Web of Science databases belong to public databases, the patients involved in the database have obtained ethical approval, users can download relevant data for free for research and publish relevant articles, and our study is based on open-source data, and the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology do not require research using publicly available data to be submitted for review to their ethics committee, so there are no ethical issues and other conflicts of interest.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shu, Z., Cao, J., Li, H. et al. Efficacy and safety of first- and second-line antibiotics for cellulitis and erysipelas: a network meta-analysis of randomized controlled trials. Arch Dermatol Res 316, 603 (2024). https://doi.org/10.1007/s00403-024-03317-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-03317-1