Abstract
The viscosity of the remelted rock compositions of the Glass House Mountains, SE Queensland, Australia, has been determined via micro-penetration in the high-viscosity regime (108–1013 Pa s). The heat capacity of these melts has also been determined from room temperature to above the glass transition. The combination of these two data sets allows the fitting of the viscosity data by the Adam-Gibbs equation using the configurational heat capacity Cpconf(Tg12) and configurational entropy Sconf(Tg12). The resulting fit parameters allow the robust extrapolation of the viscosity data to higher temperature and viscosities of 10–4 Pa s. This data can now be used in the discussion of the emplacement of the magmas of the plugs, laccoliths, sills and dykes that form the Glass House Mountains complex and the plate motion and the plume responsible for the volcano plugs. The large increase in viscosity of the evolving magma and the resulting decrease in discharge rate of the volcanic vents suggest that very little magma appeared as extrusive lavas or pyroclastic material and that the Glass House Mountains are mainly remnants of intrusive bodies exposed by erosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
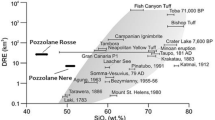
There is a growing interest in the 9–33 million years old plume track across Eastern Australia from Cape Hillsborough (QLD) to Cosgrove (Vic) (Cohen et al. 2013; Davies et al. 2015). The Noosa-Maleny-Glass House Mountains complex (hereafter called GHM) is a small suite of volcanoes in SE Queensland that is associated with this plume track (see Fig. 1). The GHM volcanoes have been dated in the Oligocene at 26 million years (Cohen et al. 2007). The shape of these plugs is similar to that of glass furnaces in Great Britain, hence the name given to them by Cook in 1770. Recent analysis of major and trace elements of rocks from the Glass House Mountains as well as Sr, Nd and Pb isotopic data by Shao et al. (2015) has shown the GHM volcanoes to be the product of an evolving magma chamber with the magma compositions progressing from basaltic lava flows in the Maleny region, to the Glass House Mountains volcanic plugs whose composition ranges from basaltic, basaltic trachyandesite to trachyandesite, trachyte and peralkaline rhyolite of comendite composition (see Fig. 2). The peraluminous rhyolite of Mt Tinbeerwah in the Noosa area is assumed to be formed by basaltic underplating causing crustal anatexis.
Although the composition and mineralogy of the volcanic plugs in the Glass House Mountains series are now known and they are assumed to be related through fractional crystallisation, little else is known of the flow and emplacement mechanisms of the magmas responsible for these volcanoes. Except for an early study (Bryan and Stevens 1973) there is no information of the emplacement temperatures of the magmas.
The viscosity of magmas of basaltic, andesitic and rhyolitic compositions have been determined (e.g. Vetere et al. 2008; Whittington et al. 2009; Di Genova et al. 2013; Webb et al. 2014; Sehlke and Whittington 2016); however, there are very few studies of the continuous change in viscosity with evolution of the magma chamber by fractional crystallisation. There is little data on the viscosity of subaluminous comenditic melts with Stevenson et al. (1995) investigating the viscosity of naturally occurring metaluminous comenditic obsidians which had not been remelted in the laboratory. Although the viscosity of magma of known composition can be estimated to within ± 1.5 log10 units using any of a range of viscosity models (e.g. Giordano et al. 2008; Misiti et al. 2011; Di Genova et al. 2013; Robert et al. 2015), it remains necessary to measure the viscosity of silicate melts in order to obtain accurate data.
