Abstract
Purpose
This study aimed to investigate the effects of subconjunctival injection of aflibercept, a soluble protein decoy for VEGFR-1 and VEGFR-2, on corneal angiogenesis and VEGFR-expressing CD11b+ cells in a mouse model of suture-induced corneal neovascularization.
Methods
Corneal neovascularization was induced in BALB/c mice by placing three sutures on the cornea. Immediately after surgery, either 200 µg aflibercept (5 µL) or an equal volume of phosphate-buffered saline (PBS) was administered into the subconjunctival space. Seven days after later, corneal new vessels were quantified through clinical examination and measurement of the CD31-stained area in corneal flat mounts. The levels of pro-angiogenic and inflammatory markers in the cornea were evaluated using RT-qPCR. The percentages of VEGFR-2+CD11b+ cells and VEGFR-3+CD11b+ cells were analyzed in the cornea, blood, and draining cervical lymph nodes (DLNs) using flow cytometry.
Results
Subconjunctival injection of aflibercept significantly reduced the growth of corneal new vessels compared to subconjunctival PBS injection. The mRNA levels of Cd31, vascular growth factors (Vegfc and Angpt1), and pro-angiogenic/inflammatory markers (Tek/Tie2, Mrc1, Mrc2, and Il6) in the cornea were downregulated by subconjunctival aflibercept. Also, the percentage of VEGFR-3+CD11b+ cells in the cornea, blood, and DLNs was decreased by aflibercept, whereas that of VEGFR-2+CD11b+ cells was unaffected.
Conclusion
Subconjunctival aflibercept administration inhibits inflammatory angiogenesis in the cornea and reduces the numbers of cornea-infiltrating and circulating VEGFR-3+CD11b+ cells.
Key messages
What is known:
• Aflibercept inhibits hemangiogenesis in the eye by interfering with the VEGF-A/VEGFR-2 signaling.
What is new:
• Subconjunctival aflibercept downregulates the VEGF-C and angiopoietin signaling in the cornea.
• Subconjunctival aflibercept reduces both circulating and cornea-infiltrating VEGFR-3+CD11b+ myeloid cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflibercept, also termed a vascular endothelial growth factor (VEGF) trap, is a recombinant fusion protein that acts as a soluble protein decoy for VEGF receptors, VEGFR-1 and VEGFR-2. Since it binds to VEGF-A with a higher affinity than its natural competitors (VEGFR-1 and VEGFR-2), aflibercept inhibits VEGF signaling and thus prevents pathologic angiogenesis [1, 2]. Based on its anti-angiogenic activity, intravitreal injection of aflibercept (Eylea®, Regeneron Pharmaceuticals, Inc., Tarrytown, NY) has been approved by FDA for treatment of retinal and choroidal neovascularization such as neovascular age-related macular degeneration, myopic choroidal neovascularization, macular edema associated with retinal vein occlusion, diabetic macular edema, and diabetic retinopathy. Especially, aflibercept has been shown to have markedly higher affinity for VEGF compared to bevacizumab and ranibizumab, widely-used monoclonal antibodies against VEGF-A [3, 4].
The cornea is devoid of both blood and lymphatic vessels and maintains its avascularity through immunologic and angiogenic privilege. An injury to the cornea can cause inflammation and disrupt its immune and angiogenic privilege, thereby leading to the growth of corneal new vessels and loss of corneal transparency [5, 6]. The mechanisms of corneal neovascularization involve both hemangiogenesis (driven by VEGF-A binding to VEGFR-1 and VEGFR-2) and lymphangiogenesis (driven by VEGF-C and VDGF-D binding to VEGFR-3) [5,6,7], Therefore, it is possible that therapies targeting VEGFs and their receptors are effective in attenuating corneal neovascularization as well as choroidal and retinal neovascular diseases. Indeed, previous studies by our group and others reported the inhibitory effects of topical, subconjunctival, or intracorneal administration of bevacizumab and ranibizumab on corneal neovascularization in animal models and human clinical studies [8,9,10,11,12,13,14,15]. In recent years, several studies have demonstrated superior or similar efficacies of aflibercept, a more potent VEGF-A inhibitor, in reducing corneal neovascularization compared to bevacizumab [16,17,18].
