Abstract
Background
Diffuse gliomas are among the most common brain tumors in adults and are associated with a dismal prognosis, especially in patients with glioblastoma. To date, tumor tissue acquisition is mandatory for conclusive diagnosis and therapeutic decision-making. In this study, we aimed to identify possible diagnostic and prognostic biomarkers in cerebrospinal fluid (CSF) and blood.
Methods
During glioma surgery at our institution, CSF and blood samples were collected from patients. Subsequently, targeted metabolomics analysis was used to detect and quantify circulating metabolites. The metabolome profiles of glioma patients were compared with those of patients in a control group who had undergone neurosurgery for other entities, such as nonglial tumors or hydrocephalus, and were correlated with established glioma diagnostic molecular markers.
Results
In this study, a total of 30 glioma patients were included, along with a control group of 21 patients without glioma. Serum metabolomic analysis did not detect any significant differences between the groups, whereas CSF-metabolome analysis revealed increased levels of six metabolites in glioma patients. Among these, the most pronounced differences were found for the biogenic amine putrescine (p = 0.00005). p-Cresol sulfate was identified as a potential CSF marker for determining isocitrate dehydrogenase (IDH) status in glioma patients (p = 0.0037).
Conclusion
CSF-metabolome profiling, unlike blood profiling, shows promise as a diagnostic tool for glioma patients with the potential to assign molecular subtypes. The next step will involve a larger multicenter study to validate these findings, with the ultimate objective of integrating CSF metabolomics analysis into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gliomas are the most common brain tumors, with an incidence varying from 4.67 to 5.73 per 100,000 persons [1, 2]. They are classified and graded according to the WHO classification of tumors of the central nervous system based on their histological appearance and molecular characteristics [3]. However, the preoperative diagnosis is mainly dependent on neuroimaging. Patient-related prognostic factors include patient age and Karnofsky Performance Score, whereas the most accurate prognostic and diagnostic indicators are tumor-related genetic markers. These include mutations in the isocitrate dehydrogenase (IDH) genes (IDH1 and IDH2), the 1p/19q codeletion, O6-methylguanine methyltransferase (MGMT) promoter methylation, cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) gene homozygous deletion, telomerase reverse transcriptase (TERT) promoter mutations, and epidermal growth factor receptor (EGFR) alterations, + 7/− 10 chromosome copy-number alterations and H3 p.K28 mutations [3]. To date, tumor tissue examinations are mandatory for the final diagnosis. The established therapeutic options include surgical resection followed by radiotherapy and chemotherapy, mostly with alkylating agents [4]. In cases of unclear lesions or deep-seated tumors, stereotactic biopsy is needed for tissue acquisition and further therapeutic decisions. Considering the currently available multimodal treatment options, the prognosis remains poor, especially in glioblastoma patients, who have an average overall survival of approximately 16 months [5].
In the past decade, the concept of liquid biopsy has gained increased importance in cancer research. Circulating tumor DNA, microRNA, long noncoding RNA, tumor-derived proteins, extracellular vesicles, and tumor cells in blood and cerebrospinal fluid (CSF) have been identified as potential diagnostic and prognostic markers in gliomas, yet this subject has received little attention thus far [6, 7]. Moreover, the topic of metabolomic studies in patients with gliomas remains largely underexplored. While a recent study on the metabolic profile of glioma tumor tissue demonstrated a correlation with the current WHO classification [8], data about circulating metabolites are needed.
In this study, we aimed to identify and quantify circulating metabolites in the blood and CSF of glioma patients by performing targeted metabolomics analysis. Furthermore, we explored the diagnostic value of the detected metabolites by analyzing their potential correlation with established molecular genetic markers.
Methods
Study design and patient selection
A collaborative project between the Department of Neurology and the Department of Neurosurgery at Hannover Medical School was initiated to identify new biomarkers in gliomas. After providing written informed consent, patients who required surgery for brain tumor removal were recruited for the study. Patients with nonglial tumors or patients who needed cranial surgery for other reasons (for example, hydrocephalus) served as controls. CSF and serum were collected from the patients intraoperatively. The CSF was taken at the beginning of the operation before the tumor was resected. Afterward, the biomaterial was immediately transferred to the Neurochemistry Laboratory of the Department of Neurology, where it was further processed and then frozen at − 80 °C. The maximum time from sampling to freezing was approximately 1 h. Neuropathological evaluation was performed according to the 2021 “World Health Organization Histological Classification of Tumors of the Central Nervous System” [3]. The study protocol was approved by the Ethics Committee at Hannover Medical School (No. 8269_BO_S_2019).
