Abstract
Behcet's syndrome (BS) is a vasculitis characterized by immune dysregulation. Biomarkers are valuable for assessing clinically atypical pathogenesis. We aimed to investigate the distribution of different biomarkers and their effects on the clinical features of patients with BS in a large-scale, real-world study. This is a retrospective, single-center study. In total, 502 patients diagnosed with BS were enrolled in this study. We analyzed the clinical features of this cohort and divided patients’ symptoms into six categories, including mucocutaneous, articular, neurological, gastrointestinal, vascular, and ocular involvements. HLA-B51 cells, autoantibodies, and subsets of immune cells from the patients were tested. Pearson’s correlation, Wilcoxon rank sum test and multivariate logistic regression were used for data analysis. Various autoantibodies were detected in the serum of 40.8% of patients with BS. The positivity rate of anti-endothelial cell antibodies (AECA) was the highest among autoantibodies and was found in 23.5% (118/502) of patients with BS. The positivity rate of HLA-B51 in patients with BS was 27.1%. Tumor necrosis factor (TNF)-α, IL-2, and IL-4 producing CD4+ T cells were positively correlated with the gastrointestinal BS. Increased IL-4+CD4+ T cell was a risk factor for gastrointestinal BS (P = 0.006, Overall rate [OR] = 2.491, 95% Confidence interval [CI]: [1.317, 5.100]). Various autoantibodies can be detected in patients with BS. HLA-B51 and AECA are the most common biomarkers. Increased IL-4+ CD4+ T cell was a risk factor for gastrointestinal involvement in BS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BS is a systemic vasculitis affecting both small and large blood vessels in the venous and arterial systems. It is a multiorgan disease with various manifestations, including mucocutaneous, articular, ocular, gastrointestinal, vascular, and neurological involvements [1, 2]. While progress has been made in understanding BS, the underlying mechanism remains unclear. One significant genetic factor in BS development is HLA-B51, and studies have shown a notably higher frequency of HLA-B51 in patients with BS [3]. Current research indicates that the interaction between HLA-B51 and endoplasmic reticulum aminopeptidase 1 contributes to immune dysregulation and BS manifestation [4,5,6].
Autoimmune inflammation plays an important role in BS pathogenesis. The profiles of antibodies in the BS have not been fully elucidated for humoral immunity. The prevalence of autoantibodies in BS appears to be lower than in other autoimmune disorders [1, 7]. However, previous studies have shown that several autoantibodies can be found in the sera of patients with BS. AECA have been shown to bind specifically to the endothelial cell membrane, potentially promoting vascular lesions in patients with BS [8,9,10]. Antinuclear antibodies (ANA), commonly found in autoimmune diseases, such as systemic lupus erythematosus, Sjogren's syndrome, and systemic sclerosis [11,12,13], have also been found in patients with BS [14, 15]. Some newly discovered antibodies, such as anti-tubulin-α-1c and gastric parietal cell antibodies, have also been detected in patients with BS [16, 17].
Adaptive immunity, specifically the subsets of CD4+ T cells, also mediates BS pathogenesis. Th1, Th2, Th17, and Treg cells play roles in promoting inflammatory responses in patients with BS by interacting with cytokines and cell surface antigens [18]. Th1 cells are activated in BS, leading to elevated levels of inflammatory interleukins, such as IL-2, IL-12, interferon (IFN)-γ, and TNF-α. Additionally, Th2 cells and IL-4, the main factors in this subset of T-helper cells, play an important role in BS pathogenesis [4, 7]. Disturbances in the balance of Th17/Treg cells have been found in BS, with elevated levels of IL-17 and inhibited activation of Treg cells [19]. Additionally, natural killer cells and neutrophils are involved in BS pathogenesis [7, 20].
Despite the growing body of research focusing on the role of different molecules in BS pathogenesis, large-scale studies summarizing the distribution of different biomarkers in patients remain limited. Furthermore, the impact of different biomarkers on the clinical manifestations of BS remains unclear. Therefore, this study aimed to explore the distribution of different biomarkers in patients with BS and their relationship with the involvement of different systems in a large sample.