In this study, we investigated the effects of subconjunctival aflibercept injection on corneal angiogenesis in a model of suture-induced inflammatory corneal neovascularization. Specifically, we analyzed the effects of aflibercept on corneal new vessel growth, the levels of pro-angiogenic and inflammatory monocyte/macrophage markers in the cornea, and the numbers of circulating and cornea-infiltrating VEGFR-expressing myeloid cells.
Methods
Animal model and treatment
The experimental protocol was approved from the Institutional Animal Care and Use Committee of Seoul National University Hospital Biomedical Research Institute (Seoul, Korea) and followed the guidelines stated by ARVO for ophthalmic and vision research.
Eight-week-old male BALB/c mice (KOATECH, Pyeongtaek, Korea) were used for the study. Under anesthesia with intraperitoneal injection of zolazepam-tiletamine (Zoletil®, Virbac, Carros, France) and topical administration of 0.5% proparacaine hydrochloride ophthalmic solution (Hanmi Pharm Co., Ltd., Seoul, Korea), the cornea was marked using a 2-mm-diameter trephine, and three 10 − 0 nylon sutures were evenly placed 120° apart from each other, through the epithelial and stromal layers, and with both points of each stitch going in and out of the cornea along the mark. The knots were left unburied.
Immediately after the placement of corneal sutures, either 200 µg aflibercept (5 µL) (Eylea®, Regeneron Pharmaceuticals, Inc.) or the same volume of phosphate-buffered saline (PBS) was injected subconjunctivally using a 33-gauge needle (Hamilton, Reno, NV). Seven days later, the mice were subjected to assays.
Clinical examination
Corneal new vessels were examined under slit-lamp biomicroscopy and photographed with a camera mounted on a microscope. The extent of corneal neovascularization was graded independently by two individuals (C.H.Y. and J.Y.O.) in a blinded manner using the standardized scale system: the growth of corneal new vessels was graded from 0 to 3 in each quadrant of the cornea, and scores for each quadrant were summed to obtain the clinical score (range 0 to 12) for each eye [19,20,21].
Histopathology
The corneas were extracted and fixed in 4% paraformaldehyde overnight at 4˚C. After washing with PBS, the tissues were treated with proteinase K for 5 min at room temperature, followed by treatment with methanol for 30 min. After PBS washing, the tissues were blocked with 2% BSA overnight at 4˚C, and then were incubated with primary antibodies against CD31 (BD Pharmingen, San Diego, CA), CD11b (BD Pharmingen), VEGFR-2 (R&D Systems, Minneapolis, MN), or VEGFR-3 (R&D Systems) for 16 h at 4˚C. The next day, the corneas were incubated with secondary antibodies overnight at 4˚C in the dark. The stained corneas were flat-mounted onto slides and examined under a fluorescence microscope (Nikon, Tokyo, Japan).
For quantification of the CD31-stained area, the digital fluorescence images of corneal flat mounts were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) [20, 22]. The total area of the cornea was manually delineated by outlining the innermost vessels of the limbal arcade and removing areas outside the delineated margin. After background subtraction and thresholding, the percentage of CD31+ area out of the total corneal area was calculated.
For quantification of VEGFR-2+CD11b+ or VEGFR-3+CD11b+ myeloid cells in the cornea, the numbers of VEGFR-2+CD11b+ and VEGFR-3+CD11b+ cells were counted in the regions adjacent to the limbus along the sutures of the cornea under a fluorescence microscope, at a magnification of X400. For each quantification, the corneal stroma with approximate size of 280 μm X 450 μm was covered.
