Abstract
Substaging of T1 urothelial cancer is associated with tumor progression and its reporting is recommended by international guidelines. However, it has not been integrated in risk stratification tools and there is no agreement on the best method to use for its reporting. We aimed to investigate the applicability, interobserver variability, and prognostic value of histological landmark based and micrometric (aggregate linear length of invasive carcinoma (ALLICA), microscopic vs. extensive system, Rete Oncologica Lombarda (ROL) system) substaging methods. A total of 79 patients with the primary diagnosis of T1 urothelial cancer treated with conventional transurethral resection and adjuvant BCG therapy between 2000 and 2020 at the Medical University of Vienna were included. The anatomical and metrical substaging systems were evaluated using agreement rate, Cohen’s kappa, Kendall’s tau, and Spearman rank correlation. Prognostic value for high-grade recurrence or T2 progression was evaluated in uni- and multivariable analysis. Applicability and reproducibility were good to moderate and varied between substaging methods. Obstacles are mainly due to fragmentation of samples. Anatomical substaging was associated with progression in univariable and multivariable analysis. In our cohort, we could only identify anatomical landmark–based substaging to be prognostic for T2 progression. A major obstacle for proper pathological assessment is fragmentation of samples due to operational procedure. Avoiding such fragmentation might improve reproducibility and significance of pathological T1 substaging of urothelial cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The standard treatment of T1 urothelial carcinoma (UC) of the bladder is complete transurethral resection (TURB) followed by adjuvant intravesical instillation with BCG. However, despite adequate treatment, roughly half of patients will experience disease recurrence and 20% progression to invasive disease [1, 2]. Given the potential high aggressiveness of this disease in some cases, the risk of both under- or overtreatment is high. Significant efforts have been made to develop risk stratification tools and identify biomarkers that can accurately predict which patients are likely to fail BCG therapy and would benefit from an upfront radical cystectomy [3]. However, none of these biomarkers could achieve a sufficiently high discrimination to be implemented in clinical practice and guide decision-making, leaving the pathological stage and grade as the main driver.
In this context, pathologic substaging of T1 disease has been investigated as an option to better stratify patients. This involved the evaluation of the invasion extent either according to anatomical landmarks using a two- or three-tiered system, or based on micrometric evaluation of the invasion often using a defined cut-off in a two-tiered system [4, 5]. Despite the growing bulk of evidence, T1 substaging is still not part of current risk stratification tools. This is partially attributable to the lack of comparative studies and validation in external cohorts [1, 4,5,6,7].
To fill this gap in knowledge, we investigated the differential applicability, interobserver variability, and prognostic value of histological and micrometric T1 substaging methods.
Materials and methods
Patient population
Patients with a primary diagnosis of T1 UC of the bladder were eligible for inclusion. Patients were treated with transurethral resection (TURB) and subsequent BCG therapy between 2000 und 2020 at the Medical University of Vienna. A second look TURB was performed in 80% of patients within 6 weeks after the diagnosis of T1 UC. Patients with previous diagnosis of UC and patients with upper tract UC were excluded.
Tissue-based analyses
Archived H&E slides from formalin-fixed and paraffin-embedded tissue samples of each case were retrieved. Cases for which no histological slides were available and cases that did not show invasion during re-evaluation were excluded. Other systematically evaluated pathological features, such as lymphovascular invasion or histological subtype, were retrieved from initial pathology reports. All slides were scanned as whole slide images with a Pannoramic 250 III Flash scanner (3DHistech, Budapest, Hungary) at a resolution of 0.243 µm/px, and subsequent histological examination and substaging were performed using the Cytomine platform (Cytomine Corporation SA, Liège, Belgium, v4.3.5-beta) [8].
