Abstract
There is a clinical need to better understand and improve post-operative pain for patients undergoing laparoscopic repair of incisional hernia. The aim of this single-centre, double-blind, randomised controlled trial was to compare post-operative pain between absorbable and non-absorbable tack fixation in patients undergoing IPOM + repair. Patients with primary incisional hernia (size 3–10 cm), were randomised to either Reliatack™ (n = 27), an articulating-arm device deploying absorbable polymer tacks, or Protack™ (n = 36), a straight-arm device deploying permanent titanium tacks. The primary outcome was reported pain on activity using a visual analogue scale at post-operative day 30. Clinical and patient-reported outcome measures (PROMs) were assessed pre-operatively (day 0), and at 1-, 6-, 30- and 365-days post-surgery. No significant differences in reported pain ‘on activity’ were found at any timepoint. Less reported pain ‘at rest’ was found on post-operative day-1 with absorbable tacks (p = 0.020). Significantly longer mesh-fixation time (p < 0.001) and the use of more knots for fascial closure (p = 0.006) and tacks for mesh-fixation (p = 0.001) were found for the absorbable tack group. There were no differences in other clinical and PROMs between groups. For the whole trial cohort (n = 63) several domains in the Short-Form-36 showed a reduction from baseline scores at day 30 that improved at day 365. At post-operative day 30, 75.0% of patients reported ‘a lot of pain’ since discharge. This study found no difference in reported pain when choosing absorbable or non-absorbable tack fixation. The utility of “early” post-operative pain assessment as a comparator following incisional hernia repair needs clarification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incisional hernia incidence increases from 12.6% at 1 year to 22.4% at 3 years post-surgery [9] and is associated with significant psychosocial morbidity [28] and healthcare costs [10]. Laparoscopic intraperitonal onlay mesh (IPOM) repair remains a recommended option for treatment of incisional hernia with a defect size of 2–6 cm [14]. Abdominal pain is the most common reason for representation to services following laparoscopic incisional hernia repair [14]. The use of pain as a comparator following incisional hernia repair techniques has not yet been standardised.
European registry data from Denmark showed a greater use of laparoscopic compared to open incisional hernia repair from 2007 to 2018 (57.5% vs. 42.5%) [14]. Data from Germany has shown a more recent trend away from the use of laparoscopic intraperitoneal onlay mesh (IPOM) repairs (33.8% in 2013 to 21% in 2019) [17]. Reporting of rare but serious complications, including bowel compromise and mesh migration, has played a role in this trend [18]. A hernia research group has identified long-term follow-up on patients that underwent IPOM repair as a research priority [24].
Decision making in recommending treatment options for incisional hernia is complex and must take into account patient and hernia factors, e.g. BMI and size of defect, alongside complication profiles. For the available options, different complication profiles have been shown; for example in laparoscopic vs. open repairs the rates of readmission and reoperation are lower [14]. Failure in the use of a standardised outcome set and under-reporting of patient-reported outcome measures (PROMs) has been highlighted in the existing evidence base when comparing incisional hernia repair techniques [12]. Balancing risks and patient wishes are important in the consent for incisional hernia repair and a greater understanding of pain following incisional hernia repair will facilitate these discussions.
The IPOM + technique includes sutured closure of the fascial defect prior to intraperitoneal mesh placement [26]. Sutured fascial closure within the laparoscopic repair has shown benefits in reducing chronic pain, seroma formation and poor cosmesis [15].
Minimum datasets for reporting on incisional hernia trials have been published. They recommend ‘pain at rest’, ‘pain on activity’ and ‘pain felt during the last week’ be assessed [21]. They do not give an indication on the best and most appropriate timing in which these assessments take place [1, 16, 19]. Serial pain assessment, key clinical outcomes and PROMs were collected pre-operatively and at four post-operative timepoints up to one year [25]. The primary outcome, reported pain at day 30 following surgery, was chosen as the use of absorbable vs non-absorbable tacks has been thought to affect the early- to mid-term post-operative pain experienced by patients. This is in the window of time in which patients present to hospital services with abdominal pain following laparoscopic repair [14].