Experiments
Sample preparation
A series of nine compositions has been chosen from the 34 rock analyses of Shao et al.’s (2015) paper on the petrogenesis of the peralkaline rhyolites of the Glass House Mountains. Melts of the compositions determined by Shao et al. (2015) were made from powders of oxides and carbonates. These powders were dried at 500 °C (1000 °C for MgO) for 12 h, weighed into a Pt90Rh10 crucible and decarbonated at 1000 °C for 12 h. The resulting material was melted at 1600 °C for 1 h, quenched to a glass, crushed in an agate mortar and remelted twice. Sample 7 (Mt. Tinbeerwah) was melted at 1625 °C. The compositions of the resulting glasses were determined by electron microprobe and are presented in Table 1. The iron speciation was determined by wet chemistry analysis after Wilson (1969) and Schuessler et al. (2008). Iron-free (haplo-)glasses were also synthesised. In these glasses, 50% of the mole fraction of FeOtot was assumed to be Fe3+ and was therefore replaced by Al2O3, and the rest was replaced by MgO. MgO was chosen over CaO as the radius of Mg is closer to that of Fe than Ca radius is (Shannon and Prewitt 1969). The compositions of these glasses were also determined by electron microprobe and are given in Table 2. The polymerisation of the melts has been described in terms of γ [∑ (X2O + YO)/ ∑ (X2O + YO + Al2O3 + Fe2O3) in mol. fraction] and NBO/T (non-bridging oxygens per tetrahedron = (2O-4 T)/T, in mol. fraction). Comparison of γ and NBO/T for the iron-bearing melts and their respective iron-free samples are in good agreement, except for 20a for which the iron-free haplo-melt has too many network modifying cations and is slightly metaluminous, whereas the iron-bearing melt is peraluminous. The viscosity and heat capacity of the series of iron-free melts were also determined. These melts are labelled as haplo-GHM samples.
Viscometry
The viscosity was determined in air by the micro-penetration technique in which a 2-mm diameter single crystal sphere of corundum is forced into the sample at a fixed load at a constant temperature. Newtonian, time-independent viscosity is determined by:
for t time (s), l indent distance (m), F applied force F (N), r radius (m) of the sphere and 0.1875 a geometrical constant (Pocklington 1940; Dingwell et al. 1992). Forces from 0.2 to 5 N were used for indent times from 15 min to 12 h. Viscosities in the range 108–1013 Pa s were determined. The samples were heated to the measurement temperature at a rate of 10 K min−1. The structure of the melt was allowed to equilibrate thermodynamically for 20 min before the force was applied and the indenter pushed into the melt. This amount of time was also needed for the temperature of the furnace to stabilise within ± 0.5 K. As these melts are highly viscous, some of the data were collected over a 12-h period with the unrelaxed penetration data being discarded. In general, mechanically relaxed penetration data were obtained ~ 100 τ after the application of the load, where
is the relaxation time calculated from the Maxwell equation (Maxwell 1867; Webb 1991) for η0 the time independent shear viscosity and \({G}_{\infty }\) the elastic shear modulus (Herzfeld and Litovitz 1959). The melt samples used for the viscosity measurements were ~ 3-mm-thick with an irregular shape varying from 6 to 10 mm in cross-section. The ends were ground parallel and polished with 1 µm Al2O3 powder before the viscosity measurements. The ± 0.06 log10 unit accuracy of the viscosity measurements was determined from the viscosity of DGG1 glass (Webb 2005) as well as from a further glass sample—NIQ (Whittington et al. 2000). The measured composition of the NIQ sample is given in Table 2.
Heat capacity
The heat capacity of the melts was determined using a Netzsch® DSC 404C at a heating rate of 20 K min−1 on samples which had first been heated to temperature above the glass transition temperature and then cooled at a rate of 20 K min−1. The heat capacity is measured against an empty Pt crucible. The heat flow is calibrated at the same heating rate as the glass measurements against a single crystal of Al2O3 and the heat capacity data from Robie et al. (1978). The heat capacity measurements are performed at temperature between 20 °C and that at which η ~ 109 Pa s. The structural relaxation time at this viscosity is ~ 0.1 s and therefore holding the melt at this temperature for 60 s before controlled cooling will allow the fictive temperature of the melt to be reset to that of the 20 K min−1 cooling rate used in the subsequent measurements.