Real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The corneal tissue was dissected into small pieces using microscissors, lysed in RNA isolation reagent (RNA Bee, Tel-Test, Friendswood, TX), and homogenized using an ultrasound sonicator (Ultrasonic Processor, Cole Parmer Instruments, Vernon Hills, IL). Total RNA was extracted from the lysates using the RNeasy Mini kit (Qiagen, Valencia, CA) and converted to first-strand cDNA by reverse transcription using the High Capacity RNA-to-cDNA™ Kit (Applied Biosystems, Carlsbad, CA). RT-qPCR amplification was performed using TaqMan® Universal PCR Master Mix (Applied Biosystems) and specific TaqMan® probe sets for Cd31, Vegfc, Angpt1 (angiopoietin-1), Vegfa, Tek/Tie2, Mrc1 (mannose receptor C-type 1), Mrc2, and Il6 (all from Applied Biosystems) in an automated instrument (ABI 7500 Real Time PCR System, Applied Biosystems). Assay ID for each TaqMan®probe was as follows: Mm01242576_m1 for Cd31; Mm00456503_m1 for Angpt1; Mm00437306_m1 for Vegfa; Mm00437310_m1 for Vegfc; Mm00443243_m1 for Tek/Tie2; Mm00485148_m1 for Mrc1; Mm00485184_m1 for Mrc2; Mm00446190_m1 for Il6. The data obtained were normalized to Gapdh (Mm99999915_g1) and expressed as fold changes relative to the controls.
Flow cytometry
Whole blood was collected using a 26-gauge needle with a heparin-precoated syringe via cardiac puncture and preserved in EDTA tube. The collected blood was treated with red blood cell lysis buffer (BD Biosciences, San Diego, CA) for 5 min and centrifuged at 2,000 rpm for 10 min. Ocular draining cervical lymph nodes (DLNs) were minced into small pieces between the frosted ends of two glass slides in RPMI 1640 medium (WelGENE, Daegu, Korea) containing 10% fetal bovine serum (Gibco, Carlsbad, CA) and 1% penicillin-streptomycin (Gibco) on ice. The resultant single-cell suspension from the blood and DLNs were stained with fluorescence-conjugated antibodies against CD11b-FITC, VEGFR-2-APC, and VEGFR-3-PE (all from eBioscience, San Diego, CA) for 30 min at 4˚C. The stained cells were analyzed using the S1000EXi Flow Cytometer (Stratedigm, San Jose, CA), and the obtained data were analyzed using the FlowJo program (Tree Star, Ashland, OR).
Statistical analysis
Prism software (GraphPad, San Diego, CA) was used for statistical tests and generation of graphs. The Shapiro-Wilk test or Kolmogorov-Smirnov test was used to determine a normal distribution of data in each group. The unpaired t-test or Mann-Whitney test was used for comparisons of mean values between two groups. One-way ANOVA followed by Tukey’s Honestly Significant Difference test or Kruskal–Wallis test followed by Dunn’s multiple-comparisons test was employed for comparisons of the means between more than two groups. The data were presented as mean ± SD. Differences were considered significant at p < 0.05.
Results
Subconjunctival aflibercept inhibits corneal new vessel growth
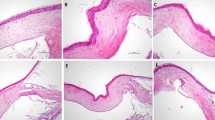
To induce inflammatory corneal neovascularization, we applied three 10 − 0 nylon sutures intrastromally onto the cornea of BALB/c mice. For treatment, we injected either 200 µg aflibercept (5 µL) or an equal volume of PBS (5 µL) into the subconjunctival space. Seven days later, the cornea was examined and extracted for histological and molecular assays, and the blood and DLNs were collected for flow cytometric analysis (Fig. 1a).
Subconjunctival aflibercept attenuates corneal neovascularization. a Experimental scheme. Corneal sutures were applied in BALB/c mice for induction of corneal neovascularization, and aflibercept (200 µg in 5 µL) (Eylea®, Regeneron Pharmaceuticals, Inc., Tarrytown, NY) or PBS (5 µL) was subconjunctivally injected. Seven days later, the corneas were clinically observed, and the corneas, blood and DLNs were collected for assays. b, c Representative corneal photographs (b) and quantification of corneal new vessels as graded by the standardized scoring system (c). d, e Representative microphotographs of whole-corneal flat mounts with CD31 immunostaining (d) and quantification of CD31-stained area (e). Mean values ± SD are shown from three independent experiments. Each circle depicts the data from an individual eye. **p < 0.01, ****p < 0.0001, as analyzed by Mann-Whitney test (c) or by unpaired t-test (e)
Consistent with previous observations [20], corneal sutures induced the growth of new vessels from the limbal arcade toward the corneal center, as examined by slit-lamp biomicroscopy (Fig. 1b). Subconjunctival aflibercept injection markedly reduced the extent of corneal neovascularization (Fig. 1b). Clinical scores of corneal neovascularization were significantly lower in aflibercept-treated eyes compared to those treated with PBS (Fig. 1c). These clinical findings were confirmed by CD31 staining of corneal whole mounts. The CD31-stained area was significantly smaller in the aflibercept-treated corneas compared to the PBS-treated controls (Fig. 1d, e).
