Abstract
While the influence of canopy trees on soils in natural and restored forest environments is well studied, the influence of understory species is not. Here, we evaluate the effects of outplanted native woody understory on invasive grass biomass and soil nutrient properties in heavily grass-invaded 30 + year-old plantations of a native N-fixing tree Acacia koa in Hawai‘i. We analyze soils from under A. koa trees with versus without planted woody understory and compare these to soils from under remnant pasture trees of the pre-deforestation dominant, Metrosideros polymorpha where passive recruitment of native woody understory has occurred since the cessation of grazing. Simultaneously, we experimentally planted understory species at three times the density used by managers to see if this could quickly decrease grass biomass and change soil nutrient dynamics. We found that invasive grass biomass declined with understory planting in surveyed and experimental sites. Yet, woody understory abundance had no effect on N cycling. Short-term N availability and nitrification potential were higher under A. koa than M. polymorpha trees regardless of understory. Net N mineralization either did not differ (~ 1 mo) between canopy species or was higher (171 day incubations) under remnant M. polymorpha where organic matter was also higher. The only influence of understory on soil was a positive correlation with loss-on-ignition (organic matter) under M. polymorpha. We also demonstrate differential controls over N cycling under the two canopy tree species. Overall, understory restoration has not changed soil characteristics even as invasive grass biomass declines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Planting out native woody understory reduces persistent invasive grasses.
-
Yet, it has no detectable effect on soil nitrogen cycling.
-
Canopy tree species drive soil N cycling in different ways, and this is independent of understory.
Introduction
Reforestation of once forested landscapes via planting of canopy trees is widespread due to local and international conservation initiatives (for example, https://www.iucn.org/theme/forests/our-work/forest-landscape-restoration/, https://www.trilliontreecampaign.org/). The resulting tree stands can be dramatically different from remnant forest stands in their canopy composition as well as their understory, particularly if canopy trees are monospecific and no effort is made toward understory restoration (for example, Powers and others 1997; Scowcroft and others 2004; Ostertag and others 2008; McClain and others 2011; Wilson and Rhemtulla 2016). Tree canopy composition can influence nutrient accumulation and cycling, which in turn can influence the successional trajectory of the ecosystem (Vitousek and Walker 1989; Powers and others 1997; Binkley and Giardina 1998; Wedderburn and Carter 1999; Hobbie and others 2006; Reed and others 2008; Omeja and others 2011; Carnol and Bazgir 2013, Yelenik 2017). In some cases, canopy composition results in the development of vegetation that inhibits the natural recruitment of native woody taxa (Royo and Carson 2006). Few studies have evaluated how active restoration of understory after canopy tree planting influences a recalcitrant understory and soil nutrient cycling, or the interactions between these.
While forest restoration with N-fixing trees is often utilized with positive outcomes (for example, Parrotta 1992; Chaer and others 2011; Hoogmoed and others 2014), planting with N-fixers may not always be beneficial, especially when in monoculture (Yelenik 2017). Nitrogen (N)-fixing woody plants can have profound consequences for understory development and soil N cycling by increasing litterfall and N availability (Hart and others 1997; Hughes and Denslow 2005), changing P limitation (for example, Allison and others 2006) or influencing secondary compounds, microbial activity and decomposition (for example, Allison and others 2006; Marchante and others 2008; Castro-Díez and others 2012). N-fixers can stall succession by shading out seedlings of other species, creating N conditions that favor fast-growing non-native species, or interfering with native understory establishment in other ways (Maron and Connors 1996; Adler and others 1998; Royo and Carson 2006; González-Muñoz and others 2012; Yelenik 2017). Non-native understory species can then generate or perpetuate high available N conditions. Invasive grasses in the understory of eastern USA forests, for example, accelerate N cycling while limiting native plant recruitment (Lee and others 2012; Rippel and others 2020). Whether resource conditions and stalled succession perpetuated by N-fixing canopies can be reversed through restoration of understory species remains unexplored.
Despite the potential importance of understory vegetation to succession and nutrient cycling (for example, Chapin 1980; Nilsson and Wardle 2005), few case studies of the plant and soil-related outcomes of understory restoration exist. Particularly lacking are studies done in the absence of other management actions. Most studies use understory removal rather than planting to evaluate the role of understory (Wardle and Zackrisson 2005; Matsushima and Chang 2007; Xiong and others 2008; Wang and others 2014) or they evaluate the role of understory after 1–2 decades of canopy and understory manipulations such as via fire or forest thinning (Laughlin and others 2011; Yelenik and others 2013; Burton and others 2017). Direct restoration of understory species could reduce undesirable species that have benefitted from high soil N by sequestering N into more conservative (slow-growing, slow N turnover) woody biomass, and shifting the composition of leaf litter. Alternatively, planted understory species may suffer competition from nitrophilous understory species, particularly if those are promoted by high N-availability under an N-fixing canopy. In this case, direct restoration may have little effect on soil nutrient dynamics. It is not clear how dense understory must be to influence soil N conditions created by canopy trees.
The planting of canopy N-fixing trees in grass dominated pastures where montane forest (~ 1600–2000 m.a.s.l.) once stood has been taking place for several decades in Hawai‘i (Baker 2009). Restoration at mid and high elevations is intended to quickly create habitat for the upslope movement of critically endangered forest birds (Paxton and others 2018). Such restoration has focused on the planting of extensive corridors of the endemic fast-growing, N-fixing tree, Acacia koa, with little attention to understory (Baker 2009). Yet some native forest birds rely on fleshy fruit bearing understory plants (Paxton and others 2018). Several studies have demonstrated that A. koa enhances short-term N availability beneath its canopy (Scowcroft and others 2004; Yelenik 2017; August-Schmidt 2018) and that passive understory regeneration does not occur under A. koa, at least not on a 40 year timescale, because of densely growing, invasive grasses that benefit from the high-N environment (Scowcroft 1992; Scowcroft and others 2007; Yelenik and others 2020, 2022a; Rehm and others 2023). Indeed the understory of Acacia koa plantations across Hawai‘i are dominated by invasive alien species primarily grasses (Baker 2009). Understory native woody plantings within A. koa stands could disrupt this relationship by creating competition with dense-growing grasses and by changing the chemistry of litter going into the soil organic matter pool (McDaniel and Ostertag 2010; Yelenik and others 2022b).
In the current study, we evaluated the responses of invasive grass biomass and soil properties (N cycling, microbial biomass, nitrification potential, organic matter) under two different native canopy tree species to varying understory woody plant densities. We focus on planted corridors of A. koa (hereafter, AK), identifying trees with and without planted understory, and compare these to nearby remnant Metrosideros polymorpha (hereafter RO for “remnant ʻōhiʻa”) trees with a range in density of native woody understory that has recruited naturally since the cessation of livestock grazing 40 years ago. The RO sites represent a more desirable management trajectory because they contain diverse native plants that attract native endangered birds (Paxton and others 2018) and help provide water capture (Takahashi and others 2011; Fortini and others 2020). M. polymorpha normally dominates intact Hawaiian forest (Mueller-Dombois and others 2013), yet is largely absent within the extensive planted A. koa corridors.
Recent planting of understory species under AK trees and natural recruitment under ROs provides a gradient of understory basal area that allows us to ask the following questions: (1) How does invasive grass abundance change as understory develops under each canopy tree? (2) How does native understory basal area influence N cycling and other soil metrics? And (3) What plant and soil variables best predict N cycling and soil metrics under the two canopy tree species? We predicted that as the basal area of planted understory increased under AK, N availability and invasive grasses would decrease, leading to soil properties resembling those under RO trees. The prediction of reduced N availability is based on an assumption (not tested here) that there would be increased N uptake by woody plants since they increase diversity of growth forms and overall net primary production. In addition they will contribute leaf litter of different quality than AK (since none are N-fixers) thus diversifying the low C:N litter inputs typical of AK (Yelenik and others 2022b).
In addition to these observational studies, we conducted an outplanting experiment to ask: (4) Can high-density understory planting reduce invasive grasses and alter soil N within 4 years? By planting at more than three times the density typically used in restoration plots (Steve Kendall, USFWS, pers. comm. 2019), we predicted that we should see reduced grass biomass and a decline in available N as mineral N is moved into woody biomass.