The configurational heat capacity Cpconf(T*) (Richet 1984; Webb 2008) is calculated from an extrapolation of the Maier and Kelley (1932) equation:
for heat capacity as a function of temperature cp(T) and temperature T in K, fit to the unrelaxed glass part of the heat capacity curve, and the measured relaxed liquid heat capacity
The limiting fictive temperature of the samples is calculated using the method of Moynihan et al. (1976) where
(Narayanaswamy 1971; Moynihan et al. 1976) where the molar heat capacity Cp (J mol−1 K−1) = molar mass (g mol−1) x cp (J g−1 K−1). Equation 5 can be rewritten as
based upon the relationship between the temperature-dependent heat capacity and the temperature-dependent fictive temperature (DeBolt et al. 1976) for Tf fictive temperature, T temperature in K, Cp heat capacity as a function of temperature, Cpg unrelaxed (glassy) heat capacity as a function of temperature, Cpe relaxed heat capacity as a function of temperature; with \({T}_{f}^{^{\prime}}\) limiting fictive temperature and T* a temperature above the glass transition region at which the heat capacity is equal to the equilibrium heat capacity (fictive temperature is equal to furnace temperature), T’ a temperature below the glass transition region at which the heat capacity is that of the unrelaxed glass. Equation 6 is shown graphically in Fig. 3 where the integral is illustrated in terms of the area under the heat capacity curve.
Results and discussion
The viscosity of the melts is listed as a function of temperature in Table 3 and shown in Figs. 4 and 5 as a function of inverse temperature. The Arrhenian equation
Viscosity of the iron-free haplo-Glass House Mountains melts and the NIQ melt as a function of inverse temperature. The data for melts H34 and H10 are shifted to the right on the X-axis by the amount given in brackets for clarity. The straight lines are Arrhenian fits to the data. The curve through the NIQ data is that of Whittington et al. (2000)
for η0 the viscosity constant in Pa s and E the activation energy for flow in kJ mol−1, T temperature in K and the gas constant R = 8.314 J mol−1 K−1 is fit to the data in the form
for AArr a constant = log10 η0, BArr activation energy in kJ mol−1. The parameters to the fit are given in Table 4.
X-ray diffraction of the iron-bearing samples indicates a minor amount of crystallization (< 3 vol%) has taken place during the viscosity and calorimetry measurements. A check on the extent of the effect of crystallization on viscosity was made plotting Tg12 (the temperature at which η = 1012 Pa s) against γ. Previous studies have shown that a smooth curve as a function of gamma is to be expected in a series of compositionally related melts, with Tg12 increasing as the melts becomes subaluminous. Figure 6 illustrates the Tg12 values determined from the viscosity data for both the iron-bearing and iron-free melts. It can be seen that the data points for the Mt. Mee (34) and Maleny (12a) composition melts are much higher than expected, thus indicating that the melts had a large volume of crystals and the viscosity data are not that of a crystal-free melt. The Einstein-Roscoe equation (e.g. Vetere et al. 2013) for the viscosity of crystal-bearing melts indicates that 40 vol% crystals are needed to increase viscosity by an order of magnitude. In the case of the Mt. Mee and Maleny samples, it is observed that the interior of the glasses contained < 3 vol% micro-crystals, and the surface of the samples was covered with a thin veneer of micro-crystals after the viscosity measurements. As the micro-penetration technique measures the viscosity of the first ~ 500 µm of the sample, it would appear that the large number of crystals seen on the surface of the samples affects the measured viscosity of these two samples.
The temperature at which viscosity is 1012 Pa s has been calculated from the Arrhenian fits to the viscosity data and listed in Table 4. These Tg12 values have also been added to the composition data of Fig. 2. The importance of network modifiers is illustrated by the three SiO2-rich samples which have Tg12 values that do not linearly vary with wt% SiO2. The Tg12 values do vary linearly if they are plotted as a function of γ – which is a measure of the non-bridging oxygens in the melt. These three melts range from metaluminous (γ > 0.5), to subaluminous (γ = 0.5) to peraluminous (γ < 0.5).