Subconjunctival aflibercept downregulates pro-angiogenic and inflammatory molecules in the cornea
We next assessed the expression of angiogenesis-related molecules in the cornea. RT-qPCR showed that the mRNA level of Cd31 encoding a panendothelial marker CD31 was increased in sutured corneas and significantly reduced by aflibercept (Fig. 2a). Similarly, mRNA levels of Vegfc and Angpt1, which encode major growth factors for lymphatic vessels [23], were upregulated in response to suture injury and downregulated following aflibercept treatment (Fig. 2b). In contrast, the level of Vegfa mRNA was furthermore increased by aflibercept (Fig. 2b).
Subconjunctival aflibercept suppresses the expression of pro-angiogenic and inflammatory molecules in the cornea. a, b, c, d RT-qPCR for Cd31 (a), vascular growth factors (Vegfc and Angpt1) (b), pro-angiogenic monocyte/macrophage markers (Tek/Tie2, Mrc1, and Mrc2) (c), and pro-inflammatory cytokine (Il6) (d) in the cornea. The mRNA levels are presented as fold changes relative to the levels in control eyes which had not received injury or treatment. Mean values ± SD are shown from two independent experiments. Each circle depicts the data from an individual mouse. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as analyzed by one-way ANOVA with Tukey’s test or by Kruskal–Wallis test with Dunn’s multiple-comparisons test (Tek in c)
We also evaluated the levels of Tek/Tie2, which encodes the angiopoietin-1 receptor, as well as Mrc1 and Mrc2, which encode markers for pro-angiogenic monocytes/macrophages. We chose to evaluate these markers because, in our previous study, we discovered a strong positive correlation between the mRNA levels of Tek/Tie2, Mrc1, and Mrc2 and the degree of corneal neovascularization [20]. The mRNA levels of Tek/Tie2, Mrc1, and Mrc2 were all elevated in the cornea by sutures and significantly repressed by aflibercept (Fig. 2c). Similar results were obtained with the mRNA levels of the pro-inflammatory cytokine, Il6 (Fig. 2d).
Taken together, the results suggested that subconjunctival aflibercept injection inhibited the development of corneal neovascularization by downregulating the production of angiogenic and inflammatory factors and suppressing pro-angiogenic monocytes/macrophages, in addition to directly blocking VEGF-A signaling.
Subconjunctival aflibercept reduces VEGFR-3+ CD11b + cells in the cornea, blood, and DLNs
Having observed the downregulation of pro-angiogenic monocyte/macrophage markers in the cornea following aflibercept treatment, we proceeded to examine the population of VEGFR-expressing myeloid cell, which are known to be highly angiogenic [24, 25]. Flow cytometric analysis demonstrated that the percentage of VEGFR-3+CD11b+ cells was increased in the blood and DLNs seven days after the placement of corneal sutures (Fig. 3a). Notably, subconjunctival injection of aflibercept significantly reduced the percentage of VEGFR-3+CD11b+ cells in both blood and DLNs (Fig. 3a). However, neither corneal sutures nor subconjunctival aflibercept injection had an impact on the percentage of VEGFR-2+CD11b+ cells in the blood or DLNs (Fig. 3b). Similar findings were observed in the cornea. The number of VEGFR-3+CD11b+ cells infiltrating the cornea was significantly reduced by subconjunctival aflibercept treatment, while the number of VEGFR-2+CD11b+ cells remained unaffected (Fig. 3c. Supplementary Fig. 1).