A final goal was to address, (5) How do soil conditions under canopy trees (AK or RO) compare to conditions in adjacent unrestored pasture? Our goals do not include comparisons to intact forests. Rather, we evaluate how soils beneath plantation AK or RO trees compare to the absence of a tree canopy. We predicted that these open sites would have lower organic matter and microbial biomass compared to either tree type due to the lower C inputs in the relative absence of tree and shrub litter.
Methods
Study Site
Hakalau Forest National Wildlife Refuge is a 13,240 ha high elevation tract on the windward side of Hawai‘i Island, USA. The area receives approximately 2700 mm rainfall annually (Giambelluca and others 2013, Hawai‘i Rainfall Atlas). The refuge includes intact forest at lower elevations (< 1600 m.a.s.l.), and large areas (~ 2000 ha) where forest was cleared. The intact forest is dominated by M. polymorpha with A. koa as the second most abundant canopy tree. Clearing for livestock occurred in the 1800s with some remnant M. polymorpha and A. koa trees left within pastures. The US Fish and Wildlife Service (USFWS) purchased the land in the 1980s, fenced and removed ungulates and in the early 1990s, planted corridors of A. koa to initiate forest recovery and facilitate development of endangered bird habitat. Yet the understory of the A. koa corridors today remains dominated by two introduced (African) perennial grasses, Cenchrus clandestinum (kikuyu) and Ehrharta stipoides (meadow rice grass). Native understory species have not regenerated in these corridors or under remnant koa (Yelenik 2017) reducing their attractiveness for native frugivorous birds. Consequently, over the past 15 years, the USFWS has begun to outplant understory species, including Cheirodendron trigynum, Coprosma rhincocarpa, Ilex anomola, Myrsine lessertiana, and Vaccinium calycinum. Metrosideros polymorpha is also sometimes planted into this understory. While we do not know the precise planting time of locations we sampled, we chose sites where understory woody plants were at least 8 years old based on size and our familiarity with plant growth rates. These approximate dates were corroborated by USFWS personnel.
In contrast to the dense grass under AK trees, RO trees vary in their understory composition from almost 100% non-native grass cover to dense ‘halos’ of native shrubs and sub-canopy trees extending to and sometimes beyond the edge of the RO tree canopy (Yelenik 2017). These ‘halo’species have recruited into the understory since the removal of ungulates (Yelenik 2017). Common understory species include many of those currently being used in understory outplanting efforts (Rehm and others 2021).
Soils across the refuge are acidic andisols of the Laupahoehoe Volcanic series (Peterson and Moore 1987). The surface soils are well-drained loams and typically have high organic matter. They overlay basalt lava flows between 10,000 and 20,000 years old (Scowcroft and others 2004).
Focal Tree and Sample Site Selection
From October 2016 through February 2017, we conducted an initial tree survey along transects established by USFWS for annual bird point-counts. Every 200 m we located potential focal trees of the two canopy types. For RO, we attempted to locate trees outside of AK corridors to isolate species-specific canopy effects. We visually estimated percent cover of understory species within a 5 m radius of the potential focal tree and then chose a non-random subset of twenty of each of these tree-centered plots stratified to ensure overstory tree types were spread across the refuge (Figure S1) and to be representative of a gradient of woody understory density under each canopy species.
Understory woody plants in AK sites were from plantings, as there is no natural recruitment there. By contrast, the understory woody plants in all RO sites were ‘naturally recruited’ since there is no active planting under ROs. Soil from six ‘open grassland’ (OP) sites was sampled as a comparison to tree-covered plots. Open sites were more than 10 m from a tree with no A. koa suckers or woody plants in a 100-m2 area around the center point. We included fewer open sites because it was difficult to find ones without A. koa root suckers, or that were not on rocky topography or covered with invasive Rubus argutus (Florida raspberry). OP sites were representative of abandoned pasture without restoration.
Vegetation and Soil Sampling
At each chosen focal tree or OP center point, we set out 5-m radius transects and surveyed all woody plants in pie wedges formed by these transects. We placed each plant encountered into a basal diameter size class identified to species. Grass cover was visually estimated by 2 or 3 observers. To sample the grass and litter biomass, we placed 1 m × 0.5 m quadrats 2.5 m away from the central plot point in each cardinal direction. We harvested grass biomass to the soil surface (considered to be soil without recognizable leaves) and separated out plant litter. The grasses and plant litter were weighed in the field, and a subsample of each returned to the laboratory to be dried for conversion to dry biomass. Subsamples were ground through 20 mm Wiley mesh and a Wig-L-bug (Crescent Dental), and analyzed for %C and %N on an elemental analyzer (Fison Instruments) at the University of Hawai‘i, Hilo Analytical Lab. Values from the four quadrats were averaged to a single value per tree.
We also took two 15 cm deep, 2.5 cm wide soil cores in two of the quadrats. The 4 cores per focal tree were combined into one bag for a single composite sample. Samples were transported to the laboratory in coolers and sieved through 2 mm mesh within a week of collection and stored in a refrigerator at 4C until the first extractions (~ 2 weeks). Soil organic content was estimated on dry subsamples from each site by measuring the loss of weight after ignition (Loss-on-ignition) in a 550 °C muffle furnace for 5 h. Values are presented as raw numbers since we did not establish a carbon correction factor for these soils, yet we consider them indicative of soil organic matter in these low clay soils.
Short-Term N Availability
We used two methods to determine short-term N availability: (1) A five-day laboratory resin capsule incubation (hereafter, resinN) begun immediately after the final soil samples were sieved, and (2) a roughly one-month (26 days) aerobic laboratory incubations (netmin) begun at the same time. For resinN, 35–40 g of field moist soil was wetted with DI water to form a paste following methods from Unibest (https://www.unibestinc.com/technology). Then a resin capsule (Unibest) was inserted into the paste and incubated for 5 days in the dark at 22 °C in the laboratory. Retrieved resin capsules were washed with a forceful stream of DI water and then dripped with 2N HCl (https://www.unibestinc.com/technology). The extracted solutions were analyzed for NH4 and NO3 at the UHH Analytical lab. Values were converted from ppm to ug mineral N captured per g resin per day. These HCl extracts were also analyzed by Unibest for other associated ions (data not reported here). For short-term netmin, sieved soils were wetted to 70% of water holding capacity (determined in the lab with funnel method) and allowed to incubate in a plastic bag (Zip-loc) for two days at room temperature in the dark before a time zero (T0) sample was taken. At that time, 10-g of soil was placed into a tin and dried overnight at 100 °C for gravimetric water determination. 20 g were extracted immediately with 2 M KCl for T0N, and an additional 20 g were placed into a specimen cup, covered, and incubated for 26 days at 20–22 °C in the dark in Hawai‘i. After 26 days, samples were extracted with 2 M KCl. Extractants were analyzed for NH4 and NO3 at the UHH Analytical lab on a flow injection analysis system (Lachat 8500 Quickchem). Short-term netmin was calculated as T1(NH4 + NO3)—T0(NH4 + NO3) divided by the dry weight of incubated soil. Net nitrification was calculated similarly.
Nitrogen availability metrics were not meant to substitute for field rates as both disturbance and storage conditions disrupt N dynamics (for example, Collier and Ruark 2021). Rather, they were meant as comparative methods across ‘treatments’ (vegetation categories) to evaluate mineralization potential.
Long-Term N Transformation, Nitrification Potential and Microbial Biomass
To measure the comparative long-term ability of soils to mineralize N, we shipped sieved, moistened soils in coolers to the University of California, Santa Barbara (UCSB), where they were kept at 4 °C for ~ three weeks. In total these soils had been field collected for ~ 10 weeks prior to the initiation of this long incubation. While we recognize that how soils are handled can strongly influence their mineralization potential (Benghi and Richter 2002), and cold storage decreases mineralization in tropical soils (Arnold and all 2008), all soils were treated equally after collection and experienced no air drying since this can dramatically change C and N mineralization (for example, Benghi and Richter 2002; Collier and Ruark 2021). Furthermore, storage time has been shown not to influence differences among soil microbial communities in different soils (Gonzalez-Quinones and others 2009). Thus, comparisons among vegetation treatments should be robust (Collier and Ruark 2021). To assess whether soils at UCSB were similar in their potential to when we collected them, we regressed the T0 mineral N value from our Hawai‘i netmin study, with the Tinitial value of each core in the UCSB long-term netmin and found that they were correlated (Figure S2) particularly for the RO cores (R2 = 0.68). The UCSB, Tinitial values are much higher because the soils had been sitting for some time (even though refrigerated), but they nonetheless correlated with the original extraction values.