The Arrhenian equation should not be extrapolated linearly in temperature. Instead the configurational heat capacity and the Adam-Gibbs equation (Richet 1984) have been used to fit the viscosity data across of the entire temperature range:
in order to calculate the temperature dependence of the viscosity at high temperatures; for AAG a constant, taken to be − 4.55 (see Giordano et al. 2008; Kleest et al. 2020 and references therein); BAG activation energy (J mol−1), Sconf(Tg12) configuration entropy (J mol−1 K−1) at the temperature for which viscosity is 1012 Pa s, Cpconf the difference in extrapolated glass heat capacity and relaxed melt heat capacity (J mol−1 K−1) at a temperature above the glass transition. This equation describes the viscosity-temperature behaviour of the melt from the temperature range of measurement to higher temperatures. The parameters BAG and Sconf(Tg12) are listed in Table 4.
This equation assumes there is no change in Sconf(Tg12) nor in Cpconf for temperatures below Tg12. The value for the parameter A is assumed to be constant for all silicate melts and thus independent of composition (Toplis et al. 1997). Based on the Maxwell relation (Eq. 2) and taking τ0 = 10–14.5 s (the vibration frequency of an atom at T−1 = 0; Martinez and Angell (2001)) and G∞ = 10 ± 0.5 GPa (Dingwell and Webb 1989), the Ae = -4.55 log10 Pa s is used in this study. The same value was used in the model of Giordano et al. (2008). The values of A found in the literature range from − 2.6 (Toplis 1998) to − 5 (see Webb 2011; Russell and Giordano 2017 for a discussion). Russell and Giordano (2017) point out that − 5 < A < − 3 is found to be the range of values used for the constant A in the literature; with – 4 < AAG < − 3 for Adam-Gibbs fits to viscosity data and – 5 < AVFT < − 4 for Vogel-Fulcher-Tammann equation fits to the viscosity data. They found the best fit value for AAG to be − 3.51 ± 0.25. The Sconf(Tg12) values obtained from the present viscosity and Cpconf data using this value of AAG are also given in Table 4. The fragility of the melts was also calculated from:
(Toplis et al. (1997)
The viscosity prediction models of Hui and Zhang (2007) and Giordano et al. (2008) in which it is assumed that the Fe2+/Fetot is constant for all melts investigated, and Duan (2014) which differentiates between Fe2+ and Fe3+, have been compared to the present viscosity data. These models estimate the viscosity of the iron-bearing and iron-free melts to within ± 2 orders of magnitude. The difference between the measured viscosity of the present melts and that predicted by the Giordano et al. (2008) model is shown in Fig. 7.
Misfit between modelled viscosity of Giordano et al. (2008) and the measured viscosities as a function of measured viscosity
The variation of Cpconf and Sconf(Tg12) as a function of γ, and also SiO2 content can be observed in Table 4. While Sconf(Tg12) does not show any clear trend as a function of composition, the Cpconf values increase with decreasing polymerisation of the melts. Giordano and Russell (2017) found the measured Cpconf values for a series of silicate melts to be a linear function of mole fraction SiO2 with \(C_p^{conf}=52.6-55.88\;X_{{SiO}_2}\) . The present Cpconf data for both the iron-bearing and iron-free melts scatter around this trend-line with \({{\text{C}}_{\text{p}}}^{{\text{conf}}}{\text{(GHM) = 50}}{.35 - 54}{\text{.43 }}{{\text{X}}_{{\text{Si}}{{\text{O}}_{2}}}}\).
Russell and Giordano (2017) found the ratio of the measured Cpconf to the fitted Sconf value to be a function of Tg12 and fragility (m). The Cpconf and Sconf determined here from heat capacity and viscosity measures and calculated with AAG = − 4.55 fall slightly off this trendline (Cpconf/Sconf(Tg12) = 0.061 m—1.018). However, the ratio obtained using the Sconf values determined from the viscosity data assuming an AAG of − 3.51 fall exactly on the trendline of Russell and Giordano (2017) (0.064 m – 0.993). The Tg12 term has been ignored here in these straight-line fits to the data as its contribution to the equation of Russel and Giordano is ~ 0.004.