Subconjunctival aflibercept reduces the percentage of circulating and cornea-infiltrating VEGFR-3+CD11b+myeloid cells. a, b Representative and quantitative flow cytometry results for VEGFR-3+CD11b+ cells (a) or VEGFR-2+CD11b+ cells (b) in the blood and DLN. (c) Enumeration of VEGFR-3+CD11b+ cells or VEGFR-2+CD11b+ cells in the corneal flat mounts immunostained with CD11b, VEGFR-2, or VEGFR-3. Mean values ± SD are shown from two independent experiments. Each circle depicts the data from an individual mouse. **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant, as analyzed by one-way ANOVA with Tukey’s test (a, b) or by unpaired t-test (c)
Therefore, the results demonstrated that subconjunctival aflibercept administration led to decreases in both cornea-infiltrating and circulating VEGFR-3+CD11b+ cells.
Discussion
Our study yielded two key observations. First, the treatment with subconjunctival aflibercept injection was effective in inhibiting corneal angiogenesis. Second, subconjunctival aflibercept downregulated the VEGF-C signaling and reduced both circulating and cornea-infiltrating VEGFR-3+CD11b+ myeloid cells.
Aflibercept is a fusion protein consisting of an IgG backbone fused to extracellular VEGFR sequences of VEGFR-1 and VEGFR-2. As a decoy receptor, aflibercept binds to VEGF-A, VEGF-B, and PIGF (placental growth factor) with higher affinity than their native receptors, VEGFR1 and VEGFR2, effectively blocking the activity of VEGF-A, VEGF-B, and PIGF [1, 2]. Especially, the binding and activation of VEGF-A and VEGFR-2 play key roles in hemangiogenesis. Thus, the inhibitory activity of aflibercept on VEGF-A/VEGFR-2 signaling is the mechanism of action of aflibercept in treating pathologic neovascularization. In the context of corneal neovascularization, recent studies, along with our study, have demonstrated that subconjunctival, topical, or intracorneal administration of aflibercept effectively inhibits corneal new vessels in animal models of high-risk corneal transplantation or suture-induced corneal neovascularization [18, 26, 27].
One interesting finding of our study is that the mRNA level of Vegfa in the cornea was significantly upregulated, while mRNA levels of Vegfc and Angpt1 were suppressed, after subconjunctival aflibercept treatment. A similar phenomenon was observed in a study by Puddu et al. [28], where they treated retinal pigment epithelial cells with aflibercept or ranibizumab for 24 h. They found that both aflibercept or ranibizumab increased VEGF-A and PlGF expression, while decreasing the expression and secretion of VEGF-C. The increased VEGF-A expression in the cornea or retinal pigment epithelial cells treated with VEGF-A inhibitors, such as aflibercept or ranibizumab, might be a result of compensatory responses of the cells to counter the lack of VEGF-A. Furthermore, the downregulation of VEGF-C by aflibercept, a VEGF-A inhibitor, suggests the complex regulatory mechanisms involving the VEGF family members in response to anti-VEGF treatments. Further research is needed to fully understand these intricate interactions.
Another notable observation from our study is that subconjunctival aflibercept treatment reduced both cornea-infiltrating and circulating VEGFR-3+CD11b+ cells after suturing injury, whereas VEGFR-2+CD11b+ cells were not affected by either suturing or aflibercept. VEGFR-expressing CD11b+ monocytes, also termed myeloid angiogenic cells, have been shown to be highly angiogenic in previous studies [24, 25]. Indeed, our group previously showed that many cornea-infiltrating CD11b+ cells in sutured corneas were monocytes/macrophages expressing VEGFR-3 [20]. Moreover, a study by Chung et al. demonstrated that VEGFR-3-specific signaling contributed to corneal angiogenesis in which macrophages played a major role [29]. Therefore, the suppression of VEGFR-3+CD11b+ monocytes by a VEGF-A inhibitor aflibercept, as well as Vegfc downregulation, might serve as one of the mechanisms underlying aflibercept’s inhibition of corneal neovascularization in our model. In line with our findings, an elegant study by Cursiefen et al. demonstrated that intraperitoneal administration of VEGF Trap, which neutralizes VEGF-A but not VEGF-C or D, completely inhibited both hemangiogenesis and lymphangiogenesis by suppressing VEGF-A-mediated recruitment of pro-angiogenic monocytes/macrophages expressing VEGF-C or D [30]. Additionally, in our study, local inhibition of VEGF-A through subconjunctival aflibercept effectively reduced the levels of systemic circulating VEGFR-3+CD11b+ cells in DLNs and blood, resulting in decreased corneal infiltration of VEGFR-3+CD11b+ cells. These findings indicate that signals released locally in the injured cornea act as chemoattractants for myeloid cells in the systemic circulation, and that the systemic mobilization of myeloid cells can be regulated by modifying the local injury signals.