Targeted metabolomic analysis
All CSF and serum samples were stored at − 80 °C. For analysis, the samples were shipped to Fraunhofer ITEM (Hannover) on dry ice and analyzed in duplicate using a targeted metabolomics kit (MxP Quant 500 kit, Biocrates Life Science AG, Innsbruck, Austria). The concentrations of metabolites were measured on an AB SCIEX 5500 QTrapTM mass spectrometer (AB SCIEX, Darmstadt, Germany). Metabolite extraction and analyses were conducted following the manufacturer’s protocol (https://biocrates.com/mxp-quant-500-kit, accessed on 05 June 2023).
Data processing: After normalization and preprocessing of the data, MetIDQTM software (Biocrates) was used for peak integration and subsequent calculation of metabolite concentrations. All analytes that were above the limit of detection (LOD) in ≥ 80% of patients in at least one group were selected for further investigation as previously described [9, 10]. Values below the LOD were replaced by the reference value provided by the manufacturer (LOD/2). Missing values were replaced by the samplewise k-nearest neighbor algorithm using MetaboAnalyst 5.0.
Statistical analysis
The metabolite concentration data were entered into Metaboanalyst 5.0 software for multivariate analyses, including t tests, where Benjamini–Hochberg correction was applied with a false discovery rate (FDR) of 0.1 to account for multiple testing. A fold-change (FC) threshold of > 2 was set for differential abundance analysis, which was visualized as a volcano plot. Classical univariate receiver-operating characteristic (ROC) curve analysis was employed to evaluate the performance of the identified metabolites as possible biomarkers. Differential abundance analysis and receiver-operating characteristic (ROC) curve analysis were performed using the open source tool MetaboAnalyst 5.0 (http://www.metaboanalyst.ca).
The abbreviations used for phosphatidylcholines (PCs), triglycerides (TGs), sphingomyelins (SMs), hexosylceramides (HexCers), and ceramides (Cers) correspond to the Biocrates nomenclature.
Age and BMI are expressed as the median ± SD, and differences between groups were analyzed using the Mann‒Whitney test. Group comparisons for sex and comorbidities were performed with Fisher’s exact test.
Results
Cohort description
A total of 30 patients who underwent surgery for gliomas were included in this study. Surgery was performed according to the standard departmental techniques as described previously [11]. In 5 patients (17%), surgery was performed due to tumor recurrence (Table 1). Neuropathological evaluation according to the 2021 “World Health Organization Histological Classification of Tumors of the Central Nervous System” [3] revealed glioblastoma IDH-wildtype (CNS WHO grade 4) in 22 patients, oligodendroglioma IDH-mutant and 1p/19q-codeleted CNS WHO grade 2 in 2 patients and CNS WHO grade 3 in one patient, diffuse astrocytoma IDH-mutant (CNS WHO grade 2) in 2 patients, and astrocytoma IDH-mutant (CNS WHO grade 3), as well as diffuse midline glioma H3 K27-altered (CNS WHO grade 4) in one patient, respectively.
One patient showed a diffuse glioma without morphological signs of malignancy. The tumor exhibited no IDH1, IDH2, TERT, H3, or BRAF mutation, and had no EGFR amplification, no MYB rearrangement, and a positive MGMT status. The epigenetic profile of the tumor corresponded to the methylation class ‘neuroepithelial tumor with EP300:BCOR(L1) fusion’ in the Heidelberg brain tumor classifier version 12.3 (www.molecularneuropathology.org/mnp/classifiers/10). IDH1/2 mutations were found in 7 patients. The MGMT gene promoter status was analyzed in 19 patients with GBM, one with neuroepithelial tumor with EP300::BCOR(L1) fusion, and one with astrocytoma CNS WHO grade 2. Hypermethylation was detected in nine patients. Table 1 displays the clinical presentation, tumor characteristics, and treatment of the patients in the glioma group. In one patient (No. 30, Table 1), no CSF samples were available.
The control group consisted of 21 patients with no glioma. The detailed patient characteristics are provided in the supplementary material (Table S1). The patients’ ages ranged between 21 and 83 years in the glioma group and between 17 and 82 years in the control group (median age: glioma: 61.5 years; control: 56 years; p = 0.06). The ratio of men to women was 20/10 in the glioma group and 11/10 in the control group. There was no significant difference in BMI between the two groups (median BMI: glioma: 25; control: 28; p = 0.05). Arterial hypertension was found in 17 patients in the glioma group and in 6 patients in the control group (p = 0.08). Diabetes mellitus was diagnosed in 3 patients in the glioma group and in 2 patients in the control group (p > 0.99). The characteristics of the patients in the overall cohort are summarized in Table 2.