Figure 8 shows the viscosity of melts 7 (Maleny) and 12 (Mt. Tinbeerwah) calculated from Eq. 9 together with the viscosity region of the input data. This figure also shows the present viscosity data for the NIQ sample, together with the high temperature datum from Whittington et al. (2000) and the curves calculated by Whittington et al. and the present Adam-Gibbs curve. The two curves are identical (within 0.1 log10 unit); and both pass though the datum of Whittington et al. (2000). This agreement between the viscosity extrapolated using the Adam-Gibbs equation and the measured low viscosity indicates the applicability of the Adam-Gibbs equation in the calculation of low viscosities, based on the high viscosity data combined with the configuration heat capacity.
Extrapolation of the measured viscosity data for the Glass House Mountains magmas. The viscosity is extrapolated using the Adam-Gibbs equation and the present heat capacity data (see Table 4). The two viscosity curves are for the least and most fragile (m) of the melts and also for the highest and lowest melt viscosities of the system. The viscosity data for the NIQ melt are also shown
As there is little or no information on the eruption temperature of the lavas of the Glass House Mountains volcanoes, it is difficult to estimate the amount of magma involved. Further, although the mafic lavas near Maleny are present, the lavas and/or pyroclastics of the rest of the Glass House Mountains volcanoes appear to have been removed by erosion (Ewart & Grenfell 1985; Cohen et al. 2007).
Taking the best-case scenario to calculate the discharge rate of a volcanic conduit, the volume flow rate of a frictionless cylindrical volcanic conduit can be calculated from:
for acceleration due to gravity 9.81 m s−2, rock density ρr (kg m−3), melt density ρm (kg m−3), radius of the vent r (m) and viscosity of the melt η (Pa s) (Philpotts 1990). The rate of flow through such a conduit calculated using the average eruption temperature of each composition melt (taken from Spera 2000), the viscosity of the present melt at that temperature, and the density of the present melt calculated from Lange and Carmichael (1987) and the assumption that the source of the magma is the upper mantle with a density of 3300 kg m−3, is shown in Table 5. The mafic lavas around Maleny are known to cover 200 km2, and to be made up of ~ 10 m thick layers to a total depth of 180 m (Ewart and Grenfell 1985; Cohen et al. 2007). The calculations of Table 5 would indicate that 68 days of continuous eruption with a 2.5-m radius vent are needed to produce 36 km3 of basaltic lava, with 4 days eruption time needed to cover the surface with a 10-m-thick layer. These calculations would infer that the basaltic eruptions were of short duration, or that the vent radius was smaller than 2.5 m. A reduction of the radius by 15% would halve the flow rate. Doubling the radius of the vent would increase the volume flow rate by more than an order of magnitude.
Conclusion
The combination of low temperature viscosity data with low temperature heat capacity data allows the use of the Adams-Gibbs equation to extrapolate the viscosity of the Glass House Mountains magma to higher temperature with great confidence. Based on the small basalt flows observed in the Maleny region it is suggested that the discharge rate of the more viscous melts would be very low and perhaps, as suggested by Ewart and Grenfell (1985) and Cohen et al. (2007) the Glass House Mountains are the remnants of unextruded magmas which formed plugs and laccoliths. The effect of water and perhaps bubbles would, however, decrease the viscosity. Stevenson et al. (1998) found that 1 wt% water decreased the viscosity of comendites by 2 or more orders of magnitude in the temperature range of interest. This would increase the discharge rate by 2 orders of magnitude. In the case of the comendite compositions (as well as the dacite and andesite) such an increased discharge rate would still remain very low when compared to that of the basalt melts.
Change history
02 March 2022
The original version of this paper was updated to add the missing compact agreement Open Access funding note.