In conclusion, we demonstrated that subconjunctival administration of aflibercept suppressed the expression of pro-angiogenic and inflammatory growth factors and cytokines in the cornea and reduced the number of pro-angiogenic VGFR-3+CD11b+ monocytes in the circulation and the cornea. Our results suggest that aflibercept holds promise as an effective therapeutic option for patients with corneal neovascularization. Further investigations are needed to optimize treatment regimens and conduct clinical trials in human patients.
References
Adams BS, Sorhaitz W, Stringham J (2022) Aflibercept. StatPearls [Internet], vol 5. StatPearls Publishing, Treasure Island
Rosso WC, Shah VA, Kim AL et al (2023) Aflibercept [EyeWiki web site]. May 24, https://eyewiki.aao.org/Aflibercept. Accessed July 24, 2023
Konner J, Dupont J (2004) Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer 4 Suppl 2S81–85. https://doi.org/10.3816/ccc.2004.s.013
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. https://doi.org/10.1007/s10456-011-9249-6
Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH (2010) Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res 29:208–248. https://doi.org/10.1016/j.preteyeres.2010.01.002
Di Zazzo A, Gaudenzi D, Yin J, Coassin M, Fernandes M, Dana R, Bonini S (2021) Corneal angiogenic privilege and its failure. Exp Eye Res 204:108457. https://doi.org/10.1016/j.exer.2021.108457
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7:359–371. https://doi.org/10.1038/nrm1911
Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG (2013) Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea 32:435–444. https://doi.org/10.1097/ICO.0b013e3182542613
Oh JY, Kim MK, Shin MS, Lee HJ, Lee JH, Wee WR (2009) The anti-inflammatory effect of subconjunctival bevacizumab on chemically burned rat corneas. Curr Eye Res 34:85–91. https://doi.org/10.1080/02713680802607740
Oh JY, Kim MK, Wee WR (2009) Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea 28:1070–1073. https://doi.org/10.1097/ICO.0b013e31819839f9
Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J, Alió JL (2010) Inhibition of corneal neovascularization by topical bevacizumab (Anti-VEGF) and sunitinib (Anti-VEGF and Anti-PDGF) in an animal model. Am J Ophthalmol 150:519–528e511. https://doi.org/10.1016/j.ajo.2010.04.024
Gupta AA, Mammo DA, Page MA (2020) Intrastromal bevacizumab in the management of corneal neovascularization: a retrospective review. Graefes Arch Clin Exp Ophthalmol 258:167–173. https://doi.org/10.1007/s00417-019-04519-4
Liarakos VS, Papaconstantinou D, Vergados I, Douvali M, Theodossiadis PG (2014) The effect of subconjunctival ranibizumab on corneal and anterior segment neovascularization: study on an animal model. Eur J Ophthalmol 24:299–308. https://doi.org/10.5301/ejo.5000391
Peng WY, He LW, Yin XF, Zhou BB, Zhou T, Zhou SY (2023) Successful regression of newly formed corneal neovascularization by subconjunctival injection of bevacizumab in patients with chemical burns. Front Med (Lausanne) 10:1210765. https://doi.org/10.3389/fmed.2023.1210765
Moon CH, Moon BG, Kim JY, Kim MJ, Tchah H (2017) Comparison of topical low-molecular-weight heparin-taurocholate and Bevacizumab for Treatment and Prevention of corneal neovascularization. Cornea 36:497–501. https://doi.org/10.1097/ico.0000000000001105
Eski MT, Teberik K, Oltulu P, Ankaralı H, Kaya M, Alpay M (2022) The effects of subconjunctival bevacizumab, ranibizumab, and aflibercept on corneal neovascularization. Hum Exp Toxicol 41:9603271221084674. https://doi.org/10.1177/09603271221084674