Anatomical landmark-based substaging was defined as T1a (invasion not involving the muscularis mucosa (MM)) and T1b (invasion involving or beyond MM). In the case of absence of MM, the vesicular vascular plexus (VVP) was used as surrogate. Cases that could not be classified as T1a or T1b by anatomical substaging were designated as T1 and excluded from further analyses. For micrometric-based substaging, the number of invasive foci was recorded and the maximum diameter of every invasive focus was measured irrespective of the orientation of this diameter in regard to the urothelial surface. The measurements on the digitalized slides were done using Cytomine [8] and QuPath [9] version 0.4.3. The aggregate linear length of invasive carcinoma (ALLICA) was then calculated as the sum of the diameter of invasive foci in millimeters. Based on focality and ALLICA, samples were categorized as using both, the “microscopic vs. extensive” system and the Rete Oncologica Lombarda (ROL) system [10, 11]. In short, the “microscopic vs. extensive” system classifies a case as microscopic (“m”) in the case of a single focal invasion of ≤ 0.5 mm, and otherwise as extensive (“e”). The ROL system classifies a case as ROL1 if the sum of all invasive foci is ≤ 1 mm, irrespective of the number of invasive foci, and otherwise as ROL2. For systematic display of applied methods, see Table 1.
All slides were re-reviewed for extent of lamina propria invasion separately by two dedicated uropathologists, blinded to each other and to the oncologic outcomes of the patient. Discrepancies in anatomical landmark–based substaging were subsequently discussed to reach a consensus result. This consensus result was used for subsequent analyses. For measurement-based substaging, no consensus measurements were made but results of both observers were used for subsequent analysis.
Our primary endpoint was reproducibility and applicability of different histology-based substaging methods. Secondary endpoints were prognostic value of substaging methods for the progression rate to T2 urothelial cancer and high-grade (HG) recurrence within 3 years after initial diagnosis. Patients who died without progression or were lost to follow-up were censored at the date of death or last follow-up visit.
Statistical analysis was done using SPSS version 25 (IBM). Group comparison was done using fisher exact test for categorical variables and Mann–Whitney U test for metrical variables. Interobserver agreement was assessed using agreement rate and Cohen’s kappa for categorical classification systems and Spearman’s correlation coefficient and Kendall’s tau for ALLICA. Progression and recurrence rate was assessed using uni- and multivariable Cox regression analysis. Receiver operating characteristic (ROC) curve was used for visualization and evaluation of a potential binary classifier based on ALLICA measurements.
The study was approved by local ethics committees and is in line with the declaration of Helsinki and its revisions.
Results
Our cohort consisted of 79 patients with de novo diagnosis of T1 urothelial cancer, who were all treated with TURB and consecutive BCG instillation. Clinical and pathological baseline data at the time of TURB is summarized in Table 2. The gender ratio (male to female) was 62:17 and the median age was 69 years (IQR 63–78) with no significant age difference between gender (p = 0.56). Histologically, all lesions were high-grade according to WHO grading and mostly conventional UC (93.7%). Lymphovascular invasion was present in 13 cases and carcinoma in situ (Cis) in 37. Detrusor muscle (muscularis propria) was present in 69 cases.
The median observational time was 34 months (IQR, 14–36), in which cancer-related death occurred only in one patient (six patients died of cancer-unrelated cause). Eleven patients (13.9%) showed progression to muscle invasive carcinoma; 25 (31.6%) patients had HG recurrence. In the group of patients who progressed to T2 cancer, the median time until progression was 8 months (IQR 5–24). In univariable analysis, none of our baseline characteristics were associated with progression rate and only presence of Cis was associated with HG recurrence (Supplementary Table 1).
Interobserver agreement of anatomical landmark–based substaging was moderate with Cohen’s kappa of 0.63 with 16 discrepancies corresponding to an agreement rate of 79.7% (Table 3). Consecutive consensus scoring was done for discrepant cases. Overall, six cases could not be categorized as either T1a or T1b (7.6%) due to insufficient specimen orientation leading to inability to access infiltration depth properly. These cases were categorized as T1 and excluded from further analyses. Anatomical substaging was not associated with HG recurrence (p = 0.78) but with T2 progression (p = 0.03) and was prognostic for progression-free survival in univariable analysis (HR 4.26; CI 95% 1.10–16.48; p = 0.083) and also in multivariable analysis including age, gender, LVI, CIS, histologic subtype, tumor size > 3 cm, and presence of muscularis propria (HR 6.89; CI 95% 1.20–39.50; p = 0.030) (Table 4 and suppl.Table 2).