Study aim
To establish a difference in post -operative pain between a choice of two spiral tack mesh-fixation devices when used to perform laparoscopic incisional hernia repair by the IPOM + technique.
Objectives
To compare post-operative pain scores between absorbable and non-absorbable tack fixation, measured using a visual analogue scale (0–10 cm), at post-operative day 30 following laparoscopic IPOM + repair.
To compare post-operative clinical outcomes between absorbable and non-absorbable tack fixation.
To compare patient-reported outcomes between absorbable and non-absorbable tack fixation.
Methods
Participants
TACKoMesh is a prospective, single-centre, double-blind parallel RCT (NCT03434301). Participants with primary incisional hernia, with defect size 3–10 cm and a minimum distance of 3 cm from costal margin or pelvic brim, underwent elective laparoscopic incisional hernia repair. For full eligibility criteria see published trial protocol [25]. Exclusion criteria included age < 18 or > 80 years old, previous attempt at repair, BMI > 40 kg/m2 and inability to close the defect during surgery [25].
Screening took place in specialist outpatient clinics at MFT. Following consent for laparoscopic incisional hernia repair, patients were provided with trial information ahead of recruitment into the trial (Research Ethics Committee reference 17/NW/0082; Integrated Research Application System project ID 213,428).
Treatment allocation
Patients were randomised to surgery with Reliatack™ (Medtronic, Medtronic.com), an articulating device deploying absorbable plastic copolymer tacks, or Protack™ (Medtronic, Medtronic.com), a straight arm device deploying non-absorbable tacks on the day of surgery using a sealed envelope technique that stratified patients according to size of hernia defect (3–6 cm or > 6–10 cm). Sealed envelopes were generated prior to trial initiation by an independent member of the trial team.
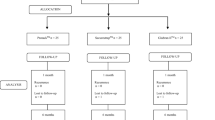
Patients were enrolled by the blinded researcher (JJP) and principal investigator (AJS). Randomisation was performed in theatre by the principal investigator (AJS). Defect size had been recorded in clinic with the use of available cross-sectional imaging or, where imaging was unavailable, clinical examination in the supine position by two independent examiners (JJP & AJS). Intra-operative details were recorded at the end of the procedure. Patients were followed up by face-to-face encounter at post-operative days 1, 6, 30, and 365 by the blinded researcher (JJP) in the inpatient and outpatient setting. Excluding the operating surgeon (AJS), all patients and members of the trials team were blind to treatment allocation throughout trial conduct and data management up unto unblinding for analysis.
Surgical procedure
Following pneumoperitoneum, adhesiolysis and fascial closure, Symbotex TM (Medtronic, Medtronic.com) mesh was secured by either absorbable or non-absorbable tack fixation.
All cases were performed by AJS [25]. Skin preparation and antimicrobial prophylaxis was standardised and recorded. Establishing pneumoperitoneum and adhesiolysis was performed at intrabdominal pressure 12-15mmHg. Fascial closure was performed using a 1 − 0 Loop Maxon with one end cut to provide extra length and an extracorporeal knot slid down using an Endoloop pusher at intrabdominal pressure 8-10mmHg. Interrupted sutures were sited sequentially to close the defect and the number of knots used was recorded in the operative data case report form. Sizing of the mesh took place, appropriate to achieve mesh overlap of 3 cm in all directions. This was followed by mesh placement and fixation with the allocated mesh-fixation device with tacks deployed in a double-crown arrangement and without the use of transfascial sutures [25]. At the end of the procedure, a recorded amount of local anaesthetic was infiltrated as a bilateral TAP block under direct visualisation with the laparoscope in addition to infiltration at port sites.
Outcomes
The primary outcome of the trial was reported pain on activity, at 30 days following surgery. This was measured using a visual analogue scale (VAS)(0–10).
To obtain pain scores, trial participants were shown the validated universal pain assessment tool (Fig. 1) [7]. The blinded researcher (JJP) then asked the following standardised questions; “Please indicate on this scale the pain that you currently experience from your incisional hernia at rest?” and “Please indicate on this scale the pain that you currently experience from your incisional hernia during activity?”.