CSF-metabolome profile of all glioma patients compared to controls
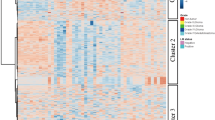
Of the 131 out of 630 detectable metabolites in CSF that were suitable for analysis, six showed a significant difference in concentration in both groups according to the FDR-corrected t test, as shown in Fig. 1. Compared with that in the control group, the concentration of putrescine significantly increased in the glioma group, with the most significant difference found for putrescine (p value of 0.00005). According to the correlation analysis, several other metabolites showed increased CSF concentrations in the glioma group, but the difference was not significant (Fig. 2A). Likewise, the heatmap that shows the relative concentrations of the top 20 metabolites for each patient illustrated that almost all significantly different metabolites were increased in concentration in most samples in the glioma group compared to those in the control group. The numeration of the glioma group in the heatmap (see figure legend) corresponds to the patients’ numbers in Table 1 (Fig. 2B). By applying ROC curve analysis for the identification of biomarker candidates, all six metabolites that were determined by t tests (Fig. 1) exhibited an area under the curve (AUC) > 0.7 (Fig. 2C). Again, putrescine also had the most notable effect, with an AUC of 0.785 (Fig. 2D). Among the 51 samples (30 glioma and 21 control) and 131 metabolites, 8 had missing values, which were managed as described previously 2.2.
Illustration of the six CSF metabolites that showed the most significant differences between the glioma and control groups according to the FDR-corrected t test. The graphs show normalized data (log10). Due to this normalization process, negative values were obtained for some metabolites. Values below the LOD were replaced by the reference value provided by the manufacturer (LOD/2)
Comparison of the CSF-metabolome profiles of glioma patients and control patients. A The top 25 metabolites correlated with glioma vs. control (Spearman rank correlation). The light red bars indicate positive correlations. B Heatmap of the top 20 metabolites (t test, using Euclidean clustering, Ward method). Each colored cell on the map corresponds to a concentration, with samples in rows and compounds in columns. Red and blue indicate higher or lower metabolite concentrations, respectively. The numbers of patients in the glioma group corresponding to Table 1 from left to right are as follows: 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 22, 16, 17, 3, 1, 18, 20, 19, 21, 22, 23, 24, 25, 26, 27, 28, 29, 2, and 13. C Table showing the univariate ROC analysis of the metabolites selected according to the volcano plot. Putrescine, xanthine, betaine, glutamate, aspartate, and acetylcarnitine (C2) showed considerable areas under the curve (> 0.7). D Example ROC curve of putrescine with an AUC of 0.785. Glu Glutamate, Asp aspartate, C0 carnitine, GABA γ-aminobutyric acid, Cys cysteine
CSF-metabolome profile of GBM subgroup compared to controls
The complementary comparison of the CSF-metabolome profiles of control patients with those of patients with primary glioblastoma (n = 18) revealed significant differences. Out of 630 detectable metabolites in the CSF, 131 were suitable for analysis, and 88 showed a significant difference in concentration between the two groups according to the FDR-corrected t test. The metabolites with the highest significance (according to p value) were putrescine, cystine, glutamate, aspartate, xanthine, and p-cresol-SO4. In the ROC curve analysis, all six metabolites exhibited an area under the curve (AUC) > 0.7. Figure 3A, B shows the concentration differences and the AUC for the two most relevant metabolites, putrescine and glutamate. Figure 3C represents the heat map of the top 20 metabolites with the largest differences between the GBM and control patients.
Comparison of the CSF-metabolome profiles of patients with primary GBM and control patients. A Normalized concentrations of putrescine and glutamate in CSF of GBM patients vs. controls. B Example ROC curve of putrescine with an area under the curve (AUC) of 0.828 and glutamate with an AUC of 0.815. C Heatmap of the top 20 metabolites (t test, using Euclidean clustering, ward method). Each colored cell on the map corresponds to a concentration, with samples in rows and compounds in columns. Red and blue indicate higher or lower metabolite concentrations, respectively. The numbers of patients in the GBM group correspond to Table 1 from left to right are as follows: 4, 6, 7, 8, 10, 12, 17, 3, 18, 20, 21, 22, 23, 25, 27, 28, 29, 2. AA arachidonic acid, Asp aspartate, Glu glutamate, GABA γ-aminobutyric acid, C0 carnitine, HCys homocysteine, PC phosphatidylcholine, TG triglyceride, HexCer hexosylceramide
The FDR-corrected T test showed no differences between the CSF-metabolomic profiles of all patients with primary glioma compared to the group of patients with glioma recurrence (Supplemental Figure S1). The same applied to the comparison of the CSF-metabolomic profiles of primary glioblastoma and glioblastoma recurrence (Supplemental Figure S2).
Comparison of the serum metabolome profiles of glioma patients and controls
Serum metabolome profiling revealed no metabolites with significant concentration differences between the two groups (Fig. 4A). In trend, few metabolites, including putrescine, showed increased concentrations in the serum of glioma patients, whereas the majority of metabolites in the serum were reduced (Fig. 4B). There were 506 out of 630 metabolites detectable > LOD in more than 80% of the samples in at least one group. No values were missing.