ALLICA measurements of invasive foci showed good correlation between observers (Pearson’s coefficient 0.81; p < 0.01, Kendall’s tau 0.69; p < 0.01) and could be applied in all cases (Table 2). Stratification based on ALLICA measurements into “m” vs. “e” and “ROL1” vs. “ROL2” as described in the literature revealed moderate interobserver agreement for the m/e system (Cohen’s kappa 0.37; agreement rate 89.9%) and better agreement for the ROL system (Cohen’s kappa 0.65; agreement rate 89.9%) (Table 3). For micrometric-based methods, no consensus score or consensus stratification was obtained. Consequently, the following analyses were performed twice and p-values are displayed for both observers. Overall, micrometric stratification did not demonstrate significant correlation with progression or recurrence, neither using the “m vs e” system (p > 0.99 both observers) nor using the ROL system (p = 0.68 and p > 0.99) (Table 4). There was no significant difference between lengths of ALLICA in relation to disease progression (p = 0.87 and p = 0.81) (Table 4), and ROC analysis revealed AUC values close to 0.5 (0.53 respectively 0.51, Supplementary Fig. 1). It was concluded that—in this dataset—useful cut-off value for micrometric substaging could be determined.
Discussion
Applicability and reliability
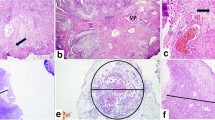
Anatomical substaging can be applied in most cases; however, six (8%) cases remained as unclassifiable due to insufficient specimen orientation resulting in tangentially cut invasive foci in which no MM or VVP can be identified (Fig. 1). Other studies assessing AS reported applicability for 40.8–100% of cases with reasons for inapplicability, if reported, like cutting artifact, impaired orientation, or absence of defined anatomical landmark [11,12,13,14,15,16]. Additionally, the VVP as a widely used surrogate for the MM is inaccurately defined and may extend throughout the thickness of the lamina propria [17]. Substaging based on a micrometric approach is reported to be more feasible, resulting in applicability rates of 96.0–100% [11, 12, 16, 18, 19]. Most of these obstacles are conditional by the procedure of TURB [20, 21]. En bloc resection might be an alternative operational procedure to enhance specimen orientation and thereby applicability, accuracy, and reliability of T1 substaging, especially, but not exclusively, for anatomical landmark-based methods [21,22,23]. Only limited data is available concerning reliability, e.g., interobserver variability. Most of the studies investigating T1 substaging do not explicitly report on this issue, even though in most cases more than one pathologist contributed to histology reading [19, 24,25,26]. Some data is available for assessment of Ta vs. T1, reporting concordance rates of 72–80%, suggesting impaired interobserver agreement between pathologists also in regard to extent of invasion and interconnected substaging [27, 28]. Other studies also reported substantial up- or down-staging of T1 cases that were evaluated for further substaging [11, 26, 29]. Data specifically for T1 substaging is only reported by Grobet-Jeandin et al. and Colombo et al. who reported an agreement rate of 89% for anatomical substaging and an agreement rate of 96% for ROL1/ROL2 substaging, but did not report a kappa value [11, 14]. These results are basically in line with our results; however, due to missing kappa statistics, a comparison is limited. Surprisingly low interobserver agreement was seen for “m” vs. “e” in our evaluation. This is probably caused by the lack of consensus on how far two foci must be apart to be separate (Fig. 2, inlay a) and the problem that also a single invasive cell, representing an additional invasive focus, might cause upstaging to “e” (Fig. 2, inlay b). The issue of perceiving invasive tumor areas proximal to each other as one or more separate foci is also a problem for systems assessing the largest as well as the sum of invasive foci, as one examiner might include “tumor-free” areas between the foci in the measurement, while another might exclude them. Moreover, the necessity of assessing multiple foci in a highly fragmented tissue further increases interobserver variability. Small invasive foci, potentially being critical to cause upstaging, might be overlooked by one, but perceived by another examiner, especially in systems like “m vs. e.” Additionally, the axis of the diameter that is measured may vary between observers and cause additional variability in the results.