Methods
Patients
This is a retrospective, single-center study conducted in Peking University People’s Hospital, Beijing, China. The cohort comprised 502 patients diagnosed with BS in the Department of Rheumatology and Immunology between 2018 and 2023. All patients fulfilled the 2014 International Criteria for Behçet’s disease (ICBD) [21], and this was their first diagnosis of BS. All patients were in their active phase of disease and receiving glucocorticoids or/and immunosuppressors or/and immunomodulators or/and biologic agents when enrolled. Patients with other autoimmune diseases were excluded. The age, disease duration, disease manifestation, and disease activity of patients with BS were recorded at their initial visit to the clinic. The patients were divided into six groups according to their clinical manifestations: mucocutaneous-only, joint, neurological, gastrointestinal, vascular involvement and ocular involvement. The Behcet's Disease Current Activity Form (BDCAF) was used to evaluate disease activity. [22]
Laboratory analysis
Indirect immunofluorescence (IIF) was used to detect ANA and AECA. Immunoblotting was performed to identify anti-SSA, anti-SSB, anti-Jo-1, anti-Scl-70, anti-U1RNP, anti-Ro-52, and anti-Sm antibodies. Enzyme-linked immunosorbent assay (ELISA) was employed to detect anti-mitochondrial antibodies M2 (AMA-M2), anti-PR3, and anti-MPO antibodies. Notably, AMA-M2 was procured from Shanghai Kexin Technology Co., Ltd., and the remaining ELISA, IIF, and immunoblotting kits were sourced from Oumeng Company. A sequencing typing from Beijing Jingzhun Medical Technology Co., Ltd., was used to determine HLA-B51 positivity. Furthermore, a Beckman AU5832 fully automated biochemical analyzer, which employs immune dispersion turbidimetry, was used to measure the levels of immunoglobulins, complement and rheumatoid factor (RF). The aforementioned tests were conducted at the Rheumatology and Immunology Laboratory, Peking University People's Hospital.
For detection of cytokines producing CD4+ T cells, peripheral blood lymphocytes were stimulated by using leukocyte activation cocktail, with BD GolgiPlug™ (BD 550583). Cells were stained intracellularly with monoclonal antibodies: BV510-IL-2 (BD 563265), APC-TNF-α (BD 340534), FITC-IFN-γ/PE-IL-4 (BD 340456), and BV421-IL-17 (BD 562933). Samples were analyzed by flow cytometry using a FACSAria II (BD), FlowJo software (Tree Star) (Figure S1) To determine the proportions of regular T cell (Treg) in the periphery, immunophenotyping of T cells was classified using conjugated anti-human murine monoclonal antibodies (mAbs) as follows: anti-CD3-PERCP, anti- CD4-FITC, anti-CD25-PE, anti- CD127 (IL-7Rα)-BV605. Tregs were defined as CD3+CD4+CD25hiCD127low. All antibodies were purchased from BD Pharmingen (San Diego, CA) and were analyzed by flow cytometry using a FACSAria II (BD), FlowJo software (Tree Star). (Figure S2) In terms of absolute numbers, detection and analysis of T lymphocyte subsets were performed in BD Trucount™ tube (BD 340334) using a single-platform flow cytometry-based absolute counting technique. Lymphocytes were gated by CD45/side scatter dot plots along with T lymphocytes (CD3+), B lymphocytes (CD19+), helper/inducer T lymphocytes (CD3+CD4+), suppressor/cytotoxic T lymphocytes (CD3+CD8+), and natural killer (NK) lymphocytes (CD3–CD16+ and/or CD56+). (Figure S3).
The serum concentration of cytokines was detected by cytometric bead array (CBA) (BD Biosciences, CA, USA). CBA samples were run on an Acuri C6 flow cytometer (BD Biosciences, CA, USA) and data were analyzed using FCAP-array software (version 3.1; BD Biosciences, CA, USA).