To set up the long-term incubation, soils were divided into multiple 10-g subsamples, each placed into a falcon tube and incubated in mason jars at 22 °C on a 12 h:12 h light dark cycle for ~ five months. N mineralization was measured by taking a Tinitial sample and samples at ~ 4–8 week intervals to a maximum of 171 days. Sampling involved extraction with 2 M KCl, as well as measurement of 24 h soil respiration rates using an infrared gas analyzer (Licor-CO2 gas analyzer) prior to KCl extraction.
We measured the relative size of the microbial nitrifier community via a nitrification assay assessed using a chlorate slurry inhibition method (Belser and Mays 1980). We incubated 5 g of moist soil for 6 h in a solution containing 0.2 M dipotassium phosphate (K2HPO4), 0.2 M monopotassium phosphate (KH2PO4), and 0.2 M ammonium sulfate ((NH4)2SO4). The goal was to provide abundant substrate to microbes, while blocking oxidation of nitrite to nitrate. We determined NO2 concentrations with a colorimetric assay at hours 0, 3 and 6 and estimated nitrification potential using the rate of increase in NO2− over the course of the assay. The assay was conducted after the soils had incubated for ~ two months.
To measure microbial biomass, we used a substrate-induced respiration (SIR) assay following Fierer and others (2003) modification of West and Sparling [1986]. The assay was performed on soil subsamples from the same bulk samples on which nitrification potential measurements. The method relies on measuring the response of microbes to an excess of labile C as yeast. Five grams of wet soil were incubated with the yeast, after which CO2 efflux rates were measured at T0 (10 min post yeast addition), and approximately 2 and 4 h later by injecting gas from the headspace of each jar into an infrared gas analyzer (Licor IRGA). Conversion of CO2 efflux rates to an index of SIR was done following Fierer and others (2003).
Experimental Understory Density Treatments
To evaluate experimentally how understory planting density affects grass biomass and soil properties, we planted understory species commonly used in restoration at Hakalau Forest around single AK trees with no previous outplanting. We selected sets of trees at seven sites that were at least 100 m apart from each other and applied three treatments: control, single density and double density. For the single-density treatment, seedlings of 5 woody plants species (four C. trigynum (ʻōlapa), four C. rhynchocarpa (pilo), three M. lessertiana (kōlea), three V. calycinum (ʻōhelo) and two I. anomala (kāwaʻu) were outplanted around focal AK with 16 individual plants planted into a roughly 28 m2 area (= 0.57stems m−2). This is almost three times the planting density currently used by the USFWS. For the high-density planting treatment, seedlings were planted at twice this density (1.14stems m−2). The highest density we could find in USFWS outplanting was 0.65 stems m−2. For examples of how these plantings look see Figure S3. Two of the species (ʻōlapa and pilo) were planted in July 2016, and the rest in October 2016 (see Yelenik and others 2022b). The understory of all trees was initially dominated by kikuyu grass with no woody plants. Outplant height averaged 40 cm. The mix of species was typical of those found naturally recruiting around RO. Nitrogen availability and grass biomass were sampled immediately prior to planting and found to not be different between the plots (Yelenik and others 2022b).
In September 2020, we harvested grass and standing woody litter to the soil surface in two 0.5 m × 0.5 m quadrats per AK tree as well as at one nearby AK tree within 10 without understory plantings as a control (n = 7). Clipped plots were located 0.5 m north and east of the focal AK. We then collected soil by taking one 3.8-cm diameter by 10 cm deep soil cores from both clipped plots, bulked the two cores into one sample per tree, and sieved the samples through 2 mm mesh. We extracted 10 g of sieved soil with 50 mL of 2 M KCl for 1 h and filtered the extracts. Extracts were analyzed at the UHH Analytical Lab for inorganic N (Lachat Quikchem 8500) and corrected for gravimetric soil moisture. We measured soil %C and N on sieved soil. In August 2021 we collected light transmittance with a light wand (Apogee MQ-301 PAR Meter, Logan, Utah) at breast height, with the wand placed into the understory plantings (or a similar location at control trees) at each of the cardinal directions. We then took three points from a nearby location with no canopy to get a full sun reading and used the ratio of the understory to the average full sun reading for light transmittance (four readings per tree).
Statistics
We used ANCOVA to evaluate the impact of canopy tree and understory woody basal area (hereafter, UWBA,) on N availability metrics, loss-on-ignition, microbial biomass, and nitrification potential. Because these analyses uniformly supported the distinct nature of the two canopy soil environments, we then analyzed controls over soil metrics separately by tree type. Long-term netmin was assessed using a mixed model ANOVA with treatment (canopy type), time and their interaction as fixed, and site (specific tree) as a random factor.
We used multiple regression to identify the best combination of continuous predictor variables for the N metrics for each tree species. Prior to running the models, we assessed the distribution of residuals of each response variable for normality. In all but two cases the normal distribution was supported. Where it was not (AK-longterm net min, RO-resinN), the lognormal or another distribution (Weibull) was chosen. We considered six potential explanatory variables from the tree down to soil microbes: focal tree basal area, UWBA, grass biomass, litter %N (hereafter litterN), organic matter as loss on ignition, and microbial biomass by SIR from our lab studies (hereafter MB). Prior to running models we ran a correlation matrix among the variables for each canopy tree species. UWBA and focal tree size did not correlate with each other for either canopy tree species (P > 0.79 for both), so both variables were included in models. Grass biomass, however, declined significantly with UWBA for both tree species. As a result, runs where grass biomass was a contributor to the best model were rerun without it to see if basal understory became a significant variable. In all cases, it did not. Because loss-on-ignition and microbial biomass were not correlated, both were entered as explanatory variables. LitterN is a mass-based %N measurement, used here as an indicator of litter quality. Analyses were conducted in JMP version 15.1.
We assessed the effects of UWBA and focal tree size on grass cover and biomass, using univariate regression analyzing AK plots and RO plots together and separately. We also used univariate regression to evaluate relationships between resinN and other N metrics, and between loss-on-ignition and UWBA and focal tree area. In all cases we report the equation that resulted in the highest R2 value. To test whether T0 extractions from fresh collections in Hawai‘i were similar to T0 values when we began incubations at UCSB, we used simple linear regression.
In the outplanting density experiment, because multiple grass, litter, and light samples were taken per tree, we used ANOVAs with treatment (double density, single density, and control) as a fixed effect and sample location nested within treatment as a random effect. For assessing the effects of planting density on grass and woody litter biomass, we had two samples per AK tree whereas for light transmittance we had four samples per tree. For soil inorganic N, C:N, moisture, and organic matter (one sample per tree because soils were bulked per tree) we used one-way ANOVAs with treatment as a fixed effect. Grass biomass was ln transformed for normality. We used post-hoc Tukey tests to specify differences between treatments when ANOVAs had a P ≤ 0.05.
To compare OP soils with the AK and RO soils, we used one-way ANOVA followed by Tukey post hoc tests. Controls over N metrics within the OP category were assessed using linear regression with each of four possible predictor variables. However, several of the predictor variables were highly correlated with one another (for example, loss-on-ignition × SIR, SIR × LitterN). This plus low sample size limited use of multiple regression and interpretation of a dominant predictor.
For all analyses, we consider that P < 0.10 indicated something worth discussing. In our analyses very few p values fell between 0.05 (standard accepted significance) and 0.10. Nonetheless we discuss those that did as ‘trends’.
Results
Grass Response to Understory Woody Plants-Survey
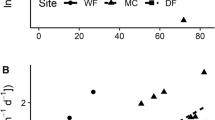
Despite our efforts to locate AK trees with high density of native woody understory, the basal area of understory under RO trees tended to be much higher than under AK trees (see differences in x-axis points between tree types, Figure 1). Indeed, the density of native woody stems under RO’s that had understory far surpassed the density of native woody understory plants under the AK trees planted with understory (RO mean = 1.74 stems m−2 [n = 18, excluding trees with zero UWBA], range 0.02 to 5.5; AK mean = 0.20 stems m−2 [n = 15, excluding zeros] range 0.01 to 0.37).