Gal-Or O, Livny E, Sella R, Nisgav Y, Weinberger D, Livnat T, Bahar I (2016) Efficacy of Subconjunctival Aflibercept Versus Bevacizumab for Prevention of corneal neovascularization in a rat model. Cornea 35:991–996. https://doi.org/10.1097/ico.0000000000000849
Ucgul RK, Celebi S, Yilmaz NS, Bukan N, Ucgul AY (2021) Intrastromal versus subconjunctival anti-VEGF agents for treatment of corneal neovascularization: a rabbit study. Eye (Lond) 35:3123–3130. https://doi.org/10.1038/s41433-020-01347-3
Li C, Zhang F, Wang Y (2010) S100A proteins in the pathogenesis of experimental corneal neovascularization. Mol Vis 16:2225–2235
Song HB, Park SY, Ko JH, Park JW, Yoon CH, Kim DH, Kim JH, Kim MK, Lee RH, Prockop DJ, Oh JY (2018) Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the Cornea by suppressing macrophage in a TSG-6-Dependent manner. Mol Ther 26:162–172. https://doi.org/10.1016/j.ymthe.2017.09.026
Lee HJ, Yoon CH, Kim HJ, Ko JH, Ryu JS, Jo DH, Kim JH, Kim D, Oh JY (2022) Ocular microbiota promotes pathological angiogenesis and inflammation in sterile injury-driven corneal neovascularization. Mucosal Immunol 15:1350–1362. https://doi.org/10.1038/s41385-022-00555-2
Rabiolo A, Bignami F, Rama P, Ferrari G (2015) VesselJ: a New Tool for Semiautomatic Measurement of corneal neovascularization. Invest Ophthalmol Vis Sci 56:8199–8206. https://doi.org/10.1167/iovs.15-17098
Lee HK, Lee SM, Lee DI (2021) Corneal lymphangiogenesis: current pathophysiological understandings and its functional role in Ocular Surface Disease. Int J Mol Sci 22. https://doi.org/10.3390/ijms222111628
Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW (2013) The role of immune-related myeloid cells in angiogenesis. Immunobiology 218:1370–1375. https://doi.org/10.1016/j.imbio.2013.06.010
Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL (2016) Macrophages: an inflammatory link between angiogenesis and Lymphangiogenesis. Microcirculation 23:95–121. https://doi.org/10.1111/micc.12259
Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, Chauhan SK, Dana R (2015) VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 99:678–686. https://doi.org/10.1097/TP.0000000000000512
Zhang W, Schönberg A, Bock F, Cursiefen C (2022) Posttransplant VEGFR1R2 Trap Eye drops inhibit corneal (Lymph)angiogenesis and improve corneal allograft survival in eyes at high risk of rejection. Transl Vis Sci Technol 11:6. https://doi.org/10.1167/tvst.11.5.6
Puddu A, Sanguineti R, Traverso CE, Viviani GL, Nicolò M (2016) Response to anti-VEGF-A treatment of retinal pigment epithelial cells in vitro. Eur J Ophthalmol 26:425–430. https://doi.org/10.5301/ejo.5000786
Chung ES, Chauhan SK, Jin Y, Nakao S, Hafezi-Moghadam A, van Rooijen N, Zhang Q, Chen L, Dana R (2009) Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol 175:1984–1992. https://doi.org/10.2353/ajpath.2009.080515
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113:1040–1050. https://doi.org/10.1172/JCI20465
Funding
This study was funded by the National Research Foundation (NRF) funded by the Korean government (MSIT) (2021R1A2C3004532).
Open Access funding enabled and organized by Seoul National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The experimental protocol was approved from the Institutional Animal Care and Use Committee of Seoul National University Hospital Biomedical Research Institute (Seoul, Korea) and followed the guidelines stated by ARVO for ophthalmic and vision research.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoon, C.H., Ko, J.H., Lee, H.J. et al. Subconjunctival aflibercept inhibits corneal angiogenesis and VEGFR-3+CD11b+ cells. Graefes Arch Clin Exp Ophthalmol (2024). https://doi.org/10.1007/s00417-024-06560-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00417-024-06560-4