Statistical analysis
Statistical analysis and graph creation were performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA), Prism 8 (GraphPad Software, San Diego, CA, USA), and R package (“UpSetR” and “pheatmap”). Spearman’s correlation analysis was used to analyze the correlations between serum markers and affected systems. Wilcoxon rank sum test was used to compare two sets of non-normal distribution data. Multivariate logistic regression analysis was used to assess the risk factors. Statistical significance was set at P < 0.05.
Results
Patients’ characteristics
In total, 502 patients diagnosed with BS were enrolled in our study, with a male-to-female ratio of 1:1.15. Patients’ median age was 38 (29, 51) years, and their median disease duration was 6.0 (2.0, 10.0) months. Patients’ characteristics are listed in Table 1. Based on their clinical manifestation, 226 (45.0%) showed mucocutaneous involvement without other systemic symptoms. Gastrointestinal symptoms were present in 86 (17.1%) patients. Ocular involvement was observed in 81 (16.1%) patients. Eighty (15.9%) patients had arthritis or arthralgia, 68 (13.5%) had cardiovascular lesions including arterial aneurysms, thromboembolisms, and valvular disease, and 33 (6.6%) had central or peripheral nervous system abnormalities. (Figure S4).
Distribution of autoantibodies in patients with BS
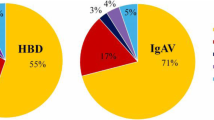
Positivity rates for HLA-B51 and autoantibodies in patients with BS are shown in Fig. 1. Several autoantibodies were detected in the serum of 40.8% (205/502) of the patients with BS. The positivity of HLA-B51 (27.1%, 136/502) was higher than all autoantibodies. The positivity rate for AECA was the highest (23.5%, 118/502) among autoantibodies, followed by ANA (16.7%, 84/502). The positivity of other antibodies accounts for < 5% of the total number of patients, including 4.0% (20/502) for RF, 2.8% (14/502) for AMA-M2, 2.0% (10/502) for anti-Ro-52 antibodies, 1.0% (5/502) for anti-SSA antibodies, 0.8% (4/502) for anti-PR3 antibodies, 0.6% (3/502) for anti-Jo-1 antibodies, 0.6% (3/502) for anti-MPO antibodies, 0.2% (1/502) for anti-Scl-70 antibodies, and 0.2% (1/502) for anti-U1RNP antibodies.
The positive rates of HLA-B51 and autoantibodies in patients with BS. Of all biomarkers, HLA-B51 had the highest positive rate of 27.1%. AECA was the autoantibody with highest positive rate (23.5%). The frequency of ANA is 16.7%. Positivity of other autoantibodies was less than 5%. AECA, anti-endothelial cell antibodies; ANA, antinuclear antibodies; AMA-M2, anti-mitochondrial antibodies M2
The positivity rate of HLA-B51 in patients with BS was 27.1% (136/502). Among patients positive for HLA-B51, the proportion of patients with BS with at least one positive autoantibody was 27.1% (13/48), while in the HLA-B51(−) group, autoantibodies were detected in 42.2% (54/128) of the patients. No significance was found between HLA-B51(+) and HLA-B51(−) groups (P = 0.066). In both HLA-B51(+) and HLA-B51(−) groups, the proportion of cases with positive AECA was the highest. (Figure S5).
Figure 2 shows the distribution of the frequencies of HLA-B51, AECA, and ANA in different phenotypes of BS. Patients with ocular (34.6%) and neurological (33.3%) involvement showed a higher positivity rate of HLA-B51. The ANA positivity rate was the highest in patients with neurological involvement (21.2%) and lowest in those with the vascular phenotype (11.8%). The frequency of AECA was the highest in patients with skin and mucosal lesions (25.7%) and the lowest in patients with neuro-BS (6.1%).