Relationship between understory woody basal area (UWBA) and two metrics of grass abundance. A Grass biomass m2 (log + 1) and B Grass % cover (Square root transformed). Each point for both metrics represents an average of four, surveyed or harvested plots per tree. N = 40 trees (20 per species). Blue = plots under planted Akacia koa (AK). Orange = plots under remnant ʻohiʻa (RO). Black dashed line is for both tree species combined.
Both invasive grass cover and biomass declined as basal area of understory increased for both tree species (Figure 1). For each tree type, increased UWBA correlated with decreased grass biomass (AK, F(1,19) = 9.58, P < 0.006; RO F(1,19) = 29.08, P < 0.0001) and cover (AK, F(1,19) = 17.20, P < 0.001; RO, F(1,19) = 14.28, P < 0.001). For AK, focal tree size did not correlate with grass biomass (P > 0.70) or cover (P > 0.45). By contrast, under RO trees, grass biomass increased with the size of the focal tree (R2 = 0.23, F(1,19) = 6.81, P < 0.018) while grass cover did not (P > 0.41).
Short-Term N Dynamics
Available nitrogen was higher in the AK soils compared to those from RO locations early after soil collection (T0) and in the five-day, resin incubation (Figure 2; T0, F(3,36) = 13.883, P < 0.0002; resinN, F(3,34) = 9.70, P < 0.0006). UWBA was not a significant covariate (P > 0.89) in the model. T0-available N was largely as nitrate, comprising 90 and 85% of available N for the AK and RO soils, respectively. Over the 26-day incubation period, netmin did not differ between AK and RO soils (Figure 2, F(3,36) = 0.175, P > 0.91), although net nitrification was higher in AK than RO soils (Figure 2, F(3,36) = 3.66, P < 0.04).
Nitrogen availability metrics under remnant ʻohiʻa (RO) and planted koa (AK) trees at Hakalau Forest NWR. Bars represent means plus/minus one s.e. with all points displayed. *indicates P < 0.05, ***indicates P < 0.001 for difference between AK and RO soils using ANCOVA. In no case was the co-variate (understory woody basal area) significant. Black dashed horizontal line on each plot indicates values for open soil (no canopy). Statistical comparisons of open to AK and RO are in text.
ResinN and T0N were correlated with one another for both tree species (AK, F(1,16) = 10.98, P < 0.004; RO F(1,18) = 25.39, P < 0.001) suggesting resins captured a labile pool that continued to be present in the immediate 2 weeks after soil collection (Figure 3A). There was also a positive correlation between resinN and short-term netmin for RO trees (Figure 3B; RO F = 21.27, P < 0.002). However, the relationship between resinN and short-term netmin was flat for AK trees (Figure 3B). Together these patterns (Figure 3) suggest different microbial/organic matter dynamics in the two canopy types.
A Relationship between nitrogen captured on resins (5 day resin-paste incubation) and T0 available (NH4 + NO3) nitrogen from field collected soil in Hawai‘i. Blue dots and numbers are AK trees. Orange are RO trees. Black line represents regression for all trees combined. B Relationship between nitrogen captured on resins (5-day incubation) and short-term netmin (26 days, in Hawai‘i). Colors are as before.
When soils from under AK trees were assessed for variables predicting short-term available N metrics using multiple regression, T0N and 26-day netmin were both best predicted by only loss-on-ignition (Table 1; T0 N F = 10.38, P < 0.0047, R2 = 0.33; 26-day netmin F = 7.35, P < 0.013, R2 = 0.26). Higher values were correlated with a higher T0 pool and higher short-term netmin. Neither focal tree size nor UWBA were significant predictors of N metrics under AK trees. Grass biomass was also not predictive of short-term metrics.
Under RO trees, the best model for T0N includes only litterN concentration (Table 1: F = 5.71, P < 0.027, R2 = 0.20) while resinN was best predicted by microbial biomass (F = 5.31, P < 0.033, R2 = 0.19). In the latter case higher microbial biomass was negatively related to resin N capture suggesting microbial immobilization is occurring. In RO soils, short-term netmin values were not well predicted by any combination of measured variables. As with AK soils, focal tree size, UWBA and grass biomass did not explain variation across RO samples.
Longer-Term N Dynamics
The Tinitial values for the long-term incubations correlated positively with T0 values on the fresh soils when extracted in Hawai‘i suggesting that despite sieving, shipping and 4 °C storage, the samples retained individual identities (Figure S2). The ANCOVA on total N mineralized showed that after 171 days, net values in RO soils exceeded AK soils (Figure 2, Effect of vegetation type, F = 6.67, P > 0.003) by ~ 35%. UWBA was not a significant predictor (P > 0.42) for either canopy type. With a mixed model including time as a repeated measure, and individual site as a random factor, canopy type was again significant (F1,37 = 5.67, P < 0.022) with RO mineralizing more than AK soils. Time was also significant with soils variably mineralizing versus immobilizing depending on the interval (Figure S4, time, F3,111 = 8.74, P < 0.001). Mineralization was highest in the latter two intervals, and lowest in the second interval (~ 1–2 months after start) when RO soils mostly immobilized N, and AK netmin values were still relatively low. The random effect of tree identity (site) was not significant.
Multiple regression models evaluating the best predictors of Tfinal [5 mo] minus Tinitial for AK soils, showed that only grass biomass is a significant predictor of long-term netmin (Table 1: F = 7.19, P < 0.0156, R2 = 0.25): greater grass biomass corresponded to higher rates. When the model was run without grass biomass, UWBA was still not significant. For RO soils, the best model included only loss-on-ignition as a weak predictor (F = 3.078, P = 0.096, R2 = 0.10), where LOI predicted higher rates.
Other functional Differences Between AK and RO Soils
Organic matter, estimated by loss-on-ignition, was significantly higher in RO than AK soils (Table 2, P < 0.05). In RO soils it increased as a function of understory woody basal area (Figure 4A, F(1,19) = 4.46, P < 0.011). This was not true in the AK soils (Figure 4A, P > 0.89). When all trees were combined the relationship was stronger (Figure 4A, F(1,38) = 10.45, P < 0.003). Multiple regression analysis suggested that UWBA and focal tree size together improved the prediction of organic matter (Figure 4B). This combined metric was predictive of loss-on-ignition for all trees together, RO trees alone and marginally for AK trees (Figure 4B; all trees F(1,38) = 30.39, P < 0.001; AK F(1,19) = 3.18, P < 0.09; RO F(1,19) = 4.66, P < 0.045). Hence as trees grow, they accumulate organic matter beneath them, and this is enhanced by accumulating understory. Also, loss-on-ignition values were not correlated with MB-SIR in either RO or AK soils (P > 0.78 and P > 0.96 for RO and AK, respectively), yet they were correlated with average CO2 respired in the long-term incubation for RO soils (data not shown, F = 9.24, P < 0.007, R2 = 0.34), a relationship that was weaker in AK soils (F = 3.26, P < 0.088, R2 = 0.15).
A Relationship between loss-on-ignition (%) and understory woody basal area (UWBA) under each species alone and combined. B Relationship between organic matter measured as loss-on-ignition and all woody basal area (focal tree + UWBA). Best fit equations use log x for both graphs. Colors as in previous figures.
Microbial biomass (MB) was generally higher in RO than in AK soils despite wide variation within canopy types (F = 3.06, P = 0.06) (Table 2). UWBA was not correlated with MB across the two types together (P > 0.77). Within the AK soils, grass biomass and litterN were the best predictors of MB, both having a positive relationship (P = 0.02). No models were significant in predicting MB for RO sites.
Nitrification potential (NitPot) of soils was substantially higher in AK than RO soils (Table 2, F = 6.47, P < 0.0039, R2 = 0.22; Figure 5) and basal understory again was not a significant covariate (P > 0.90). Grass biomass was the only predictor of NitPot under AK trees (F = 3.82, P = 0.067, R2 = 0.13) where more grass biomass correlated with lower nitrification potential. Under the RO trees, litterN was the most important predictor of NitPot with increasing litterN corresponding with higher NitPot (Figure 5; F(1,19) = 11.30, P = 0.004, Figure 5). Yet litterN had an asymptotic relationship with NitPot across both tree species (Figure 5, all trees F(1,38) = 13.75, P < 0.001) suggesting that high mineral N under AK was saturating nitrifying microbes but something else (C?) was limiting their growth.