Comparison of the serum metabolome profiles of glioma patients and control patients. A Volcano plot showing no significant differences for the investigated metabolites. Each point represents a metabolite. B Plot of correlation coefficients of the top 25 metabolites and their relative concentrations between the two groups. Metabolites with red bars are upregulated in the glioma group compared to the control group, and metabolites with blue bars are downregulated. C14 Tetradecanoylcarnitine, FA 18:2 octadecadienoic acid, GLCAS glycolithocholic acid sulfate, TDCA taurodeoxycholic acid, DHEAS dehydroepiandrosterone sulfate, C18 octadecanoylcarnitine, Trp tryptophan, Thr threonine, Pro proline, 3-IAA 3-indoleacetic acid, t4-OH-Pro trans-4-hydroxyproline, Gly glycine, Ser serine
However, if only the serum metabolome profile of patients with primary GBM was considered in comparison to control patients, significant differences were found. Of the 506 out of 630 detectable metabolites in the serum that were suitable for analysis, cortisol and DHEAS showed a significant higher concentration in the primary GBM group according to the FDR-corrected t test (Supplemental Figure S3).
CSF-metabolome profile of IDH-mutant glioma compared to wild-type glioma
When comparing the CSF-metabolome profiles of patients with IDH-mutated glioma and those with wild-type glioma, 18 metabolites showed considerable differences in concentration according to the volcano plot (FC and raw p value) (Fig. 5A). These differences were not reflected by the FDR-corrected t test, as the set limits were not met.
CSF-metabolome profile of patients with IDH-mutant gliomas (n = 7) compared to patients with wild-type gliomas (n = 22). A Volcano plot. Each point represents a metabolite; red (1/160) represents upregulated metabolites in IDH-mutant glioma compared with IDH wild-type glioma, blue (17/160) represents downregulated metabolites, and gray represents metabolites with no difference between the IDH-mutant and IDH wild-type glioma groups [raw p value < 0.05 and fold change (FC) > 2]. B Representative representation of the metabolite p-cresol sulfate (p-Cresol-SO4), which showed the most significant difference in concentration in the group comparison and was significantly increased in the glioma wild-type group (FDR-corrected t test). C According to the univariate ROC curve analysis, among the metabolites selected according to the volcano plot, hexoses (H1), p-cresol sulfate (p-cresol-SO4), glycolithocholic acid sulfate (GLCAS), ceramide (d18:1/18:0), and homocysteine (HCys) had considerable AUCs (> 0.83). AA arachidonic acid, C0 carnitine, Glu glutamate, HexCer hexosylceramides, OH-GlutAcid 3-Hydroxyglutaric acid, PC aa/ae glycerophospholipids, SM (OH) sphingolipids, SM sphingomyelin, Suc succinic acid, WT wild type
3-Hydroxyglutaric acid (OH-GlutAcid) was significantly increased in the CSF of patients with IDH-mutated glioma, whereas the other 17 metabolites, especially p-cresol sulfate (p-cresol-SO4), homocysteine (HCys), glycolithocholic acid sulfate (GLCAS), hexoses (H1), and ceramide (d18:1/18:0), were decreased. P-Crescol-SO4 showed the most significant difference in concentration (Fig. 5B, C) and proved to be a potentially suitable biomarker in the ROC analysis, with an AUC of 0.8442.
For this analysis, 160/630 metabolites were selected as described previously in 2.2. Among 29 samples (22 IDH WT, 7 IDH MUT) and 160 compounds, there were 7 missing values.
CSF-metabolome profile of patients with a hypermethylated MGMT promoter compared to patients without MGMT promoter hypermethylation
In the CSF of patients with GBM with a methylated MGMT promoter, three triglycerides, TG (20:3_36:3), TG (16:0_38:3), and TG (18:2_36:2), were found to be significantly elevated according to the volcano plot. These differences were not reflected by the FDR-corrected t test, as the set limits were not met. For this analysis, 198/630 metabolites were selected as described in Sect. 2.2. Among 21 (9 with MGMT+ and 12 with MGMT) samples and 180 compounds, 6 had missing values.
Discussion
Here, we analyzed the serum and CSF-metabolome profiles of 30 glioma patients and compared them with profiles from 21 control patients, thereby contributing to the understanding of metabolomic changes in gliomas and potentially improving diagnostic methods. No significant differences were found in the serum profiles of glioma patients compared to controls, suggesting that serum may not be suitable for this purpose (Fig. 4). It should be mentioned that individual significant metabolites could be detected if only the sera of the glioblastoma patients were considered in comparison to the control group (Supplemental Figure S3). Here, the significantly increased cortisol value was particularly noticeable, which is most likely due to the oral dexamethasone medication required by glioblastoma patients (Table 1) or the increased stress reaction following the usually more complex glioblastoma operation.