References
Bryan WB, Stevens NC (1973) Holo-crystalline pantellerite from Mt. Ngun-Ngun, Glass House Mountains, Queensland. Australia Am J Sci 273:947–957
Cohen BE, Knesel KM, Vasconcelos PM, Schellart WP (2013) Tracking the Australian plate motion through the Cenozoic: contraints from 40Ar/39Ar geochronology. Tectonics 32:1371–1383
Cohen BE, Vasconcelos PM, Knesel KM (2007) 40Ar/39Ar constraints on the timing of Oligocene intraplate volcanism in southeast Queensland, Australia. Aust J Earth Sci 54:1205–2125
Davies DR, Rawlinson N, Campbell ICH (2015) Lithospheric controls on magma composition along Earth’s longest continental hotspot track. Nature 525:511–514
DeBolt MA, Easteal AJ, Macedo PB, Moynihan CT (1976) Analysis of structural relaxation in glass using rate heating data. J Am Ceram Soc 59:16–21
Di Genova D, Romano C, Hess KU, Vona A, Poe PT, Giordano D, Dingwell DB, Behrens H (2013) The rheology of peralkaline rhyolites from Pantelleria Island. J Volc Geotherm Res 249:201–216
Dingwell DB, Knoche R, Webb SL (1992) The effect of B2O3 on the viscosity of haplogranitic liquids. Am Mineral 77:457–461
Dingwell DB, Webb SL (1989) Structural relaxation in silicate melts and non-Newtonian Melt rheology in geological processes. Phys Chem Minerals 16:508–516
Duan X (2014) A model for calculating the viscosity of natural iron-bearing silicate melts over a wide range of temperatures, pressure, oxygen fugacites, and compositions. Am Mineral 99:2378–2388
Ewart A, Grenfell AT (1985) Cainozoic volcanic centres in southeastern Queensland: with special reference to the Main Range, Bunya Mountains, and the volcanic centres of the northern Brisbane coastal region. Papers, Department of Geology, University of Queensland, 11:1–57
Giordano D, Russell JK (2017) The heat capacity of hydrous multicomponent natural melts and glasses. Chem Geol 461:96–103
Giordano D, Russell JK, Dingwell DB (2008) Viscosity of magmatic liquids: a model. EPSL 271:123–134
Herzfeld TA, Litovitz KF (1959) Absorption and dispersion of ultrasonic waves. pp 535, Academic Press, New York
Hui H, Zhang Y (2007) Toward a general viscosity equation for natural anhydrous and hydrous silicate melts. Geochim Cosmochim Acta 71:403–416
Kleest C, Webb SL, Fanara S (2020) Rheology of melts from the Colli Albani volcanic district (Italy): a case study. Contrib Mineral Petrol 175:82
Lange RA, Carmichael ISE (1987) Densities of Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-TiO2-SiO2 liquids – new measurements and derived partial molar properties. Geochim Cosmochim Acta 51:2931–2946
Maier CG, Kelley KK (1932) An equation for the representation of high-temperature heat content data. J Am Chem Soc 54:3243–3246
Martinez LM, Angell CA (2001) A thermodynamic connection to the fragility of glass-forming liquids. Nature 410:663–667
Maxwell JC (1867) On the dynamical theory of gases. Proc Roy SocLondon 15:167–171
Misiti V, Vetere F, Freda C, Scarlato P, Behrens H, Mangiacapra A, Dingwell DB (2011) A general viscosity model of Campi Flegrei (Italy) melts. Chem Geol 290:50–59
Moynihan CT, Easteal AJ, DeBolt MA, Tucker J (1976) Dependence of fictive temperature of glass on cooling rate. J Am Ceram Soc 59:12–16
Narayanaswamy OS (1971) Model of structural relaxation in glass. J Am Ceram Soc 54:491–498
Philpotts AR (1990) Principles of Igneous and Metamorphic Petrology. Prentice Hall
Pocklington HC (1940) Rough measurement of high viscosities. Proc Cambridge Phil Soc 36:507–508
Richet P (1984) Viscosity and configurational entropy of silicate melts. Geochim Cosmochim Acta 48:471–483
Robert G, Knipping JL, Scherbarth S, Robertson TE, Stechern A, Behrens H, Whittington AG (2015) Heat capacity and viscosity of basaltic melts with H2O ± F ± CO2. Chem Geol 418:51–65