The positivity of HLA-B51, AECA and ANA in patients with BS with different systemic involvements. The positive rate of HLA-B51 was highest in ocular BS (34.6%) and lowest in intestinal BS (19.7%). The positive rate of AECA was highest in patients with mucocutaneous involvement alone (25.7%) and was lowest in Neuro-BS patients (6.1%). The positive rate of ANA was the highest in neuro-BS patients (21.2%) and was the lowest in vascular phenotype (11.8%)
Immunologic changes in BS with gastrointestinal involvement
Gastrointestinal involvement of patients was positively associated with the proportion of TNF-α+CD4+ T cells (P = 0.008), IL-2+CD4+ T cells (P = 0.027), and IL-4+CD4+ T cells (P = 0.003) among the total CD4+ T cells in PBMCs. Ocular involvement was positively associated with the absolute number of NK cells in peripheral blood (P = 0.046). The results were shown as heatmap (Fig. 3). No correlations were found between serum immunoglobulins or complement and systemic involvement in patients with BS.
The relation between different systemic involvement and the proportion of TNF-α, IFN-γ, IL-2, IL-4, IL-17 producing CD4+ T cells and Tregs among total CD4+ T cells, the absolute number of CD4+ T cells, CD8+ T cells, B cells and NK cells, and the level of serum immunoglobulins and complement in peripheral blood. The proportion of TNF-α, IL-2 and IL-4 producing CD4.+T cells were positively associated with gastrointestinal involvement of BS. The absolute number of NK cells was positively associated with ocular involvement of BS. The depth of the color represents the magnitude of the Spearman Rank correlation coefficient (Src). *P < 0.05, **P < 0.01
When comparing patients with BS with and without gastrointestinal involvement, the results showed that patients with gastrointestinal manifestation had a higher proportion of TNF-α+CD4+ T cells (47.50[39.50, 57.70] vs. 41.30[33.90, 48.55], P = 0.012), IL-2+CD4+ T cells (55.40[44.20, 70.90] vs. 46.80[38.60, 58.00], P = 0.038), and IL-4+CD4+ T cells (1.90[1.46, 2.25] vs. 1.60[1.03, 2.09], P = 0.009) among CD4+ T lymphocytes. Further analyzing these cytokines, the results showed that the serum level of TNF-α (17.17[4.57, 34.64] vs. 7.31[3.22, 14.89], P = 0.025), IL-2 (12.90[7.93, 31.18] vs. 7.31[2.58, 11.70], P = 0.044) and IL-4 (13.00[6.92, 28.66] vs. 7.98[2.96, 12.38], P = 0.021) were significantly increased in patients with gastrointestinal involvement. (Table 2) In addition, the absolute number of NK cells in patients with ocular involvement was significantly higher than that in patients without ocular involvement (188.0[1507.0, 405.5] vs. 169.0[112.5, 282.5], P = 0.031).
Risk regression of patients with BS with gastrointestinal involvement
The above subset of CD4+ T cells, age, sex and disease course, were included in the risk assessment analysis to explore the predictors of gastrointestinal involvement in BS. Multivariate logistic regression analysis indicated that an elevated proportion of IL-4+CD4+ T cells was a risk factor for gastrointestinal involvement in BS (β = 0.952, SE = 0.345, Wald = 7.595, P = 0.006, OR = 2.491, 95%CI: [1.317–5.100]). However, other variables were not significant in the regression (Table 3).
Discussion
BS is an autoimmune disease involving multiple cells and molecules, with various clinical manifestations ranging from recurrent aphthous ulcers to arterial aneurysms. Although several biomarkers have been identified in patients with BS, no specific biomarkers are currently available for its diagnosis, as the criteria of the 2014 ICBD and 1990 International Study Group [23] are mainly based on manifestation. Additionally, the impact of different biomarkers on the clinical characteristics of patients remains unclear. In this study, we aimed to address these knowledge gaps by comprehensively analyzing a large sample of patients with BS. Specifically, we assessed the prevalence of various autoantibodies in patients with different systemic involvements and examined the relationship between biomarkers and clinical manifestations. Through this study, we hope to provide valuable insights into the role of biomarkers in BS and contribute to the development of more accurate diagnostic tools and tailored treatment approaches.