Universal Pain Assessment Tool (18)
Secondary objectives were to assess pain scores at rest and on activity on days 1, 6 and 365 post-surgery, and to compare key clinical and PROMs between treatment arms. A full list of secondary outcome measures can be found within the published trial protocol [25]. All pain scores were collected in the same manner as for the primary outcome. The operative data recorded included details on mesh size, number of tacks used, number of knots used, time to perform mesh fixation (recorded as the time from deploying first to last spiral tack) and total length of surgery. Key clinical outcomes and complications were collected in trial specific case report forms with the same blinded researcher at face-to-face patient reviews which included a clinical examination. Complications were subsequently graded as per the Clavien-Dindo classification [6]. Additional PROMs were obtained via validated questionnaires, the Short Form 36 (SF-36) and Carolina Comfort Score (CCS), and a trial-specific questionnaire designed for the TACKoMesh trial that included standard questions that have been used in other comparative trials and were approved by the local ethics and trial steering committee. The SF-36 was issued at three timepoints; pre-operative day 0, and post-operative days 30 and 365. The CCS was issued at two post-operative time points; day 30 and day 365. The TACKoMesh PROMs questionnaire was issued once at post-operative day 30. It included non-validated questions on trial participants’ pain experience and recovery to normal activity since discharge from the hospital.
Sample size
A power calculation was performed using similar studies as a reference [3,4,5; 8]. A change in VAS score of 1.5 was deemed to be clinically significant based on the interpretation of previous results and timing of pain assessments. A sample size of 74 patients was required to detect a significant difference of 1.5 in VAS pain score between treatment groups with 80% power, and alpha of 0.05, SD of 2 and 20% drop out.
Statistics
Statistical analysis was performed in RStudio v1.4.1106. For comparisons between groups, t-test, Wilcoxon Rank Sum test, and Fisher’s Exact test were used based upon the type of data and its distribution. In cases where repeat data were collected at multiple timepoints, and the assumptions were valid, repeated measures ANOVA was used. Where missing data was encountered, the patient was removed from that particular analysis, with the exception of SF-36 and CCS data, where validated instructions on imputation of missing data were followed.
Results
Demographics
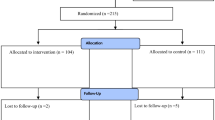
Sixty-seven patients were randomised to treatment with absorbable (Reliatack™)(n = 30) or non-absorbable (Protack™)(n = 37) tack fixation (Fig. 2). Four patients were converted to open retro-rectus repair due to dense intraabdominal adhesions and patient safety considerations for proceeding laparoscopically; one experienced an iatrogenic small bowel injury. They were removed from ongoing follow-up as they did not receive either method of spiral tack mesh-fixation. Recruitment began in July 2017 and surgeries took place between July 2017 and March 2020.
Patient groups were evenly matched on baseline demographics (Table 1) and proposed risk factors for incisional hernia formation in all areas except age [23] (Appendix 1).
Response rate for the primary outcome at 30 days was 95.5%. Other case report form response rates varied from 87.3 to 100.0%. For the SF-36, response rates at days 0, 30 and 365 were 73.0%, 90.5% and 85.7% respectively. For the CCS, response rates on days 30 and 365 were 92.1% and 85.7% respectively. All available data was included in analysis.
The trial was running at the time of the COVID-19 outbreak. Prolonged reduced access to elective operating theatre space within the NHS led to early closure of trial. A small number of patients were undergoing trial follow-up at the time of the coronavirus lockdown. For these patients, available primary and secondary outcome measures were collected via telephone. Those outcomes requiring face-to-face contact were delayed until the lifting of restrictions. Follow up took place until March 2021.
VAS pain scores: absorbable (Reliatack™) vs. non-absorbable (Protack™)
There was no significant difference in VAS pain score on activity at any timepoint between the two treatment arms (Fig. 3). There was significantly less reported pain at rest on post-operative day 1 with absorbable tacks (p = 0.020), with a diminishing trend towards less pain at 6- and 30-days post-surgery which did not reach statistical significance (Fig. 3). There were no significant differences between groups in the use of patient-controlled analgesia or analgesic regime on 1-, 6- or 30-days post-surgery.