Prognostic value
Two large meta-analyses of anatomical substaging reported a significant prognostic value for disease recurrence and progression rate; however, some studies included reported negative results [4, 30]. Notably, also Cis, LVI, tumor size > 3 cm, and multiple tumors were prognostic for recurrence or progression [30]. In a study of 239 patients with T1 tumors, Grobet-Jeandin et al. showed that anatomical substaging remained the only prognostic factor for progression in a multivariable model including Cis and size > 3 cm [14]. Asimakopoulos et al. who used a three-tier-based anatomical substaging, showed only prognostic value in univariable analysis [13]. In contrast, other studies looking at anatomical substaging based on MM invasion could not show prognostic value for tumor progression [15, 29, 31]. Those results are partially in line with our findings, as we also show prognostic value of anatomical substaging for tumor progression in univariable and multivariable analysis including age, gender, LVI, CIS, histologic subtype, tumor size > 3 cm, and presence of muscularis propria, but not for recurrence. A general obstacle that we encountered in anatomical landmark—based substaging is tissue orientation, particularly in tangentially cut invasive foci, and identification of MM or VVP, an issue already reported by others and possibly a major factor for inconsistent results [32].
In regard to micrometric-based substaging methods, there is quite a variability in proposed systems or rather the applied cut off values for dichotomization. One commonly used system is the two tiered “m vs. e” system in which cases with a single invasive focus smaller than 0.5 mm are categorized as “m” and everything else as “e” [10]. Several studies showed prognostic value for this categorization, while De Marco et al., Budina et al., or Colombo et al., in line with our results, did not [10, 11, 13, 15, 16, 25, 29, 31]. However, 0.5 mm is by far not the only cut-off proposed. Leivo et al. suggested a 2.3-mm cut-off for which they could show a 30% false positivity rate in ROC analysis of tumor progression, while Budina et al. suggested 8.915 mm with a 13.3% false positivity rate [16, 31]. Of note, both studies did not report an AUC value. Applying these proposed cut-offs to our data reveals higher false positivity rates of 69–67% and 38–37% for 2.3 mm and 9.3 mm, respectively (suppl. Table 4). In regard to the consequence of radical cystectomy, we would strive for a rather high specificity, respectively, low false positivity rate, probably less than 10%. This would be a cut-off of 35.0 mm (OS1), respectively, 36.5 mm (OS2) in our data, a value only seven, respectively, six patients surpassed. Furthermore, our data (Supplementary Fig. 2) showed that especially in cases with large invasive tumor, the ALLICA measurements deviate more between observers, indicating lower reliability of higher cut-off values, but which in turn are the ones that correspond to lower false positivity rates. The ROL system, which is based on a cut-off value of 1 mm for dichotomization into ROL1 and ROL2 and in contrast to the “m vs. e” system, does not take the number of invasive foci into account, is probably slightly more applicable in daily routine, as 1 mm can be evaluated during microscopic examination without requiring digital microscopy [11]. Notably, this approach was already prospectively evaluated and was found prognostic for progression in a multivariable analysis [19]. However, in our cohort, also, the ROL system was not prognostic for progression or recurrence.