Relationship between nitrification potential and %N of plant litter. Blue dots are AK trees. Orange dots are RO trees. Best fit for each tree type alone = linear. Black line represents polynomial/quadratic fit for all 40 points (both tree types combined) showing a leveling of nitrification potential near 1.4% litter N.
Experimental Density Outplanting
Four years after planting native woody understory at two densities under AK we saw substantial differences in grass biomass compared to controls (no planting). Invasive grass biomass in plots where understory was planted at double density (213 ± 52 gm m−2) was half that of plots planted at single density (407 ± 40 g m−2), and a quarter of the grass biomass found in control plots (822 ± 52 gm m−2) (F = 199.22, P = 0.0006). Leaf litter of woody species did not differ between plots (Table 3).
Despite strong effects of experimental plantings on grass biomass, we found no differences in soil inorganic nitrogen (similar to T0N methodologically), bulk soil C:N, or loss-on-ignition between treatments (Table 3). Light transmittance, however, showed strong differences among treatments with double-density plots having a third of the light, and single-density plots having half of the light as the unplanted control plots (Table 3). In addition, soil moisture showed a trend toward differences among treatments: lowest values were in high-density plots, then low-density, then control plots (Table 3).
Characterization of Open Sites
The six open sites scattered between the corridors of AK had significantly lower LOI than either tree type. Microbial biomass was intermediate between AK and RO soils (Table 2). Nitrification potential in the open resembled AK soils (Table 2).
Open soils had low T0N, resinN and short-term netmin (Figure 2), the former two measurements being more like those in RO rather than AK soils. The low sample size and high correlation among predictor variables (MB and loss-on-ignition were strongly correlated as were litterN and MB) precluded use of multiple regression. Of the four single variables that could correlate with the N metrics, grass biomass was the only significant predictor for T0 and resinN (Table 4) with both correlations being negative. By contrast, for long-term netmin, MB and litterN were strong positive predictors (Table 4).
Discussion
Our data support that invasive grass abundance in reforestation settings can be reduced through planting native woody understory (for example, Holl 1999; McDaniel and Ostertag 2010). Our data further support that the dramatic decline in grass biomass in high-density planting results from light limitation rather than changes in soil N. This is consistent with studies demonstrating a positive correlation of invasive grass biomass with understory light (Holl 1999; Aide and others 2000; Cole and Weltzin 2004; Huebner 2010; McDaniel and Ostertag 2010) or showing that invasive understory grasses can be reduced through experimental shading (for example, Funk and McDaniel 2010). Our data also provide evidence that planting understory at much higher densities would more consistently and rapidly decrease grass biomass. Because recruitment of native woody plants occurs with grass reductions of approximately 50% (Rehm and others 2023), we might expect natural regeneration to take place in areas planted at higher densities as woody individuals become reproductive.
Our study also supports existing literature, including two studies from nearby sites (Scowcroft and others 2004; Yelenik 2017), documenting strong tree canopy species effects on soil nutrient dynamics consistent with expectations of N-fixing versus non-fixing canopies (Finzi and others 1998; Hobbie and others 2006). Yet, our data do not support a role of understory restoration in influencing soil conditions despite the reduction in invasive grasses: we found no relationship between soil N metrics and understory basal area. The best predictors of N metrics vary by canopy tree type and suggest that the net N dynamics are primarily limited by carbon under AK trees but by nitrogen under the RO trees.
Why no Effect of Planted Understory on N Cycling?
Studies in forest ecosystems have documented the role of individual understory species or leaf traits in influencing N availability and cycling (Chapin 1980; Nilsson and Wardle 2005; Wardle and Zackrisson 2005; Laughlin and others 2011; Yelenik and others 2013). Additionally, studies of invasive understory shrubs and grasses support that understory plant species can dramatically change N cycling (for example, Kourtev and others 1999; Mack and D’Antonio 2003; Hawkes and others 2005; Lee and others 2012; Stark and Norton 2015). These studies focus on naturally recruiting species (native or invasive), that in all cases increase rates of N cycling. We could not find studies evaluating how actively restored understory species influence N cycling or that showed understory species slowing N cycling. One exception is Pan and others (2018), where aerial seeding created two different understory compositions, which in turn created two different nutrient and microbial biomass conditions: a grass-dominated understory showed greater microbial biomass C and N and greater SOM than a non-grass understory. The general dearth of studies is evidence that the role of understory restoration in affecting forest soils is understudied.
In our study, grass biomass was not an important influence on short-term N cycling, yet it showed a negative correlation with nitrification potential, suggesting a longer-term influence. This latter finding is contradictory to studies showing positive effects of invasive grasses on nitrification (Hawkes and others 2005; Lee and others 2012), and could reflect the perennial nature of grass N uptake in our system as opposed to the annual grasses in Hawkes and others (2005) and Lee and others (2012). Consistent year-round grass N uptake, along with labile carbon from the grasses feeding ammonifying microbes, could reduce N for nitrifying microbes thus limiting nitrification potential. Our results are consistent with studies suggesting warm-season grasses can inhibit nitrification (Subbarao and others 2015). Still, grass biomass was a significant, although weak, positive predictor of long-term netmin under AK trees. This could reflect that grass biomass responds to higher soil fertility as indicated by higher long-term netmin, or that grasses enhance long-term netmin by alleviating carbon limitation for ammonifiers.
An important goal of our study was to evaluate if woody understory restoration could reverse the high N availability typically found under A. koa. Although studies from other tropical ecosystems have shown that using N-fixing trees in restoration can lead to successful outcomes for canopy development and forest biomass (for example, Parrotta 1992; Chaer and others 2011; Hoogmoed and others 2014), our previous work demonstrates that the planting of A. koa monocultures in Hawai‘i can stall secondary succession (Yelenik 2017; Yelenik and others 2022a, 2022b; Rehm and others 2023). We had previously hypothesized that this stalled succession was due in part to increased soil N (Yelenik 2017). A large body of literature documents efforts to slow down N cycling in enriched ecosystems by adding forms of carbon such as leaf litter, sawdust, sugar, or rice hulls that would enhance microbial competition for nitrogen and thereby reduce available N to plants (for example, Blumenthal and others 2003; Perry and others 2010; Steers and others 2011; Cole and others 2021; Yelenik and others 2022b) and then presumably change plant competitive relationships. Our selected AK sites contained woody understory that was at least 8 years old. The outplanted woody species produce litter that has a higher CN than A. koa and grasses (Banko and others 2022), so it could potentially reduce available soil N, yet we did not observe this.
There are many possible reasons why an influence of understory was not observed. First we did not control for understory composition, and the species planted into these sites vary in the timing of their growth, N uptake, and litterfall as well as leaf C:N (Table S1). Grogan and Jonasson [2003] document strong differences between understory species in the amount and timing of N uptake. Wardle and Zackrisson [2005] also document strong differences among understory shrub species in influencing soil carbon and N dynamics. Had we focused only on sampling beneath those planted species producing the highest C:N litter, such as planted ʻōhiʻa (M. polymorpha) or L. tameiameia (Table S1), we might have detected understory effects. Secondly, the influences of the woody understory individuals might be very localized, and given the generally low density of understory plants in AK, the signal would be diluted by pooling samples across quadrats under a tree. If carbon in microbial biomass is limited under AK by inaccessibility of C, then litter of other species with initially higher C:N, might facilitate a temporary localized increase in MB. A sustained and larger increase in MB and soil organic matter under AK, which is what we were looking for in denser UWBA plots, would require adding enough C relative to N to overcome microbial C limitation consistently across a reasonable area. Third, even if the planted understory was contributing a higher C:N litter compared to A. koa and grass, it may take decades for that input to be detected in a large SOM pool. In previous work, 2.5 years of manual addition of large amounts of high C:N litter under AK trees did not change soil mineral N but did slightly raise soil C:N (Yelenik and others 2022b). Thus, more time might be needed to detect a consistent effect given the low density of plantings in our study. Lastly, the canopy of A. koa was relatively consistent in its coverage. It provides a higher total dissolved nitrogen (TDN) to dissolved organic carbon (DOC) ratio in throughfall and green and brown litter leachate than the RO canopy (K. Dowdy, UCSB, unpublished) which would favor C limitation to microbes.