‘VAS Pain Scores at Rest’ and ‘VAS Pain Scores on Activity’ at All Trial Timepoints (Day) for patients separated by Treatment Arm. Boxplots showing median (bold line), interquartile range (box), values within 1.5x IQR (whiskers) and outliers (dots) for patients separated by treatment arm; Protack™ [Red] versus Reliatack™ [White]. Where the difference in reported pain scores was significant between the groups (p < 0.05) this is indicated by red brackets and a star
Additional outcome measures: absorbable (Reliatack™) vs. non-absorbable (Protack™)
A summary of operative data is supplied in Table 2. All procedures were recorded as ‘Clean’, and the volume of predicted blood loss was comparable between the groups.
More knots (3 [2.5-4] vs. 2 [1–3]) and tacks (28 [26–30] vs. 23 [20–27]) were used and the time to perform mesh fixation (430 [330-473.5] vs. 171 [130-270.5] seconds) was longer in the absorbable tack group (p < 0.001). Length of hospital stay and incidence of hernia recurrence, seroma formation, surgical site infection, and Clavien-Dindo Grading of complications were comparable between treatment groups (Table 3). The SF-36, the TACKoMesh patient questionnaire and CCS questionnaire all showed no significant differences between groups (Appendix 2).
Hernia size as a covariate
As part of randomisation to treatment arm, patients were stratified based upon defect size: (i) 3–6 cm (n = 44) and, (ii) > 6–10 cm (n = 19). Patients with a hernia > 6–10 cm showed an increased rate of hernia recurrence (26.3% vs. 6.8%, p = 0.047) and seroma formation (73.7% vs. 43.2%, p = 0.031) and a larger proportion of higher classed seromas identified (p = 0.021) when compared to patients with a 3–6 cm hernia. No significant differences were found in reported pain scores at rest or on activity at all time points, total length of stay, and incidence of surgical site infection and Clavien-Dindo Grade III-IV complication.
Clinical outcomes for the entire trial cohort
The reported VAS pain scores at rest and activity for the entire trial cohort (Appendix 3) showed an initial worsening in reported pain on post-operative day 1 that reduced at day 6 to near pre-operative levels, with improvement, for many, by day 30. By day 365 an overall improvement is seen in reported pain at rest and on activity by comparison to pre-operative levels for almost all patients.
Pain at rest at one year following surgery was reported by 7 (11.1%) patients. Patients with persistent pain at 1 year reported higher pain pre-operatively at rest (5 [2–7] vs. 0 [0–3] p = 0.018) and on activity (8.5 [5–10] vs. 4 [2–8] p = 0.018).
Sutured closure of the fascial defect was performed in all cases with complete closure obtained in 95.2% cases. More than one hernia defect was identified at laparoscopy in 13 (20.6%) cases; with instances of up to four separate defects noted, a ‘Swiss cheese’ hernia [11]. There was one instance of two pieces of mesh being required and one instance of a piece being altered prior to placement. Adequate mesh overlap (> 3 cm) was achieved in all cases. In two cases, additional laparoscopic port placement was required to facilitate mesh siting.
9/63 (14.3%) patients experienced a Clavien-Dindo Grade III to V complication (Table 3). Two patients required ultrasound-guided seroma intervention with one going on to require an abdominal wall washout under general anaesthesia. Five patients required an unplanned admission to critical care for respiratory support in the post-operative period. Three patients required emergency surgery during one year of follow-up for (i) iatrogenic small bowel injury, (ii) incarcerated port site hernia, and (iii) incarcerated hernia recurrence. There was one 30-day mortality owing to pulmonary embolus. Grade 2 complications were surgical site infection, lower respiratory tract infection, urinary tract infection and secondary haemorrhage requiring transfusion. Grade 1 complications included urinary retention, paralytic ileus, and constipation.