In this study, we observed that HLA-B51 had the highest positivity rate among all biomarkers tested. This finding is consistent with those of previous studies, as HLA-B51 is widely recognized as the gene most strongly associated with BS [3, 24, 25]. However, in our study, HLA-B51 incidence was relatively lower than that of previous studies. This discrepancy may be attributed to geographical variations, and we believe that the positivity rate of HLA-B51 in patients with BS in northern China was lower than that reported in regions with major epidemics, such as the Middle East and far-East Asia [1]. Moreover, we found that in the patients included in our study, HLA-B51 was more prevalent in patients with ocular involvement. This is consistent with the findings of many other researchers, who have also observed a relationship between HLA-B51 and uveitis in BS [26, 27]. Our findings further support the existing evidence linking HLA-B51 with ocular manifestations in BS. Previous study found that HLA-B51(−) patients had serious systemic involvement [28]. And in this study, positive rate of autoantibodies was higher in HLA-B51(−) group, which may indicate more severe disease. But no significant difference was found. The association between HLA-B51 and other biomarkers, as well as its impact on disease severity, necessitates further investigation.
Although previous studies have shown that autoantibodies are uncommon in patients with BS, our study revealed that several autoantibodies were detected in nearly half of the patient sera. Notably, the proportion of cases with isolated positivity for AECA was significantly higher than that for other autoantibodies in both the HLA-B51(+) and HLA-B51(−) groups. AECA is a commonly observed serological marker in various systemic vasculitis conditions, such as Takayasu arteritis and ANCA associated systemic vasculitis [29], and can bind to endothelial cell membranes, thereby damaging multiple endothelial cells [8]. Previous studies demonstrated the presence of AECA in patients with BS [10, 16]. In our study, AECA exhibited the highest positivity rate among the detected autoantibodies. We hypothesized that AECA contributes to the onset of BS by attacking the vascular endothelial cells of affected individuals and can potentially serve as a diagnostic marker for BS and may represent a therapeutic target in the future, warranting further investigation. Notably, Souza et al. indicated that IgG-AECA might serve as a marker for neuro-BS. [30] However, our study observed the lowest positivity rate for AECA among patients with neurological lesions. Therefore, additional research is necessary to elucidate the effect of AECA on the nervous system in patients with BS.
The involvement of the gastrointestinal system in BS has gained significant attention owing to its association with increased morbidity and mortality in patients with BS [31]. Despite ongoing clinical advancements, no specific markers are currently available to diagnose or evaluate intestinal BS. Although conventional treatments for gastrointestinal involvement have proven beneficial for many patients, a subset of individuals remain refractory to these treatments, highlighting the need for new therapeutic targets [32]. In this study, we observed correlations between BS, gastrointestinal involvement, and various inflammatory factors. TNF-α predominantly secreted by Th1 cells, has been identified as a cytokine that mediates inflammatory responses in the immune microenvironment. Scientists have discovered that TNF-α, along with other cytokines associated with inflammation, accumulates in the gut and contributes to damage and structural changes in the bowel seen in inflammatory bowel disease [33]. Monoclonal antibodies targeting TNF-α (for example, infliximab and adalimumab) have shown efficacy in treating refractory gastrointestinal disease in BS [34]. In our study, we found higher proportion of TNF-α+CD4+ T cells and higher serum level of TNF-α in patients with gastrointestinal manifestations, underscoring the importance of TNF-α in developing a gastrointestinal lesion in BS. Another Th1-secreting inflammatory cytokine, IL-2, has been implicated in BS. Previous studies have demonstrated that IL-2 interacts with other cytokines to promote the inflammatory cascade in BS. [35, 36] In this study, we observed BS patients with gastrointestinal involvement had higher proportion of IL-2+CD4+ T cells and higher serum level of IL-2. These findings suggest that IL-2 plays a role in mediating the manifestation of gastrointestinal tract lesions in patients with BS, thus providing a foundation for further investigation of the pathogenesis of intestinal BS.