Insights into Canopy Influences on Soil
Our data support that organic matter entering the soil under these two canopy types contributes to differences in loss-on-ignition, microbial biomass and N cycling, a result consistent with literature on canopy tree effects (for example, Parrotta 1992; Binkley and Giardina 1998; Finzi and others 1998; Reich and others 2005; Hobbie and others 2006). The high mineral N in the short-term pools (T0 and resin-N) under AK is consistent with its N-fixing status, leaf C:N ratios that are ~ half of those in M. polymorpha, and fast N loss from phyllodes during decomposition compared to M. polymorpha (Scowcroft and others 2004; Yelenik and others 2022b). Our data support an N cycling model whereby high N in A. koa litter along with low DOC:TDN in throughfall (K. Dowdy, UCSB, unpublished) favors nitrifying microbes over ammonifiers. Regarding throughfall, other studies have demonstrated that nitrogen in throughfall can affect N cycling (De Schrijver and others 2007; Carnol and Bazgir 2013). The high nitrification potential in AK soils is consistent with a high influx of mineral N, providing a competitive advantage to nitrifying microbes in the relative absence of labile C to fuel biomass of ammonifying microbes. Our results are also consistent with a recent decomposition study that showed that A. koa leaf litter loses N more rapidly than M. polymorpha litter. It has much more N to release per unit biomass in early stages of decomposition, yet, has lower overall decomposition of C compared to M. polymorpha: rapid loss of soluble C and N, is followed by the very slow loss of lignin-rich C (Yelenik and others 2022b). The significant explanatory power of loss-on-ignition for short-term N availability under AK supports that mineralizing microbes are C-limited and is consistent with the lower microbial biomass under AK trees (Table 2). Additionally, Acacia species with phyllodes like A. koa have been demonstrated to contain alkaloids, phenolics and other secondary compounds (Constantinides and Fownes 1994; Das and Joy 2009) that can limit microbial activity, soil enzyme activity, decomposition, and nutrient cycling (Kraus and others 2003; Talbot and Treseder 2012). Also, A. koa phyllodes tend to be lignin-rich in comparison to ʻōhiʻa leaves (August-Schmidt 2018). High N conditions have been linked to slow decomposition of high-lignin litter (Carreiro and others 2000; Perakis and others 2012).
A Temporal Perspective
Deforestation can rapidly reduce surface soil organic matter with slow but consistent recovery after reforestation (for example, Veldkamp and others 2020). In the 1800s, a large portion of what is now Hakalau Forest was logged, so presumably, the AK plantings and open sites began at a similar point of soil organic matter that had been depleted through deforestation. The absence of woody inputs for over 100 years combined with intensive ungulate grazing likely perpetuated soil carbon loss (Scowcroft and others 2004; Hart and others 2020). While loss-on-ignition in AK soils was significantly lower than in RO soils, it is higher than in open sites and increases with woody basal area (Figure 4B), suggesting that organic matter is building with reforestation as one might expect given steady decomposition of AK leaves (Scowcroft 1997; Yelenik and others 2022b). Then, as trees grow and UWBA approaches that seen under RO trees, organic matter should accumulate (Figure 4B). This would be consistent with the findings of Li and Matthews (2010), who saw significant organic matter build up after establishment of secondary forest (dominated by non-native trees, including Terminalia myriocarpa, Psidium cattleianum, and Spathodea campanulata) on former sugar cane lands in Hawai‘i after 22 years.
The strong collinearity among explanatory variables for N metrics in open sites, suggests less complex controls over carbon and nitrogen dynamics there. This is likely because the input of carbon in the open is largely from grasses rather than grass plus woody inputs as in RO and AK sites. The tendency toward higher MB in the open compared to the AK sites, despite lower organic matter, suggests something about the AK canopy is suppressing microbial growth.
The soil organic matter beneath RO trees represents a more complex history because there were likely organic matter inputs during the period of livestock grazing due to RO leaf and root inputs as well as retention of tree-associated hydrological features that could influence soil development. Pasture trees are well known to locally influence soil microbial activity and soil C dynamics (for example, Rhoades and others 1998; Galicia and García-Oliva 2008). Hence, the higher organic matter under RO today is likely partially historic. Yet the formation of halos of woody understory around these trees is recent (post-ungulate exclusion/past 30–40 years; Yelenik 2017) and the positive correlation of UWBA with loss-on-ignition under the RO trees suggests organic matter builds up as understory develops. Because understory stem densities are much higher under RO, it is possible that if planting is done at higher density, organic matter will increase under AK trees.
Implications for Management
While our results do not show a reduction in inorganic N availability with development of native woody species under A. koa corridors, they do demonstrate a negative effect of understory woody plants on invasive grasses. Our high-density experimental planting was particularly effective in reducing grass biomass and creating dense patches of understory vegetation (Figure S3, Table 3). Other work demonstrates that grasses strongly suppress native plant recruitment and reduce establishment sites for incoming woody seeds (Rehm and others 2019, 2023; Yelenik and others 2022a). Thus, management actions that reduce grasses could promote recovery of native species. Both of our experimental planting densities reduced grass biomass, and both were higher than is typically planted at this site. Thus, planting at higher densities is beneficial to the rapid development of a native-dominated understory.
Our results further suggest that soil organic matter and nutrient dynamics will vary between tree species for some time regardless of understory likely because of the high N in A.koa litter and carbon limitation to microbes under A. koa. These differences have implications for the short-term accumulation of soil carbon, which was, on average, 10% lower under AK than RO trees. The high mineral N perpetuated by the AK canopy promotes kikuyu grass (Yelenik and others 2020) and other non-native species including Ehrharta stipoides and Rubus argutus, yet is a reasonable place for native woody plants if outplanted seedlings are tall enough to be above the grass canopy (Yelenik and others 2022b). Thus, a reduction in mineral N and increasing shade by outplanting understory could reduce the growth of opportunistic invasive species. Our results also provide evidence that changes in soil N will be less effective than lowering light to decrease invasive grasses and enhance natural recovery of woody plants.
Data Availability
The data and metadata will be stored in the USDA Forest Service Data Research Archive. Yelenik, Stephanie; D'Antonio, Carla; Rehm, Evan; Elgersma, Cheryl. 2024. Tree, understory, soil, and light data for Hakalau Forest National Wildlife Refuge, Hawai‘i Island, 2016–2020. Fort Collins, CO: Forest Service Research Data Archive. https://doi.org/10.2737/RDS-2024-0036
References
Adler PB, D’Antonio CM, Tunison JT. 1998. Understory succession following a dieback of Myrica faya in Hawai‘i Volcanoes National Park. Pac Sci 52:69.
Aide TM, Zimmerman JK, Pascarella JB, Rivera L, Marcano-Vega H. 2000. Forest regeneration in a chronosequence of tropical abandoned pastures: implications for restoration ecology. Restor Ecol 8:328–338.
Allison SD, Nielsen C, Hughes RF. 2006. Elevated enzyme activities in soils under the invasive nitrogen-fixing tree Falcataria moluccana. Soil Biol Biochem 38:1537–1544.
Arnold J, Corre MD, Veldkamp E. 2008. Cold storage and laboratory incubation of intact soil cores do not reflect in-situ nitrogen cycling rates of tropical forest soils. Soil Biol Biochem 40:2480–2483.
August-Schmidt E. 2018. Mechanisms of community assembly beneath N-fixing trees in a Hawaiian dry woodland. University of California, Santa Barbara ProQuest Dissertation Publishing, 13423031.
Baker PJ. 2009. Koa (Acacia koa) ecology and silviculture. United States Department of agriculture forest service. Placerville: Pacific Southwest Research Station.
Banko PC, Peck RW, Yelenik SG, Paxton EH, Bonaccorso F, Montoya-Aiona K, Hughes RF, Perakis S. 2022. Hypotheses and lessons from a native moth outbreak in a low-diversity, tropical rainforest. Ecosphere 13:e3926.
Benbi DK, Richter J. 2002. A critical review of some approaches to modelling nitrogen mineralization. Biol Fertil Soils 35:168–183.
Binkley D, Giardina C. 1998. Why do tree species affect soils? The warp and woof of tree-soil interactions. In: Van Breemen N, Ed. Plant-induced soil changes: processes and feedbacks, . Dordrecht: Springer Netherlands. pp 89–106. https://doi.org/10.1007/978-94-017-2691-7_5.