For most domains of the SF-36, scores at post-operative day 30 were reduced compared to pre-operative scores. Scores at day 365 were higher (Appendix 4) These findings were most marked for the ‘Physical health’ and ‘Emotional health’ domains. ‘Emotional well-being’ and ‘General health’ were least influenced by surgery. Patients who reported persistent pain at day 365 tended to provide lower pre-operative QOL scores compared to patients with no pain following treatment. This was most marked for the ‘Social function’ domain (50 [31–50] vs. 75 [63–100] p = 0.006).
At day 30, patients were asked qualitative questions regarding their recovery (Appendix 5). In response to the ‘yes/no’ question, ‘Since your discharge from hospital did you experience a lot of pain?’, 45/60 (75.0%) patients responded ‘yes’ with 6 (10.0%) of these patients going on to select the option to describe the pain as ‘severe to unbearable’. Of other notable PROMs, it was ‘1–2 weeks’ before 50.0% of responders could ‘cook or clean’ and ‘walk without painkillers’, and it was ‘> 3 weeks’ before 84.6% and 78.4% of responders ‘returned to work’ and ‘report being fully recovered’ respectively.
Discussion
The pain experienced following incisional hernia repair needs greater understanding to best facilitate patient selection and counselling. There was a vogue to using absorbable tacks at the time of study design with a suggestion that absorbable tacks cause less pain and are less likely to cause bowel or visceral injury. No difference in reported pain on activity at post-operative day 30 was found when making a choice between absorbable and non-absorbable tack fixation. This study provides data on the laparoscopic IPOM + repair with clinical and patient-reported outcomes. The study closed early owing to the outbreak of COVID-19, it is subsequently underpowered, and that is a key limitation of this study.
Consistency in outcomes reporting is needed for incisional hernia studies [22]. A standardised outcome set recommends assessing chronic pain at 3 months, 1 and 5 years but gave no recommendation on the timing of early post-operative pain assessment [21]. Abdominal pain is the most common reason for representation to healthcare services following laparoscopic incisional hernia repair [14] Within this trial there were no significant differences in reported pain ‘on activity’ between treatment arms at all post-operative time points up to one year. When comparing pain scores at rest, there was a trend towards less pain with absorbable tacks at all post-operative timepoints, and a significant difference at post-operative day 1. This observation, coupled with findings that patients in the absorbable tack group reported more pain pre-operatively and had more sutures and tacks used during surgery, may suggest some improvements in post-operative pain when choosing absorbable tacks. This study did not reach power and the finding needs further clarification.
Recent guidelines on reporting for incisional hernia trials suggest future assessors specify ‘pain at the hernia site’ and ask for a score on a scale of 0–10 for ‘pain at rest (lying down)’, ‘pain during activities (walking, cycling, sports)’, and ‘pain felt during the last week’ [21]. Pre-operative pain is known to be a predictor of post-operative pain and reduced quality of life following ventral hernia repair [27]. There is no current recommendation on recording pre-operative pain and its impact on subsequent pain assessment and analysis. Standardisation of pain assessments following incisional hernia repair techniques would greatly facilitate future comparisons [14]. Pain scoring seems multifactorial and consistency in reporting would enhance understanding of the patient experience and its utility in practice.
This study found that mesh-fixation time was significantly longer in the absorbable tack fixation group (430 [330-473.5] vs. 171 [130-270.5] seconds). This is likely to be a product of technical differences between the design of the two tacking devices. Reliatack™ has an articulating arm and reloadable tack cartridges, which leads to increased operating time articulating the device and increased scrub time reloading the cartridges.
Recurrence rates within this study are marginally higher than quoted in the literature [2]. At the design and initiation of this trial, guidelines on the laparoscopic repair of ventral hernia allowed for a hernia with a defect of 3–10 cm in size to undergo surgery with a need for mesh overlap of > 3 cm. More recent publications have seen guidance change to hernia with defect 2–6 cm [14] and mesh overlap to > 5 cm [2]. This study found a greater risk of incisional hernia recurrence with defects sized 6–10 cm compared with 3–6 cm (26.3% vs. 6.8%, p = 0.047). Supporting this recent update to guidance.