In our study, we also found the proportion of IL-4+CD4+ T cells and serum level of IL-4 were increased in patients with gastrointestinal involvement in BS, and increased levels of IL-4+CD4+ T cells were identified as a risk factor for gastrointestinal involvement in BS. IL-4 is a cytokine associated with the Th2 immune response. Previous studies have demonstrated that IL-4, along with IL-13 (another member of the Th2 cytokine family), plays a crucial role in allergic inflammation and fibroproliferative disorders [37, 38]. Some studies have observed upregulated levels of IL-4 in active BS [39, 40]. However, the role of IL-4 in gastrointestinal involvement in BS has been relatively unexplored. Our results provide evidence that IL-4 plays a significant role in the mechanism underlying gastrointestinal tract lesions in patients with BS. Furthermore, IL-4 could potentially serve as a biomarker of gastrointestinal diseases in patients with BS. The findings regarding TNF-α, IL-2, and IL-4 collectively highlight the involvement of inflammatory cytokines in the development of intestinal BS. This study lays a solid foundation for further investigation into BS pathogenesis.
This study also found that the absolute number pf peripheral NK cells in patients with ocular involvement was higher than that in patients without ocular involvement. Previous studies have shown that NK cells were increased in BS patients with uveitis and may exert pathogenic effects through cytotoxicity and regulation of helper T cells [41]. The results of this study are consistent with the previous.
There are limitations in this study. First, this is a retrospective study conducted in single-center and the sample source should be expanded further in the future. Second, we did not follow patients over time to see how different biomarkers affected their clinical performance. Further follow-up of patients with BS is needed in the future for more in-depth exploration.
Summarily, various autoantibodies can be detected in patients with BS, in which AECA is the most common autoantibody. HLA-B51 was the predominant biomarker observed at the highest frequency in patients with BS. Increased proportion of TNF-α+, IL-2+, and IL-4+CD4+ T cells, and serum concentration of TNF-α, IL-2, and IL-4 are associated with the gastrointestinal manifestation of BS. Elevated levels of IL-4+CD4+ T cells are a risk factor for developing gastrointestinal involvement in BS.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BS:
-
Behcet’s syndrome
- AECA:
-
Anti-endothelial cell antibodies
- ANA:
-
Antinuclear antibodies
- TNF:
-
Tumor necrosis factor
- IFN:
-
Interfreon
- ICBD:
-
International Criteria for Behçet’s disease
- AMA-M2:
-
Anti-mitochondrial antibodies M2
References
Yazici Y, Hatemi G, Bodaghi B, et al. Behçet syndrome. Nat Rev Dis Primers. 2021;7(1):67. https://doi.org/10.1038/s41572-021-00301-1.
Bettiol A, Prisco D, Emmi G. Behçet: the syndrome. Rheumatology (Oxford). 2020;59(Suppl 3):iii101–7. https://doi.org/10.1093/rheumatology/kez626.
Takeno M. The association of Behçet’s syndrome with HLA-B51 as understood in 2021. Curr Opin Rheumatol. 2022;34(1):4–9. https://doi.org/10.1097/bor.0000000000000846.
van der Houwen TB, van Hagen PM, van Laar JAM. Immunopathogenesis of Behçet’s disease and treatment modalities. Semin Arthritis Rheum. 2022;52: 151956. https://doi.org/10.1016/j.semarthrit.2022.151956.
Cavers A, Kugler MC, Ozguler Y, et al. Behçet’s disease risk-variant HLA-B51/ERAP1-Hap10 alters human CD8 T cell immunity. Ann Rheum Dis. 2022;81(11):1603–11. https://doi.org/10.1136/ard-2022-222277.
Hatemi G, Seyahi E, Fresko I, et al. Behçet’s syndrome: one year in review 2023. Clin Exp Rheumatol. 2023;41(10):1945–54. https://doi.org/10.55563/clinexprheumatol/7kdo9x.
Tong B, Liu X, Xiao J, et al. Immunopathogenesis of Behcet’s Disease. Front Immunol. 2019;10:665. https://doi.org/10.3389/fimmu.2019.00665.
Legendre P, Régent A, Thiebault M, et al. Anti-endothelial cell antibodies in vasculitis: a systematic review. Autoimmun Rev. 2017;16(2):146–53. https://doi.org/10.1016/j.autrev.2016.12.012.