In the CSF, 6 out of the 131 detectable metabolites were significantly increased in the glioma group. These included the neurotransmitter glutamate and the biogenic amine putrescine. Among the metabolites detected, putrescine appears to be the most promising biomarker candidate for differentiating between gliomas and non-malignant brain tumors, as indicated by an AUC of 0.785 (Figs. 1, 2). When considering only the glioblastoma patients within the glioma group, similar results were detected regarding the differential CSF-metabolome profile compared to the control cohort (Fig. 3). Putrescine, a metabolic precursor of spermidine and spermine, is primarily synthesized by the enzyme ornithine decarboxylase [12]. Since the 1980s, studies have shown elevated putrescine concentrations and ornithine decarboxylase activity in brain tumors [13, 14], with some indications suggesting a correlation between polyamine levels and tumor malignancy [15]. In 2001, Ernestus and colleagues identified ornithine decarboxylase activity as a marker of brain tumor malignancy; ornithine decarboxylase activity was notably greater in gliomas and increased with tumor severity, although polyamine concentrations did not consistently correlate with the degree of malignancy in their study of 670 patients [16]. Recent research has further explored the role of polyamines in cancer and autoimmunity. Notably, reduced polyamine levels are observed in autoimmune disorders such as systemic lupus erythematosus and are correlated with increased inflammatory responses [17, 18]. This finding is in line with the suggestion that polyamines inhibit antitumor responses in tumors such as malignant gliomas [19]. Antineoplastic therapies targeting polyamine synthesis and uptake have shown promising effects, for instance, reducing tumor size in neuroblastoma xenograft models [19]. Polyamines also influence the immune microenvironment of solid tumors, particularly through the recruitment of immunosuppressive tumor-associated macrophages (TAMs), which express [20,21,22] the enzyme arginase-1 involved in putrescine production, to CNS tumors [23, 24]. Due to its chemical properties as an amine, it appears to function as a buffering substance, thereby promoting the survival of anti-inflammatory and therefore immunosuppressive tumor-associated myeloid cells [25]. These findings reinforce the significant role of polyamines in tumor biology, especially in CNS malignancies, suggesting that putrescine could serve as both a diagnostic CSF marker and a therapeutic target in malignant glioma [25].
In addition to putrescine, the CSF-metabolome profile analysis of patients with gliomas showed significant differences in the concentrations of aspartate, glutamate, and acylcarnitine compared to those in the control group. The elevated glutamate levels in the glioma group align with the previous findings that glutamate signaling promotes glioma invasion and growth [26]. Glioma cells often release large amounts of glutamate through glutaminase, which converts glutamine to glutamate [27], coupled with potential impairment in extracellular glutamate removal [28]. Consequently, the glutamatergic axis has been considered a possible therapeutic target for glioma treatment, including for the investigation of tumor treatments aimed at disrupting neuroglioma signaling [29]. Aspartate becomes crucial when glucose and glutamine, the primary carbon sources in rapidly proliferating cells, are depleted. High aspartate levels are essential for maintaining key metabolic pathways such as purine/pyrimidine synthesis and the generation of nonessential amino acids [30]. Elevated aspartate concentrations have previously been reported in human glioma tissue metabolomic studies [31].
Moreover, cancer can significantly alter lipid metabolism [32,33,34,35]. In 2020, researchers at Ohio State University reported that the enzyme diacylglycerol acyltransferase 1 is upregulated in glioblastoma [36]. Inhibition of this enzyme induced cell apoptosis in glioblastoma cells and reduced tumor growth both in vitro and in vivo. Notably, knocking down diacylglycerol acyltransferase 1 caused a marked increase in acylcarnitines, which transport fatty acids into mitochondria for oxidation and energy production in neuronal energy metabolism [37]. Our analysis revealed significantly elevated concentrations of acylcarnitine in patients with malignant glioma compared to controls, suggesting increased lipid metabolism in glioma patients.
To our knowledge, this study represents the largest cohort of patients with gliomas, including glioblastoma, to whom comprehensive metabolomic analysis was performed. A 2018 study by Ballester and colleagues compared CSF from 23 brain tumor patients, including those with brain metastases from lung cancer or breast cancer, with that from 8 controls [38]. However, their CSF samples were obtained by lumbar puncture or via puncture of an Ommaya reservoir, not intraoperatively, as in our study. They identified 43 metabolites with concentration differences in tumor patients, focusing on glycine, arginine, choline, and nitrogen metabolism alterations [38]. Tricarboxylic acid cycle metabolites, including malic acid and succinate, were elevated in the CSF of CNS tumor patients, especially in those with IDH-mutant gliomas. Our study also revealed elevated acylcarnitine levels in glioma patients’ CSF but only in IDH wild-type tumors [38]. A 2022 publication on diffuse glioma tumor tissue metabolome identified distinct metabolites affected by tumor histology, IDH1 mutation status, or therapy. IDH1 wild-type gliomas had higher neurotransmitter levels, possibly linked to their poorer prognosis [39]. In our study, CSF glutamate differentiated malignant glioma patients from controls but showed no differences between IDH-mutated and IDH wild-type gliomas. However, p-cresol sulfate, homocysteine, glycolithocholic acid sulfate, ceramide (d18:1/18:0), and hexoses were significantly elevated in the CSF of patients with IDH wild-type gliomas (Fig. 5). Only 3-hydroxyglutaric acid was increased in IDH-mutated gliomas. p-Cresol sulfate is a sulfate conjugate of the bacterial metabolite p-cresol, which is a uremic toxin and originates when gut bacteria ferment proteins in the large intestine. While there is a well-established link between high blood levels of p-cresol sulfate and mortality from cardiovascular disease [40], a positive correlation of this metabolite with the development of tumor diseases could only be drawn for clear cell renal cell carcinoma [41]. The role of p-cresol sulfate in neurological and neuro-oncological tumor diseases is still unclear, although gut microbiome connections are suspected [42]. The decrease in homocysteine in IDH1-mutated glioma patients might be due to a shift toward glutathione synthesis in the hypoxic tumor environment to counterbalance reactive oxygen species [43].