The WHO and the AJCC both recommend substaging of T1 UC, however without a specific method to be used. Similarly, the recent ISUP consensus report acknowledges the value of T1 substaging and advocates its reporting in routine pathology but also does not make a recommendation concerning the method to be used due to lack of consensus [33]. In our study, we could find anatomical landmark–based substaging to be superior and failed to show association with recurrence or progression for micrometric-based substaging methods. Nonetheless, we think that reporting extensiveness additionally to depth of invasion can help in clinical decision-making but potential limitations and shortcomings should be communicated.
Noteworthy limitations of this study are a comparably low case number of 79 patients spanning a time period from 2000 to 2020 and the retrospective design. During these 20 years, management of patients with urothelial cancer changed of course, potentially influencing outcome. However, this cohort consists only of patients who had TURB and consecutive BCG treatment. We also do not have data on distant metastasis, and cancer-specific death occurred only once during our follow-up period of 3 years, making it impossible to draw conclusion concerning risk for metastasis or cancer-specific survival.
Lastly, it must be noted that all our cases were high-grade according to WHO grading. This is in line with the expected low incidence [34]. Together with the reported non-association with outcome, this might be a hint that this grading is probably not useful in the setting of invasive UC [34].
Conclusion
In this investigation, no classification system could consistently reproduce its significance for recurrence, and only anatomical landmark–based substaging showed prognostic value for T2 progression. Additionally, all systems suffer shortcomings in reproducibility and, to varying degrees, applicability in everyday practice. This is mostly due to the inherent problem of tissue fragmentation and concomitant lack of orientation associated with conventional TURB resection. En bloc resection might be an alternative approach, retaining tumor integrity, thereby improving pathology work-up and concomitantly the assessment of invasion extent–based T1 substaging systems. Future studies investigating invasion extent–based substaging and its prognostic value in en bloc resection specimen are needed to elucidate this hypothesis, which might lead to a more robust histopathology-based substaging system improving patient management and outcome. Nonetheless, until that day, despite all potential shortcomings, we think that reporting of extensiveness and depth of invasion can help in clinical decision-making and should not be omitted but caveats must be communicated.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALLICA:
-
Aggregate linear length of invasion
- BCG:
-
Bacillus Calmette-Guerin
- Cis:
-
Carcinoma in situ
- TURB:
-
Transurethral resection of bladder cancer
- e:
-
Extensive
- HG:
-
High grade
- m:
-
Microscopic
- MM:
-
Muscularis mucosa
- OS1/2:
-
Observer 1/2
- ROL:
-
Rete Oncologica Lombarda
- UC:
-
Urothelial cancer
- VVP:
-
Vesicular vascular plexus
References
Powles T, Bellmunt J, Comperat E et al (2022) Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up ☆. Ann Oncol 33(3):244–258. https://doi.org/10.1016/j.annonc.2021.11.012
D’Andrea D, Abufaraj M, Susani M et al (2018) Accurate prediction of progression to muscle-invasive disease in patients with pT1G3 bladder cancer: a clinical decision-making tool. Urol Oncol Semin Orig Investig 36(5):239.e1-239.e7. https://doi.org/10.1016/j.urolonc.2018.01.018
Lonati C, Baumeister P, Afferi L et al (2022) Survival outcomes after immediate radical cystectomy versus conservative management with Bacillus Calmette-Guérin among T1 high-grade micropapillary bladder cancer patients: results from a multicentre collaboration. Eur Urol Focus 8(5):1270–1277. https://doi.org/10.1016/j.euf.2021.07.015