The study found high rates of seroma formation with this technique. This could be a product of the chosen method of fascial closure, interrupted suturing. Alternatively, the increased rates of detection could be owing to trial follow-up conditions. The majority were asymptomatic, detected at an early trial visit and resolved without intervention. This study, like others, showed that persistent seromas and seromas requiring intervention present a risk of preceding infective complications and hernia recurrence [13, 20].
A benefit to laparoscopic methods of incisional hernia repair identified by this study was the ability of a surgeon to identify multiple hernia defects during laparoscopy. An additional opinion from this trial was the success of this technique in the treatment of often difficult to fix incisional hernia, e.g. flank hernia. It is also proposed that laparoscopic techniques require less abdominal wall dissection and distortion to native anatomy. This should afford a greater choice of surgical options for the repeat attempt at repair in the instance of hernia recurrence.
During the outbreak of COVID-19 and announcement that the UK was going into lockdown, three trial participants were inpatients following surgery and one patient had been operated on the week prior. This had an impact on the trial follow-up for these patients given that new restrictions were placed on patient contact for research purposes. Follow-up milestones were maintained for pain scores and other information that could be obtained via telephone consultation. The impact of COVID, particularly the early closure of the trial, is a key limitation.
It seems the IPOM + repair has fallen out of favour. As a treatment option for the hernia surgeon there is a sub-population of patients that might benefit from its outcome profile. Longer term data is needed for the IPOM + repair [24].
Conclusion
This study found no difference in reported pain on activity following elective laparoscopic incisional hernia repair, with IPOM + repair, when choosing absorbable or non-absorbable tack fixation. The utility of “early” post-operative pain assessment as a comparator following incisional hernia repair needs clarification.
Abbreviations
- ANOVA:
-
analysis of variance
- BMI:
-
Body mass index
- CCS:
-
Carolina Comfort Score
- IPOM:
-
Intraperitoneal onlay mesh
- MFT:
-
Manchester University NHS Foundation Trust
- NHS:
-
National Health Service
- PROMs:
-
Patient–reported outcome measures
- QOL:
-
Quality of life
- RCT:
-
Randomised controlled trial
- SD:
-
Standard deviation
- SF–36:
-
Short Form 36
- VAS:
-
Visual analogue scale
References
Ahmed MA, Tawfic QA, Schlachta CM, Alkhamesi NA (2018) Pain and Surgical outcomes reporting after laparoscopic ventral hernia repair in relation to mesh fixation technique: a systematic review and Meta-analysis of Randomized clinical trials. J Laparoendosc Adv Surg Tech A 28(Nov):1298–1315. https://doi.org/10.1089/lap.2017.0609
Bittner R, Bansal Baink, Berrevoet VK, Bingener-Casey F, Chen J, Chowbey Dchenj, De Beaux Pdietzua, Ferzli A, Fortelny G, Hoffmann R, Ji Hiskanderm, Jorgensen Z, Kirchhoff Lnkhullarr, KÖCkerling P, Leblanc Fkukletaj, Li K, Meytes Jlomantodmayerf, Misra V, Morales-Conde M, Niebuhr S, Radvinsky H, Ramshaw D, B., Ranev, D., Reinpold, W., Sharma, A., Schrittwieser, R., Stechemesser, B., Sutedja, B., Tang, J., Warren, J., Weyhe, D., Wiegering, A., Woeste, G., And, Yao Q (2019) Update of Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS))-Part A. Surg Endosc 33, 10 (Oct), 3069–3139. https://doi.org/10.1007/s00464-019-06907-7
Boldo E, Perez De Lucia Armellesa, Martin G, Miralles Faraciljp, J.M., Martinez, D., And, Escrig J (2008) Pain after laparascopic bilateral hernioplasty: early results of a prospective randomized double-blind study comparing fibrin versus staples. Surg Endosc 22(May):1206–1209. https://doi.org/10.1007/s00464-007-9587-z