Blumenthal DM, Jordan NR, Russelle MP. 2003. Soil carbon addition controls weeds and facilitates prairie restoration. Ecol Appl 13:605–615.
Burton JI, Perakis SS, McKenzie SC, Lawrence CE, Puettmann KJ. 2017. Intraspecific variability and reaction norms of forest understorey plant species traits. Tjoelker M, editor. Funct Ecol 31:1881–1893.
Carnol M, Bazgir M. 2013. Nutrient return to the forest floor through litter and throughfall under 7 forest species after conversion from Norway spruce. For Ecol Manag 309:66–75.
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF. 2000. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365.
Castro-Díez P, Fierro-Brunnenmeister N, González-Muñoz N, Gallardo A. 2012. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 350:179–191.
Chaer GM, Resende AS, Campello EFC, de Faria SM, Boddey RM. 2011. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol 31(2):139–149.
Chapin FS. 1980. The mineral-nutrition of wild plants. Ann Rev Ecol Syst 11:233–260.
Cole PG, Weltzin JF. 2004. Environmental correlates of the distribution and abundance of Microstegium vimineum, in East Tennessee. Southeast Nat 3:545–562.
Cole EJ, Barker AV, Zandvakili OR, Sadeghpour A, Xing B, Hashemi M, Allan-Perkins E, Jung G. 2021. Soil nutrient and nematode community changes in response to hardwood charcoal application. Commun Soil Sci Plant Anal 52:917–925.
Collier SM, Ruark MD. 2021. Pre-incubation soil handling can influence comparability of potentially mineralizable nitrogen results. Commun Soil Sci Plant Anal 52:1121–1131.
Constantinides M, Fownes JH. 1994. Nitrogen mineralization from leaves and litter of tropical plants: relationship to nitrogen, lignin and soluble polyphenol concentrations. Soil Biol Biochem 26:49–55.
Das S, Joy VC. 2009. Chemical quality impacts of tropical forest tree leaf litters on the growth and fecundity of soil Collembola. Eur J Soil Biol 45:448–454.
De Schrijver A, Geudens G, Augusto L, Staelens J, Mertens J, Wuyts K, Gielis L, Verheyen K. 2007. The effect of forest type on throughfall deposition and seepage flux: a review. Oecologia 153:663–674.
Fierer N, Schimel JP, Holden PA. 2003. Influence of drying-rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71.
Finzi AC, Van Breemen N, Canham CD. 1998. Canopy tree-soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl 8:440–446.
Fortini LB, Leopold C, Perkins K, Chadwick O, Yelenik S, Jacobi J, Bishaw KEI, Gregg M, Rosa S. 2020. Local to landscape-level controls of water fluxes through Hawaiian forests: Effects of invasive animals and plants on soil infiltration capacity across substrate and moisture gradients. Hawai‘i Cooperative Studies Unit Technical Report HCSU-095.
Funk JL, McDaniel S. 2010. Altering light availability to restore invaded forest: the predictive role of plant traits. Restor Ecol 18:865–872.
Galicia L, García-Oliva F. 2008. Remnant tree effects on soil microbial carbon and nitrogen in tropical seasonal pasture in western Mexico. Eur J Soil Biol 44:290–297.
Giambelluca TW, Chen Q, Frazier AG, Price JP, Chen Y-L, Chu P-S, Eischeid JK, Delparte DM. 2013. Online rainfall atlas of Hawai‘i. Bull Am Meteorol Soc 94:313–316.
González-Muñoz N, Costa-Tenorio M, Espigares T. 2012. Invasion of alien Acacia dealbata on Spanish Quercus robur forests: impact on soils and vegetation. For Ecol Manag 269:214–221.
Gonzalez-Quiñones V, Banning NC, Ballesta RJ, Murphy DV. 2009. Influence of cold storage on soil microbial community level physiological profiles and implications for soil quality monitoring. Soil Biol Biochem 41:1574–1576.
Grogan P, Jonasson S. 2003. Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology 84:202–218.
Hart SC, Binkley D, Perry DA. 1997. Influence of red alder on soil nitrogen transformations in two conifer forests of contrasting productivity. Soil Biol Biochem 29:1111–1123.
Hart PJ, Ibanez T, Uehana S, Pang-Ching J. 2020. Forest regeneration following ungulate removal in a montane Hawaiian wet forest. Restor Ecol 28:757–765.
Hawkes CV, Wren IF, Herman DJ, Firestone MK. 2005. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8:976–985.
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P. 2006. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297.
Holl KD. 1999. Factors limiting tropical rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31:229–242.
Hoogmoed M, Cunningham SC, Baker P, Beringer J, Cavagnaro TR. 2014. N-fixing trees in restoration plantings: effects on nitrogen supply and soil microbial communities. Soil Biol Biochem 77:203–212.
Huebner CD. 2010. Establishment of an invasive grass in closed-canopy deciduous forests across local and regional environmental gradients. Biol Invasions 12:2069–2080.
Hughes RF, Denslow JS. 2005. Invasion by a N-2-fixing tree alters function and structure in wet lowland forests of Hawaii. Ecol Appl 15:1615–1628.
Kourtev PS, Huang WZ, Ehrenfeld JG. 1999. Differences in earthworm densities and nitrogen dynamics in soils under exotic and native plant species. Biol Invasions 1:237–245.
Kraus TEC, Dahlgren RA, Zasoski RJ. 2003. Tannins in nutrient dynamics of forest ecosystems—a review. Plant Soil 256:41–66.
Laughlin DC, Moore MM, Fulé PZ. 2011. A century of increasing pine density and associated shifts in understory plant strategies. Ecology 92:556–561.
Lee MR, Flory SL, Phillips RP. 2012. Positive feedbacks to growth of an invasive grass through alteration of nitrogen cycling. Oecologia 170:457–465.
Li Y, Mathews BW. 2010. Effect of conversion of sugarcane plantation to forest and pasture on soil carbon in Hawaii. Plant Soil 335:245–253.
Mack MC, D’Antonio CM. 2003. Exotic grasses alter controls over soil nitrogen dynamics in a hawaiian woodland. Ecol Appl 13:154–166.
Marchante E, Kjøller A, Struwe S, Freitas H. 2008. Short-and long-term impacts of Acacia longifolia invasion on the belowground processes of a Mediterranean coastal dune ecosystem. Appl Soil Ecol 40:210–217.
Maron JL, Connors PG. 1996. A native nitrogen-fixing shrub facilitates weed invasion. Oecologia 105:302–312.
Matsushima M, Chang SX. 2007. Effects of understory removal, N fertilization, and litter layer removal on soil N cycling in a 13-year-old white spruce plantation infested with Canada bluejoint grass. Plant Soil 292:243–258.
McClain CD, Holl KD, Wood DM. 2011. Successional models as guides for restoration of Riparian forest understory. Restor Ecol 19:280–289.
McDaniel S, Ostertag R. 2010. Strategic light manipulation as a restoration strategy to reduce alien grasses and encourage native regeneration in Hawaiian mesic forests. Appl Veg Sci 13:280–290.
Mueller-Dombois D, Jacobi JD, Boehmer HJ, Price JP. 2013. ʻŌhiʻa Lehua rainforest: born among Hawaiian volcanoes, evolved in isolation: the story of a dynamic ecosystem with relevance to forests worldwide. Honolulu, HI: Friends of the Joseph Rock Herbarium http://pubs.er.usgs.gov/publication/70048519.
Nilsson M-C, Wardle DA. 2005. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front Ecol Environ 3:421–428.
Omeja PA, Chapman CA, Obua J, Lwanga JS, Jacob AL, Wanyama F, Mugenyi R. 2011. Intensive tree planting facilitates tropical forest biodiversity and biomass accumulation in Kibale National Park, Uganda. For Ecol Manag 261:703–709.
Ostertag R, Giardina CP, Cordell S. 2008. Understory colonization of Eucalyptus plantations in Hawaii in relation to light and nutrient levels. Restor Ecol 16:475–485.
Pan P, Zhao F, Ning J, Zhang L, Ouyang X, Zang H. 2018. Impact of understory vegetation on soil carbon and nitrogen dynamic in aerially seeded Pinus massoniana plantations. Paz-Ferreiro J, editor. PLoS ONE 13:e0191952.
Parrotta JA. 1992. The role of plantation forests in rehabilitating degraded tropical ecosystems. Agric Ecosyst Environ 41:115–133.