KardoustParizi M, Enikeev D, Glybochko PV et al (2020) Prognostic value of T1 substaging on oncological outcomes in patients with non-muscle-invasive bladder urothelial carcinoma: a systematic literature review and meta-analysis. World J Urol 38(6):1437–1449. https://doi.org/10.1007/s00345-019-02936-y
Compérat E, Amin MB, Epstein JI et al (2021) The genitourinary pathology society update on classification of variant histologies, T1 substaging, molecular taxonomy, and immunotherapy and PD-L1 testing implications of urothelial cancers. Adv Anat Pathol 28(4):196–208. https://doi.org/10.1097/PAP.0000000000000309
Gontero P, Birtle A, Capoun O et al (2024) European association of Urology guidelines on non–muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ)—A summary of the 2024 guidelines update. Eur Urol 31:1–48. https://doi.org/10.1016/j.eururo.2024.07.027
Cussenot O, Renard-Penna R, Montagne S et al (2023) Clinical performance of magnetic resonance imaging and biomarkers for prostate cancer diagnosis in men at high genetic risk. BJU Int 131(6):745–754. https://doi.org/10.1111/bju.15968
Marée R, Rollus L, Stévens B et al (2016) Collaborative analysis of multi-gigapixel imaging data using Cytomine. Bioinformatics 32(9):1395–1401. https://doi.org/10.1093/bioinformatics/btw013
Bankhead P, Loughrey MB, Fernández JA et al (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7(1):16878. https://doi.org/10.1038/s41598-017-17204-5
Van Der Aa MNM, Van Leenders GJLH, Steyerberg EW et al (2005) A new system for substaging pT1 papillary bladder cancer: a prognostic evaluation. Hum Pathol 36(9):981–986. https://doi.org/10.1016/j.humpath.2005.06.017
Colombo R, Hurle R, Moschini M et al (2018) Feasibility and clinical roles of different substaging systems at first and second transurethral resection in patients with T1 high-grade bladder cancer. Eur Urol Focus 4(1):87–93. https://doi.org/10.1016/j.euf.2016.06.004
Patriarca C, Hurle R, Moschini M et al (2016) Usefulness of pT1 substaging in papillary urothelial bladder carcinoma. Diagn Pathol 11(1):1–9. https://doi.org/10.1186/s13000-016-0466-6
Asimakopoulos AD, Colalillo G, Telesca R et al (2021) T1 bladder cancer: comparison of the prognostic impact of two substaging systems on disease recurrence and progression and suggestion of a novel nomogram. Front Surg 8(August):1–13. https://doi.org/10.3389/fsurg.2021.704902
Grobet-Jeandin E, Wirth GJ, Benamran D, Dupont A, Tille JC, Iselin CE (2022) Substaging of pT1 urothelial bladder carcinoma predicts tumor progression and overall survival. Urol Int 106(2):130–137. https://doi.org/10.1159/000515650
De Marco V, Cerruto MA, D’Elia C et al (2014) Prognostic role of substaging in T1G3 transitional cell carcinoma of the urinary bladder. Mol Clin Oncol 2(4):575–580. https://doi.org/10.3892/mco.2014.290
Leivo MZ, Sahoo D, Hamilton Z et al (2018) Analysis of T1 bladder cancer on biopsy and transurethral resection specimens. Am J Surg Pathol 42(1):e1–e10. https://doi.org/10.1097/PAS.0000000000000964
Paner GP, Montironi R, Amin MB (2017) Challenges in pathologic staging of bladder cancer: proposals for fresh approaches of assessing pathologic stage in light of recent studies and observations pertaining to bladder histoanatomic variances. Adv Anat Pathol 24(3):113–127. https://doi.org/10.1097/PAP.0000000000000152
Van De Putte EEF, Behrendt MA, Pigot GLS, Van Der Kwast TH, Van Rhijn BWG (2015) Prognostic significance of substage and WHO classification systems in T1 urothelial carcinoma of the bladder. Curr Opin Urol 25(5):427–435. https://doi.org/10.1097/MOU.0000000000000202
Valeri M, Contieri R, Fasulo V et al (2023) Prospective validation of the ROL system in substaging pT1 high-grade urothelial carcinoma: results from a mono-institutional confirmatory analysis in BCG treated patients. Cancers (Basel) 15(3):1–11. https://doi.org/10.3390/cancers15030934