Robie RA, Hemingway BS, Fischer JR (1978) Thermodynamic properties of minerals and related substances at 29815 K and 1 bar (105 Pa) pressure and at higher temperatures. US Geolog Surv Bull 1452:428–446
Russell JK, Giordano D (2017) Modelling configurational entropy of silicate melts. Chem Geol 461:140–151
Schuessler JA, Botcharnikov RE, Behrens H, Misiti V, Freda C (2008) Oxidation state of iron in hydrous phono-tephritic melts. Am Mineral 93:1493–1504
Sehlke A, Whittington AG (2016) The viscosity of planetary tholeiitic melts: a configurational entropy model. Geochim Cosmochim Acta 191:277–299
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Cryst B25:925–946
Shao F, Nui Y, Regelous M, Zhu DC (2015) Petrogenesis of peralkaline rhyolites in an intra-plate setting: Glass House Mountains, southeast Queensland, Australia. Lithos 216:196–210
Spera F (2000) Physical Properties of magma. In: Sigurdsson H (ed) Encyclopedia of Volcanoes. Academic Press, Amsterdam, pp 171–190
Stevenson RJ, Dingwell DB, Webb SL, Bagdassarov NS (1995) The equivalence of enthalpy and shear stress relaxation in rhyolitic obsidians and quantification of the liquid-glass transition in volcanic processes. J Volc Geotherm Res 68:297–306
Stevenson RJ, Bagdassarov NS, Dingwell DB, Romano C (1998) The influence of trace amounts of water on the viscosity of rhyolites. Bull Volcanol 60:89–97
Toplis MJ (1998) Energy barriers to viscous flow and the prediction of glass transition temperatures of molten silicates. Am Mineral 83:480–490
Toplis MJ, Dingwell DB, Hess KU, Lenci T (1997) Viscosity, fragility, and configurational entropy of melts along the join SiO2-NaAlSiO4. Am Mineral 82:979–990
Vetere F, Behrens H, Schuessler JA, Holtz F, Misiti V, Borchers L (2008) Viscosity of andesite melts and its implication for magma mixing prior to Unzen 1991–1995 eruption. J Volc Geotherm Res 172:208–217
Vetere F, Sato H, Ishebashi H, De Rosa R, Donato P (2013) Viscosity changes during crystallization of a shoshonitic magma: new insights on lava flow emplacement. J Min Petrol Sci 108:144–160
Webb S (2005) Structure and rheology of iron-bearing Na2O-Al2O3-SiO2 melts. Europ J Mineral 17:223–232
Webb SL (1991) Shear and volume relaxation in Na2Si2O5. Am Mineral 9:1449–1454
Webb SL (2008) Configurational heat capacity of Na2O-CaO-Al2O3-SiO2 melts. Chem Geol 256:92–101
Webb SL, Murton BJ, Wheeler AJ (2014) Rheology and the Fe3+-chlorine reaction in basaltic melts. Chem Geol 366:24–31
Webb SL (2011) Configurational heat capacity and viscosity of (Mg, Ca, Sr, Ba)O-Al2O3-SiO2 melts. Europ J Mineral 23:487–497
Whittington A, Richet P, Holtz F (2000) Water and the viscosity of depolymerized aluminosilicate melts. Geochim Cosmochim Acta 64:3725–3736
Whittington AG, Hellwig BM, Behrens H, Joachim B, Stechern A, Vetere F (2009) The viscosity of hydrous dacitic liquids: implications for the rheology of evolving silic magmas. Bull 71:185–199
Wilson AD (1969) The micro-determination of ferrous iron in silicate minerals by a volumetric and a colorimetric method. Analyst 85:823
Acknowledgements
I wish to thank Bettina Schlieper-Ludewig for the samples and viscosity data. I would like to thank the reviewers and K. Cashman and D. Giordano for their their comments which greatly improved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by DFG grant WE 1810/14–1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: K.V. Cashman
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Webb, S.L. Viscosity of evolving magmas: a case study of the Glass House Mountains, Australia. Bull Volcanol 83, 76 (2021). https://doi.org/10.1007/s00445-021-01495-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00445-021-01495-8