Brügger L, Bloesch M, Kurmann Ipaktchir, Candinas A, D., And, Beldi G (2012) Objective hypoesthesia and pain after transabdominal preperitoneal hernioplasty: a prospective, randomized study comparing tissue adhesive versus spiral tacks. Surg Endosc 26(Apr):1079–1085. https://doi.org/10.1007/s00464-011-2003-8
Colak E, Ozlem N, Aktimur Kucukgo, R., Kesmer, S., And, Yildirim K (2015) Prospective randomized trial of mesh fixation with absorbable versus nonabsorbable tacker in laparoscopic ventral incisional hernia repair. Int J Clin Exp Med 8:11, 21611–21616
Dindo D, Demartines, N., And, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(Aug):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Dugashvili G, Van Den Berghe L, Menabde G, Janelidze M, Marks L (2017) Use of the universal pain assessment tool for evaluating pain associated with TMD in youngsters with an intellectual disability. Med Oral Patol Oral Cir Bucal 22:1. https://doi.org/10.4317/medoral.21584
Eriksen JR, Bisgaard T, Assaadzadeh S, Jorgensen LN, Rosenberg J (2011) Randomized clinical trial of fibrin sealant versus titanium tacks for mesh fixation in laparoscopic umbilical hernia repair. Br J Surg 98(Nov):1537–1545. https://doi.org/10.1002/bjs.7646
Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich, A., Werner, J., Büchler, M.W., and, Diener Mk (2014) Incisional hernia rate 3 years after midline laparotomy. Br J Surg 101(Jan):51–54. https://doi.org/10.1002/bjs.9364
Gillion JF, Sanders D, Miserez, M., And, Muysoms F (2016) The economic burden of incisional ventral hernia repair: a multicentric cost analysis. Hernia 20(Dec):819–830. https://doi.org/10.1007/s10029-016-1480-z
Grapotte A, Cimpean S (2022) Laparoscopic intraperitoneal onlay mesh (IPOM) Swiss cheese ventral Incisional Hernia repair with urinary bladder mobilization: a Case Report. Am J Case Rep 23(Nov 18):e937606. https://doi.org/10.12659/ajcr.937606
Harji D, Antoniou Thomasc, Chandraratan SA, Griffiths H, Henniford B, Horgan BT, López-cano Lköckerlingf, Miserez Mmasseyl, Muysoms Mmontgomerya, Reinpold Fpoulosebk, W., And, Smart N (2021) A systematic review of outcome reporting in incisional hernia surgery. BJS Open 5(Mar 5). https://doi.org/10.1093/bjsopen/zrab006
HE C, Lu J, Lee Ongmw, Tan DJK, K.Y., and, Chia CLK (2020) Seroma prevention strategies in laparoscopic ventral hernia repair: a systematic review. Hernia 24(Aug):717–731. https://doi.org/10.1007/s10029-019-02098-1
Henriksen NA, Friis-Andersen H, Jorgensen LN, Helgstrand F (2021) Open versus laparoscopic incisional hernia repair: nationwide database study. BJS Open 5:1. https://doi.org/10.1093/bjsopen/zraa010
Huang X, Shao X, Cheng, T., And, Li J (2024) Laparoscopic intraperitoneal onlay mesh (IPOM) with fascial repair (IPOM-plus) for ventral and incisional hernia: a systematic review and meta-analysis. Hernia 28(Apr):385–400. https://doi.org/10.1007/s10029-024-02983-4
Khan Rma, Bughio M, Ali B, Hajibandeh, S., And, Hajibandeh S (2018) Absorbable versus non-absorbable tacks for mesh fixation in laparoscopic ventral hernia repair: a systematic review and meta-analysis. Int J Surg 53(May):184–192. https://doi.org/10.1016/j.ijsu.2018.03.042
Köckerling F, Mayer Hoffmannh, Zarras F, Reinpold K, Fortelny W, Weyhe R, Lammers D, B., Adolf, D., And, Schug-Pass C (2021) What are the trends in incisional hernia repair? Real-world data over 10 years from the Herniamed registry. Hernia 25, 2 (Apr), 255–265. https://doi.org/10.1007/s10029-020-02319-y
Manzini G, Henne-Bruns D, Kremer M (2019) Severe complications after mesh migration following abdominal hernial repair: report of two cases and review of literature. GMS Interdiscip Plast Reconstr Surg DGPW 8:Doc09. https://doi.org/10.3205/iprs000135