Platz CE, Cohen MB, Jones MP, Olson DB, Lynch CF (1996) Is microstaging of early invasive cancer of the urinary bladder possible or useful? Mod Pathol 9(11):1035–1039. http://www.ncbi.nlm.nih.gov/pubmed/8933512
Gallioli A, Diana P, Fontana M et al (2022) En bloc versus conventional transurethral resection of bladder tumors: a single-center prospective randomized noninferiority trial. Eur Urol Oncol 5(4):440–448. https://doi.org/10.1016/j.euo.2022.05.001
Yang H, Lin J, Gao P et al (2020) Is the en bloc transurethral resection more effective than conventional transurethral resection for non-muscle-invasive bladder cancer? A systematic review and meta-analysis. Urol Int 104(5–6):402–409. https://doi.org/10.1159/000503734
Teoh JYC, D’Andrea D, Gallioli A et al (2023) En bloc resection of bladder tumour: the rebirth of past through reminiscence. World J Urol 41(10):2599–2606. https://doi.org/10.1007/s00345-023-04547-0
Orsola A, Trias I, Raventós CX et al (2005) Initial high-grade T1 urothelial cell carcinoma: feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur Urol 48(2):231–238. https://doi.org/10.1016/j.eururo.2005.04.013
Fransen van de Putte EE, Otto W, Hartmann A et al (2018) Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer. Urol Oncol Semin Orig Investig 36(8):361.e7-361.e13. https://doi.org/10.1016/j.urolonc.2018.05.007
Sahan A, Gerin F, Garayev A et al (2020) The impact of tumor invasion to muscularis mucosae-vascular plexus on patient outcome in pT1 bladder urothelial carcinoma. Arch Ital di Urol e Androl 92(3):239–243. https://doi.org/10.4081/AIUA.2020.3.239
Compérat E, Egevad L, Lopez-Beltran A et al (2013) An interobserver reproducibility study on invasiveness of bladder cancer using virtual microscopy and heatmaps. Histopathology 63(6):756–766. https://doi.org/10.1111/his.12214
Bol MGW, Baak JPA, Buhr-Wildhagen S et al (2003) Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J Urol 169(4):1291–1294. https://doi.org/10.1097/01.ju.0000055471.78783.ae
Van Rhijn BWG, Van Der Kwast TH, Alkhateeb SS et al (2012) A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 61(2):378–384. https://doi.org/10.1016/j.eururo.2011.10.026
Martin-Doyle W, Leow JJ, Orsola A, Chang SL, Bellmunt J (2015) Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 33(6):643–650. https://doi.org/10.1200/JCO.2014.57.6967
Budina A, Farahani SJ, Lal P, Nayak A (2022) Subcategorization of T1 bladder cancer on biopsy and transurethral resection specimens for predicting progression. Arch Pathol Lab Med 146(9):1131–1139. https://doi.org/10.5858/arpa.2021-0175-OA
Lopez-Beltran A, Cheng L (2021) Stage T1 bladder cancer: diagnostic criteria and pitfalls. Pathology 53(1):67–85. https://doi.org/10.1016/j.pathol.2020.09.014
Lopez-Beltran A, Raspollini MR, Hansel D et al (2024) International Society of Urological Pathology (ISUP) consensus conference on current issues in bladder cancer: working group 3: subcategorization of T1 bladder cancer. Am J Surg Pathol 48(1):e24–e31. https://doi.org/10.1097/PAS.0000000000002121
Paner GP, Kamat A, Netto GJ et al (2024) International Society of Urological Pathology (ISUP) consensus conference on current issues in bladder cancer. Working group 2. Am J Surg Pathol 48(1):e11–e23. https://doi.org/10.1097/PAS.0000000000002077
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Johannes Kläger, André Oszwald, Eva Compérat, Maximilian C. Koeller, Gabriel Wasinger, and David D’Andrea. The first draft of the manuscript was written by Johannes Kläger and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The submitted work was approved by the local ethics committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kläger, J., Koeller, M.C., Oszwald, A. et al. A single-center retrospective comparison of pT1 substaging methods in bladder cancer. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03907-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-024-03907-4