In our study, the CSF-metabolomic profile of patients with MGMT promoter hypermethylation showed only minor alterations. Specifically, the levels of three triglycerides [TG (20:3_36:3), TG (16:0_38:3), and TG (18:2_36:2)] were significantly greater in patients with MGMT promoter hypermethylation than in patients without MGMT promoter hypermethylation (Fig. 6). To date, there has been limited research in this area. However, a recent publication proposed an enrichment of metabolites related to glycerophospholipid metabolism and sphingolipid metabolism, findings that are in line with our results [44].
In addition to the important findings that could influence the diagnostic landscape for gliomas, this study has certain limitations. In particular, the number of patients in the sub-analyses on IDH mutation status and MGMT methylation status was limited and the glioma group included both patients with primary glioma and those with tumor recurrence. However, it should be noted that the CSF-metabolome profiles of primary glioma vs. glioma recurrence, as well as those of primary GBM vs. GBM recurrence, did not show any significant differences. In addition to the tumor diagnosis itself, the patients within the glioma cohort differed in terms of tumor location, tumor volume, required steroid dose, extent of tumor necrosis, or possible neurological comorbidities. While tumor localization, neurological comorbidities, or the presence of epileptic seizures likely had less impact in the cohort presented in this study, the influence of oral steroids on the metabolome profile, in particular, must be considered. This likely applies specifically to the differences detected in the serum metabolome profiles of control and GBM patients. A further limitation concerns the control cohort, which was rather heterogeneous. A certain impact of the underlying diseases in the control group on the metabolome profile in the CSF cannot be ruled out. However, at least in patients with vestibular/trigeminal schwannoma—conditions distant from the CSF compartment—or in patients with hydrocephalus or idiopathic intracranial hypertension, a minor influence can be assumed. The same consideration applies to patients with non-tumorous changes such as colloid or arachnoid cysts. In future studies, the metabolome profiles of non-malignant brain tumor diseases, such as meningioma, should be compared with those of malignant tumor diseases in a differentiated manner, if possible.
CSF-metabolome profile of glioma patients with a hypermethylated MGMT promoter (MGMT+) (n = 9) compared to patients without MGMT promoter hypermethylation (MGMT-) (n = 12). A Volcano plot. Each point represents a metabolite, red points (3/131) represent upregulated metabolites in the MGMT+ group compared with the MGMT– group, and gray points represent metabolites with no difference between the MGMT+ and MGMT– groups [raw p value < 0.1 and fold change (FC) > 2]. B Exemplary illustration of the metabolite TG (20:3_36:3) and comparison of its concentrations using an FDR-corrected t test
Conclusions
This study successfully demonstrated that the CSF-metabolome profile of glioma and especially GBM patients significantly differed from that of controls. In particular, putrescine was identified as a potential diagnostic biomarker for gliomas. In addition, it was possible to correlate the metabolome signature in the CSF with the IDH status within the glioma group. The next steps will involve validating these results in a larger, multicenter cohort and investigating whether differences in subgroups would have an instructive impact on outcome prediction.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Abbreviations
- 3-IAA:
-
3-Indoleacetic acid
- AUC:
-
Area under the curve
- BSC:
-
Best supportive care
- BMI:
-
Body mass index
- C0:
-
Carnitine
- C14:
-
Tetradecanoylcarnitine
- C18:
-
Octadecanoylcarnitine
- CRTx:
-
Chemoradiotherapy
- CSF:
-
Cerebrospinal fluid
- CTx:
-
Chemotherapy
- DHEAS:
-
Dehydroepiandrosterone sulfate
- FA 18:2:
-
Octadecadienoic acid
- FDR:
-
False discovery rate
- F:
-
Female
- GABA:
-
γ-Aminobutyric acid
- GBM:
-
Glioblastoma
- GLCAS:
-
Glycolithocholic acid sulfate
- Glu:
-
Glutamate
- Gly:
-
Glycine
- GTR:
-
Gross total resection
- HCys:
-
Homocysteine
- HexCer:
-
Hexosylceramides
- H1:
-
Hexoses
- IDH:
-
Isocitrate dehydrogenase
- KPS:
-
Karnofsky performance score