Muysoms F, Vander Mijnsbrugge G, Pletinckx P, Boldo E, Jacobs I, Michiels, M., And, Ceulemans R (2013) Randomized clinical trial of mesh fixation with double crown versus sutures and tackers in laparoscopic ventral hernia repair. Hernia 17(Oct):603–612. https://doi.org/10.1007/s10029-013-1084-9
Nguyen DH, Nguyen MT, Askenasy EP, Kao LS, Liang MK (2014) Primary fascial closure with laparoscopic ventral hernia repair: systematic review. World J Surg 38(Dec):3097–3104. https://doi.org/10.1007/s00268-014-2722-9
Parker SG, Halligan S, Berrevoet F, De Beaux AC, East B, Eker HH, Jensen KK, Jorgensen LN, Morales-Conde Montgomerya, Miserez S, Renard M, Sanders Y, Simons DL, Slade M, Windsor Dtorkingtonjblackwellsdamesn, A.C.J., And, Mallett S (2021) Reporting guideline for interventional trials of primary and incisional ventral hernia repair. Br J Surg 108(Sep 27):1050–1055. https://doi.org/10.1093/bjs/znab157
Parker SG, Halligan S, Erotocritou M, Boulton Woodcpj, Plumb RW, Windsor Aao, A.C.J., and, Mallett S (2019) A systematic methodological review of non-randomised interventional studies of elective ventral hernia repair: clear definitions and a standardised minimum dataset are needed. Hernia 23(Oct):859–872. https://doi.org/10.1007/s10029-019-01979-9
Sanders DL, Kingsnorth AN (2012) The modern management of incisional hernias. Bmj 344(May 9e2843. https://doi.org/10.1136/bmj.e2843
Scrimgeour DSG, Allan M, East Knightsr, Blackwell B, Dames S, Light Nlaidlawl, Horgan D, Smart L, De Beaux NJ, A., and, Wilson MSJ (2022) A modified Delphi process to establish research priorities in hernia surgery. Hernia 26, 3 (Jun), 751–759. https://doi.org/10.1007/s10029-021-02519-0
Sheen AJ, Pilkington JJ, Baltatzis M, Tyurkylmaz A, Stathakis P, Jamdar, S., And, Siriwardena AK (2018) Comparison of mesh fixation techniques in Elective Laparoscopic Repair of Incisional Hernia-ReliaTack™ v ProTack™ (TACKoMesh) - a double-blind randomised controlled trial. BMC Surg 18(Jul 11):1. https://doi.org/10.1186/s12893-018-0378-3
Suwa K, Okamoto, T., And, Yanaga K (2016) Closure versus non-closure of fascial defects in laparoscopic ventral and incisional hernia repairs: a review of the literature. Surg Today 46 7(Jul):764–773. https://doi.org/10.1007/s00595-015-1219-y
Tsirline VB, Colavita PD, Belyansky I, Zemlyak AY, Lincourt AE, Heniford BT (2013) Preoperative pain is the strongest predictor of postoperative pain and diminished quality of life after ventral hernia repair. Am Surg 79(Aug):8. https://doi.org/10.1177/000313481307900828
Van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF (2012) Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg 204(Aug):144–150. https://doi.org/10.1016/j.amjsurg.2012.01.012
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors have no competing interest in the publication of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Precis The TACKoMesh RCT found no difference in reported pain at 30 days following laparoscopic incisional hernia IPOM + repair when choosing absorbable and non-absorbable tack fixation.
A summary of this work has been presented in 2022 at both the American Hernia Society Annual Congress in Charlotte, USA and European Hernia Society Annual Congress in Manchester, UK.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pilkington, J.J., Pritchett, J., Fullwood, C. et al. TACKoMesh – A randomised controlled trial comparing absorbable versus non-absorbable tack fixation in laparoscopic IPOM + repair of primary incisional hernia using post-operative pain and quality of life - Reliatack™ versus Protack™. Hernia (2024). https://doi.org/10.1007/s10029-024-03111-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10029-024-03111-y