Ooka S, Nakano H, Matsuda T, et al. Proteomic surveillance of autoantigens in patients with Behcet’s disease by a proteomic approach. Microbiol Immunol. 2010;54(6):354–61. https://doi.org/10.1111/j.1348-0421.2010.00215.x.
Hussain M, Chen P, Zhang Y, et al. Moesin expression is correlated with its involvement in patients with Behcet’s disease. Arch Med Sci. 2020;16(4):924–30. https://doi.org/10.5114/aoms.2020.92911.
Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–79. https://doi.org/10.1038/s41584-020-0480-7.
Stochmal A, Czuwara J, Trojanowska M, et al. Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol. 2020;58(1):40–51. https://doi.org/10.1007/s12016-018-8718-8.
Hernández-Molina G, Nuñez-Alvarez C, Avila-Casado C, et al. Usefulness of IgA Anti-α-fodrin antibodies in combination with rheumatoid factor and/or antinuclear antibodies as substitute immunological criterion in sjögren syndrome with negative anti-SSA/SSB antibodies. J Rheumatol. 2016;43(10):1852–7. https://doi.org/10.3899/jrheum.151315.
Güngörer V, Polat MC, Çelikel E, et al. Factors associated with the development of thrombosis in pediatric Behçet disease. J Clin Rheumatol. 2023;29(4):e19–24. https://doi.org/10.1097/rhu.0000000000001930.
Moroi Y, Takeuchi A, Mori M, et al. Antinuclear antibody in Behçet’s disease. J Rheumatol. 1982;9(5):809–10.
Amin MM, Abdel Latif OM. Anti-tubulin-alpha-1c antibody as a marker of value in Behçet syndrome. Clin Rheumatol. 2022;41(6):1759–67. https://doi.org/10.1007/s10067-021-06025-7.
Chiang CP, Wu YH, Chang JY, et al. Hematinic deficiencies and hyperhomocysteinemia in gastric parietal cell antibody-positive or gastric and thyroid autoantibodies-negative Behcet’s disease patients. J Formos Med Assoc. 2019;118(1 Pt 2):347–53. https://doi.org/10.1016/j.jfma.2018.05.017.
Al-Obeidi AF, Nowatzky J. Immunopathogenesis of Behçet’s disease. Clin Immunol. 2023;253: 109661. https://doi.org/10.1016/j.clim.2023.109661.
Filleron A, Tran TA, Hubert A, et al. Regulatory T cell/Th17 balance in the pathogenesis of paediatric Behçet disease. Rheumatology (Oxford). 2021;61(1):422–9. https://doi.org/10.1093/rheumatology/keab253.
Le Joncour A, Martos R, Loyau S, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2019;78(9):1274–82. https://doi.org/10.1136/annrheumdis-2018-214335.
The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–47. https://doi.org/10.1111/jdv.12107.
Bhakta BB, Brennan P, James TE, et al. Behçet’s disease: evaluation of a new instrument to measure clinical activity. Rheumatology (Oxford). 1999;38(8):728–33. https://doi.org/10.1093/rheumatology/38.8.728.
Criteria for diagnosis of Behçet's disease. International study group for Behçet's disease. Lancet, 1990;335(8697):1078-1080.
Greco A, De Virgilio A, Ralli M, et al. Behçet’s disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17(6):567–75. https://doi.org/10.1016/j.autrev.2017.12.006.
Bodis G, Toth V, Schwarting A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Methods Mol Biol. 2018;1802:11–29. https://doi.org/10.1007/978-1-4939-8546-3_2.
Horie Y, Meguro A, Ohta T, et al. HLA-B51 carriers are susceptible to ocular symptoms of Behçet disease and the association between the two becomes stronger towards the east along the silk road: a literature survey. Ocul Immunol Inflamm. 2017;25(1):37–40. https://doi.org/10.3109/09273948.2015.1136422.