- LOD:
-
Limit of detection
- M:
-
Male
- MGMT:
-
O6-Methylguanine methyltransferase
- Mut:
-
mutant
- N/A:
-
Not applicable
- OH-GlutAcid:
-
3-Hydroxyglutaric acid
- p-Cresol-SO4:
-
p-Cresol sulfate
- Pro:
-
Proline
- ROC:
-
Receiver operating characteristic
- RTx:
-
Radiotherapy
- Ser:
-
Serine
- SM (OH):
-
Sphingolipids (Hydroxylated)
- SM:
-
Sphingomyelin
- STB:
-
Stereotactic biopsy
- STR:
-
Subtotal resection
- Suc:
-
Succinic acid
- TAMs:
-
Tumor-associated macrophages
- TDCA:
-
Taurodeoxycholic acid
- TG:
-
Triglyceride
- Thr:
-
Threonine
- Trp:
-
Tryptophan
- TTF:
-
Tumor-treating fields
- t4-OH-Pro:
-
Trans-4-hydroxyproline
- WT:
-
Wild type
References
Larjavaara S, Mäntylä R, Salminen T, Haapasalo H, Raitanen J, Jääskeläinen J, Auvinen A (2007) Incidence of gliomas by anatomic location. Neuro Oncol 9(3):319–325
Gousias K, Markou M, Voulgaris S, Goussia A, Voulgari P, Bai M, Polyzoidis K, Kyritsis A, Alamanos Y (2009) Descriptive epidemiology of cerebral gliomas in northwest Greece and study of potential predisposing factors, 2005–2007. Neuroepidemiology 33(2):89–95
Figarella-Branger D, Appay R, Metais A, Tauziède-Espariat A, Colin C, Rousseau A, Varlet P (2022) The 2021 WHO classification of tumours of the central nervous system. Ann Pathol 42(5):367–382
Weller M, van den Bent M, Preusser M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
Stupp R, Taillibert S, Kanner A et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318(23):2306–2316
Jones J, Nguyen H, Drummond K, Morokoff A (2021) Circulating biomarkers for glioma: a review. Neurosurgery 88(3):E221
Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA (2021) Prognostic and predictive biomarkers in gliomas. Int J Mol Sci 22(19):10373
Bjorkblom B, Wibom C, Eriksson M, Bergenheim AT, Sjöberg RL, Jonsson P, Brännström T, Antti H, Sandström M, Melin B (2022) Distinct metabolic hallmarks of WHO classified adult glioma subtypes. Neuro Oncol 24(9):1454–1468
Ratuszny D, Sühs KW, Novoselova N, Kuhn M, Kaever V, Skripuletz T, Pessler F, Stangel M (2019) Identification of cerebrospinal fluid metabolites as biomarkers for enterovirus meningitis. Int J Mol Sci 20(2):337
Al-Mekhlafi A, Sühs KW, Schuchardt S, Kuhn M, Müller-Vahl K, Trebst C, Skripuletz T, Klawonn F, Stangel M, Pessler F (2021) Elevated free phosphatidylcholine levels in cerebrospinal fluid distinguish bacterial from viral CNS infections. Cells 10(5):1115
Hong B, Wiese B, Bremer M, Heissler HE, Heidenreich F, Krauss JK, Nakamura M (2013) Multiple microsurgical resections for repeated recurrence of glioblastoma multiforme. Am J Clin Oncol 36(3):261–268
Pegg AE (1986) Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J 234(2):249–262
Scalabrino G, Ferioli ME (1985) Degree of enhancement of polyamine biosynthetic decarboxylase activities in human tumors: a useful new index of degree of malignancy. Cancer Detect Prev 8(1–2):11–16
Harik SI, Sutton CH (1979) Putrescine as a biochemical marker of malignant brain tumors. Cancer Res 39(12):5010–5015
Ernestus RI, Röhn G, Schröder R, Klug N, Hossmann KA, Paschen W (1992) Activity of ornithine decarboxylase (ODC) and polyamine levels as biochemical markers of malignancy in human brain tumors. Acta Histochem Suppl 42:159–164
Ernestus RI, Röhn G, Schröder R, Els T, Klekner A, Paschen W, Klug N (2001) Polyamine metabolism in brain tumours: diagnostic relevance of quantitative biochemistry. J Neurol Neurosurg Psychiatry 71(1):88–92
Song J, Shan Z, Mao J, Teng W (2019) Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinol (Oxf) 90(5):727–736
Kim HA, Lee HS, Shin TH, Jung JY, Baek WY, Park HJ, Lee G, Paik MJ, Suh CH (2018) Polyamine patterns in plasma of patients with systemic lupus erythematosus and fever. Lupus 27(6):930–938
Gamble LD, Purgato S, Murray J, Xiao L, Yu DMT, Hanssen KM et al (2019) Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci Transl Med 11(477):eaau1099
Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP et al (2017) Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol 19(6):796–807
Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S et al (2020) Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181(7):1643 e17-1660 e17
Feng Y, Ye Z, Song F, He Y, Liu J et al (2022) The role of TAMs in Tumor microenvironment and new research progress. Stem Cells Int 2022:5775696
Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB et al (2018) Arginase: a multifaceted enzyme important in health and disease. Physiol Rev 98(2):641–665
Brooks HB, Phillips MA (1997) Characterization of the reaction mechanism for Trypanosoma brucei ornithine decarboxylase by multiwavelength stopped-flow spectroscopy. Biochemistry 36(49):15147–15155
Miska J, Rashidi A, Lee-Chang C, Gao P, Lopez-Rosas A, Zhang P et al (2021) Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv 7(8):eabc8929
Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T et al (2019) Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573(7775):532–538
Yao PS, Kang DZ, Lin RY, Ye B, Wang W, Ye ZC (2014) Glutamate/glutamine metabolism coupling between astrocytes and glioma cells: neuroprotection and inhibition of glioma growth. Biochem Biophys Res Commun 450(1):295–299
Corbetta C, Di Ianni N, Bruzzone MG, Patanè M, Pollo B, Cantini G et al (2019) Altered function of the glutamate-aspartate transporter GLAST, a potential therapeutic target in glioblastoma. Int J Cancer 144(10):2539–2554
Kumaria A, Ashkan K (2023) Novel therapeutic strategies in glioma targeting glutamatergic neurotransmission. Brain Res 1818:148515
Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN et al (2018) Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab 28(5):706 e6-720 e6
Lee JE, Jeun SS, Kim SH, Yoo CY, Baek HM, Yang SH et al (2019) Metabolic profiling of human gliomas assessed with NMR. J Clin Neurosci 68:275–280
Guo D, Bell EH, Chakravarti A (2013) Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol 2(3):289–299
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7(10):763–777
Srivastava NK, Pradhan S, Gowda GA, Kumar R (2010) In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: one possible diagnostic view. NMR Biomed 23(2):113–122
Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T et al (2009) Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res 50(Suppl):S9-14
Cheng X, Geng F, Pan M, Wu X, Zhong Y, Wang C et al (2020) Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab 32(2):229 e8-242 e8
Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC (2010) Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem 114(3):820–831
Ballester LY, Lu G, Zorofchian S, Vantaku V, Putluri V, Yan Y et al (2018) Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol Commun 6(1):85
Trautwein C, Zizmare L, Mäurer I, Bender B, Bayer B, Ernemann U et al (2022) Tissue metabolites in diffuse glioma and their modulations by IDH1 mutation, histology, and treatment. JCI Insight 7(3)
Lin CJ, Wu V, Wu PC, Wu CJ (2015) Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 10(7):e0132589
Wu TK, Wei CW, Pan YR, Hsu RJ, Wu CY, Yu YL (2019) The uremic toxin p-cresyl sulfate induces proliferation and migration of clear cell renal cell carcinoma via microRNA-21/ HIF-1alpha axis signals. Sci Rep 9(1):3207
Reichard CA, Naelitz BD, Wang Z, Jia X, Li J, Stampfer MJ, Klein EA, Hazen SL, Sharifi N (2022) Gut microbiome-dependent metabolic pathways and risk of lethal prostate cancer: prospective analysis of a PLCO cancer screening trial cohort. Cancer Epidemiol Biomark Prev 31(1):192–199
Sowers ML, Sowers LC (2022) Glioblastoma and methionine addiction. Int J Mol Sci 23(13):7156
Chen X, Sun J, Li Y, Jiang W, Li Z, Mao J, Zhou L, Chen S, Tan G (2023) Proteomic and metabolomic analyses illustrate the mechanisms of expression of the O(6)-methylguanine-DNA methyltransferase gene in glioblastoma. CNS Neurosci Ther 30(2):e14415
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the Comprehensive Cancer Center Lower Saxony to Nora Möhn and Hauke Thiesler.
Author information
Authors and Affiliations
Contributions
NM, HFH, TS, and JKK: conceptualization. SS, NM, and SN: methodology. NM, HFH, PS, LGL, FT, ME, and SS: sample and data acquisition. NM, HFH, SN, SS, and HT: data analysis and interpretation. NM, HFH, and SN: writing—original draft. PS, LGL, KS, HH, HT, FF, CH, TS, and JKK: writing—review. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee at Hannover Medical School (No. 8269_BO_S_2019). All patients provided written informed consent for participation.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Möhn, N., Hounchonou, H.F., Nay, S. et al. Metabolomic profile of cerebrospinal fluid from patients with diffuse gliomas. J Neurol (2024). https://doi.org/10.1007/s00415-024-12667-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12667-9