Paxton EH, Yelenik SG, Borneman TE, Rose ET, Camp RJ, Kendall SJ. 2018. Rapid colonization of a Hawaiian restoration forest by a diverse avian community. Restor Ecol 26:165–173.
Perakis SS, Matkins JJ, Hibbs DE. 2012. N 2-fixing red alder indirectly accelerates ecosystem nitrogen cycling. Ecosystems 15:1182–1193.
Perry LG, Blumenthal DM, Monaco TA, Paschke MW, Redente EF. 2010. Immobilizing nitrogen to control plant invasion. Oecologia 163:13–24.
Peterson DW, Moore RB. 1987. Geologic history and evolution of geologic concepts. In: Island of Hawaii. Vol. 1350. pp 149–89.
Powers JS, Haggar JP, Fisher RF. 1997. The effect of overstory composition on understory woody regeneration and species richness in 7-year-old plantations in Costa Rica. For Ecol Manag 99:43–54.
Reed SC, Cleveland CC, Townsend AR. 2008. Tree species control rates of free-living nitrogen fixation in a tropical rain forest. Ecology 89:2924–2934.
Rehm EM, Thomas MK, Yelenik SG, Bouck DL, D’Antonio CM. 2019. Bryophyte abundance, composition and importance to woody plant recruitment in natural and restoration forests. For Ecol Manag 444:405–413.
Rehm EM, Yelenik SG, D’Antonio C. 2021. Freezing temperatures restrict woody plant recruitment and restoration efforts in abandoned montane pastures. Glob Ecol Conserv 26:e01462.
Rehm EM, D’Antonio CM, Yelenik SG. 2023. Crossing the threshold: invasive grasses inhibit forest restoration on Hawaiian Islands. Ecol Appl 33(4):e2841.
Reich PB, Oleksyn J, Modrzynski J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG. 2005. Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–818.
Rhoades CC, Eckert GE, Coleman DC. 1998. Effect of pasture trees on soil nitrogen and organic matter: implications for tropical montane forest restoration. Restor Ecol 6:262–270.
Rippel TM, Iosue CL, Succi PJ, Wykoff DD, Chapman SK. 2020. Comparing the impacts of an invasive grass on nitrogen cycling and ammonia-oxidizing prokaryotes in high-nitrogen forests, open fields, and wetlands. Plant Soil 449:65–77.
Royo AA, Carson WP. 2006. On the formation of dense understory layers in forests worldwide: consequences and implications for forest dynamics, biodiversity, and succession. Can J for Res 36:1345–1362.
Scowcroft PG. 1997. Mass and nutrient dynamics of decaying litter from Passiflora mollissima and selected native species in a Hawaiian montane rain forest. J Trop Ecol 13:407–426.
Scowcroft PG, Haraguchi JE, Hue NV. 2004. Reforestation and topography affect montane soil properties, nitrogen pools, and nitrogen transformations in Hawaii. Soil Sci Soc Am J 68:959–968.
Scowcroft PG, Friday JB, Idol T, Dudley N, Haraguchi J, Meason D. 2007. Growth response of Acacia koa trees to thinning, grass control, and phosphorus fertilization in a secondary forest in Hawai‘i. For Ecol and Manag 239:69–80.
Scowcroft PG.1992. Role of decaying logs and other organic seedbeds in natural regeneration of Hawaiian forest species on abandoned montane pasture. In: Conrad, Engene C.; Newell, Leonard A., tech. cords. Proceedings on the session on tropical forestry for people of the Pacific, XVII Pacific Science Congress Gen. Tech. Rep. PSW-GTR-129. US Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station 129: 67–73.
Stark JM, Norton JM. 2015. The invasive annual cheatgrass increases nitrogen availability in 24-year-old replicated field plots. Oecologia 177:799–809.
Steers RJ, Funk JL, Allen EB. 2011. Can resource-use traits predict native vs. exotic plant success in carbon amended soils? Ecol Appl 21:1211–1224.
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata J-C, Kishii M, Braun H-J. 2015. Suppression of soil nitrification by plants. Plant Sci 233:155–164.
Takahashi M, Giambelluca TW, Mudd RG, DeLay JK, Nullet MA, Asner GP. 2011. Rainfall partitioning and cloud water interception in native forest and invaded forest in Hawai‘i Volcanoes National Park. Hydrol Process 25:448–464.
Talbot JM, Treseder KK. 2012. Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354.
Veldkamp E, Schmidt M, Powers JS, Corre MD. 2020. Deforestation and reforestation impacts on soils in the tropics. Nature Rev Earth Environ 1:590–605.
Vitousek PM, Walker LR. 1989. Biological invasion by Myrica faya in Hawai‘i: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–265.
Wang F, Zou B, Li H, Li Z. 2014. The effect of understory removal on microclimate and soil properties in two subtropical lumber plantations. J for Res 19:238–243.
Wardle DA, Zackrisson O. 2005. Effects of species and functional group loss on island ecosystem properties. Nature 435:806–810.
Wedderburn ME, Carter J. 1999. Litter decomposition by four functional tree types for use in silvopastoral systems. Soil Biol Biochem 31:455–461.
West AW, Sparling GP. 1986. Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. J Microbiol Methods 5:177–189.
Wilson SJ, Rhemtulla JM. 2016. Acceleration and novelty: community restoration speeds recovery and transforms species composition in Andean cloud forest. Ecol Appl 26:203–218.
Xiong Y, Xia H, Li Z, Cai X, Fu S. 2008. Impacts of litter and understory removal on soil properties in a subtropical Acacia mangium plantation in China. Plant Soil 304:179–188.
Yelenik SG. 2017. Linking dominant Hawaiian tree species to understory development in recovering pastures via impacts on soils and litter. Restor Ecol 25:42–52.
Yelenik S, Perakis S, Hibbs D. 2013. Regional constraints to biological nitrogen fixation in post-fire forest communities. Ecology 94:739–750.
Yelenik SG, D’Antonio CM, Rehm EM, Caldwell IR. 2020. Multiple feedbacks due to biotic interactions across trophic levels can lead to persistent novel conditions that hinder restoration. In: Traveset A, Richardson DM, Eds. Plant invasions: the role of biotic interactions, . Wallingford: CABI. pp 402–420. https://doi.org/10.1079/9781789242171.0402.
Yelenik S, Rose E, Paxton EH. 2022a. Trophic interactions and feedbacks maintain intact and degraded states of Hawaiian tropical forests. Ecosphere. https://doi.org/10.1002/ecs2.3884.
Yelenik SG, Rehm EM, D’Antonio CM. 2022b. Can the impact of canopy trees on soil and understory be altered using litter additions? Ecol Appl. https://doi.org/10.1002/eap.2477.
Acknowledgements
This work was supported by NSF grant DEB 1557177 and associated REU supplements, and the USDA Forest Service Rocky Mountain Research Station. The authors would like to thank the USFWS for site access, the Univ. of Hawaii-Hilo, and the Institute of Pacific Islands Forestry (USFS) and the USGS Pacific Islands Research Center for logistical support. Assistance with field and lab work was provided by D. Bouck, E. Rose, K. Davidson, O. Bonilla, M. Brown, A. Egbert, D. Schuyler, C. Yanger, J. Kuwahara-Hu, S. Schoepflin, M. Puanani Smith, and L. August-Schmidt. M. Nolan provided some statistical advice. CD’A is grateful to G. Runte for help with figures in R. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this publication should not be construed to represent any official USDA determination or policy. Our fieldwork took place in the ahupuaʻa of Honohina, the moku of Hilo, on the mokupuni of Hawai‘i, which are the ancestral, traditional, and contemporary lands of the Hawaiian people and we are grateful to have had access to these sites and to have learned from the forest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
CDʻA, SGY, and ER designed the study. All authors participated in field sampling. CD’A and CE conducted the lab assays, incubations, and analyses. CD’A and SGY conducted the data analysis. CD’A wrote the manuscript. ER and SGY edited.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Antonio, C.M., Rehm, E., Elgersma, C. et al. Influence of Native Woody Understory on Invasive Grasses and Soil Nitrogen Dynamics Under Plantation and Remnant Montane Tropical Trees. Ecosystems 27, 797–814 (2024). https://doi.org/10.1007/s10021-024-00922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-024-00922-z