Su G, Zhong Z, Zhou Q, et al. Identification of novel risk Loci for Behçet’s disease-related uveitis in a chinese population in a genome-wide association study. Arthritis Rheumatol. 2022;74(4):671–81. https://doi.org/10.1002/art.41998.
Hamzaoui A, Houman MH, Massouadia M, et al. Contribution of Hla-B51 in the susceptibility and specific clinical features of Behcet’s disease in Tunisian patients. Eur J Intern Med. 2012;23(4):347–9. https://doi.org/10.1016/j.ejim.2011.12.011.
Karasawa R, Yudoh K, Ozaki S, et al. Anti-endothelial cell antibodies (AECA) in patients with systemic vasculitis: our research using proteomics. Expert Opin Biol Ther. 2011;11(1):77–87. https://doi.org/10.1517/14712598.2011.540234.
Souza RC, Lage L, Goldesntein-Schainberg C, et al. Anti-endothelial cell antibodies and central nervous system involvement in Behçet’s disease. Clinics (Sao Paulo). 2007;62(6):685–90. https://doi.org/10.1590/s1807-59322007000600005.
Skef W, Hamilton MJ, Arayssi T. Gastrointestinal Behçet’s disease: a review. World J Gastroenterol. 2015;21(13):3801–12. https://doi.org/10.3748/wjg.v21.i13.3801.
He K, Wu D. The treatment principles and targets for intestinal Behcet’s disease. Therap Adv Gastroenterol. 2023;16:17562848231167284. https://doi.org/10.1177/17562848231167283.
Jang DI, Lee AH, Shin HY, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22052719.
Zhang M, Liu J, Liu T, et al. The efficacy and safety of anti-tumor necrosis factor agents in the treatment of intestinal Behcet’s disease, a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(4):608–19. https://doi.org/10.1111/jgh.15754.
Novak T, Hamedi M, Bergmeier LA, et al. Saliva and serum cytokine profiles during oral ulceration in Behçet’s disease. Front Immunol. 2021;12: 724900. https://doi.org/10.3389/fimmu.2021.724900.
Akkurt ZM, Bozkurt M, Uçmak D, et al. Serum cytokine levels in Behçet’s disease. J Clin Lab Anal. 2015;29(4):317–20. https://doi.org/10.1002/jcla.21772.
Iwaszko M, Biały S, Bogunia-Kubik K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells. 2021. https://doi.org/10.3390/cells10113000.
Nguyen JK, Austin E, Huang A, et al. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res. 2020;312(2):81–92. https://doi.org/10.1007/s00403-019-01972-3.
Dalghous AM, Freysdottir J, Fortune F. Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet’s disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD. Scand J Rheumatol. 2006;35(6):472–425. https://doi.org/10.1080/03009740600905380.
Cingu AK, Turkcu FM, Aktas S, et al. Serum IL-4, IL-12, IL-13, IL-27, and IL-33 levels in active and inactive ocular Behcet’s disease. Int Ophthalmol. 2020;40(12):3441–51. https://doi.org/10.1007/s10792-020-01530-1.
Lin S, Xu Z, Lin Z, et al. Advances in pathogenesis and treatment of ocular involvement in Behcet’s disease. Front Immunol. 2023;14:1206959. https://doi.org/10.3389/fimmu.2023.1206959.
Acknowledgements
We thank the English editor of Editage for polishing the language of this article. We express our gratitude to all the study participants and the members of Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis.
Funding
This study was supported by the Bethune-Puai Medical Research Fund (PAYJ-023).
Author information
Authors and Affiliations
Contributions
F.S., Y.L., J.Z., and X.W. contributed to laboratory testing of biomarkers. All authors were involved in drafting or revising the article critically for important intellectual content and approved the final version to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Peking University People’s Hospital (approval no.2023PHB163-001). All participants included in this study provided written informed content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Sun, F., Li, Y. et al. Profile of immunological biomarkers in Behcet’s syndrome: a large-scale single-center real-world study. Clin Exp Med 24, 201 (2024). https://doi.org/10.1007/s10238-024-01462-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01462-5