Abstract
Purpose
The high prevalence of allergic conjunctivitis in Japan necessitates novel, easy-to-use treatment options for prophylactic use. We evaluated the safety and efficacy of a newly-developed 0.5% epinastine topical eyelid cream to prevent the development of allergic conjunctivitis.
Study design
This was a phase 3, single-centre, double-masked, intra-patient randomised trial in asymptomatic adults (aged 20–65 years) with seasonal allergic conjunctivitis in Japan.
Methods
The left and right eyes of eligible patients were randomised to receive a topical application of either 0.5% epinastine cream (~ 30 mg per dose) to one eye or placebo cream to the other (on the outer skin of the upper and lower eyelids) after a conjunctival antigen challenge (CAC) test. Symptom severity was assessed up to 24 h post-treatment. Primary efficacy endpoints were mean ocular itching and conjunctival hyperaemia severity scores in each eye; safety endpoints included adverse events (AEs) and adverse drug reaction (ADRs).
Results
In total, 30 patients (60 eyes) were included in the study. The 0.5% epinastine topical eyelid cream reduced mean ocular itching scores (difference in least squares means ± standard error, − 1.12 ± 0.214; p < 0.0001) and mean conjunctival hyperaemia scores (− 0.54 ± 0.197; p = 0.0097) 24 h after treatment versus placebo. The 0.5% epinastine topical eyelid cream was well tolerated, with no AEs or ADRs reported.
Conclusion
With its novel route of administration, 0.5% epinastine topical eyelid cream may be considered a unique, easy-to-use, once-daily treatment option to prevent the onset of seasonal allergic conjunctivitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic conjunctivitis refers to a group of disorders caused by perennial and seasonal hypersensitivity, associated with type I hypersensitivity reactions, and accompanied by subjective and objective symptoms [1,2,3]. The most common type is seasonal allergic conjunctivitis, a non-proliferative disease caused by seasonal exposure to airborne antigens and characterised by conjunctival hyperaemia, itching and ocular discharge [1,2,3].
The global prevalence of allergic conjunctivitis varies widely between regions but is particularly high in Japan [4], where the prevalence was recently estimated to be 48.7% [5]. In this study, the most common form of allergic conjunctivitis in Japan was seasonal allergic conjunctivitis (estimated prevalence, 45.4%), caused specifically by Japanese cedar and cypress pollen (37.4%) [5]. Although allergic conjunctivitis is rarely associated with structural damage or permanent vision loss, it is a burdensome condition that negatively impacts daily activities, emotional wellbeing and overall quality of life [6].
International guidelines recognise a stepwise approach to managing allergic conjunctivitis [7], ranging from allergen identification and avoidance, nonpharmacological options and pharmacological therapy for patients with more severe allergic conjunctivitis [1, 2, 8,9,10]. Anti-allergic eye drops are the first-line treatment and include histamine H1-receptor antagonists (e.g. levocabastine, emedastine), mast cell stabilisers (e.g. cromolyn, lodoxamide) and dual-acting agents (e.g. epinastine, ketotifen) [1,2,3, 8, 9]. Tacrolimus or pimecrolimus are available for the treatment of the eyelid skin in atopic dermatitis [7].
In Japan, the recommended dosing frequency for most anti-allergic eye drops is 2–4 times daily; however, many patients with allergic conjunctivitis use these agents on an as-needed basis and only after the onset of symptoms [11, 12]. Eye drop instillation can be challenging, particularly when patients experience visual field loss, feel discomfort, or touch the eye drop bottle to the eye immediately after administration [13,14,15]. Several studies demonstrate the benefits of preventative use (regular use that is compliant with labelled dosing, regardless of symptoms) versus as-needed use of anti-allergy medications for allergic conjunctivitis, including improved symptomatic relief, inflammatory control and quality of life [12, 16], thereby alleviating current symptoms (if present) and reducing the frequency of future exacerbations.
In addition to repositioning anti-allergy medication as a longer-term treatment strategy for allergic conjunctivitis, there remains an unmet clinical need in Japan for an easy-to-use, once-daily dosing, and/or topical application that facilitates prophylactic use and maximises treatment adherence [17]. Epinastine is a dual H1-receptor antagonist and mast cell stabiliser, and previous studies have established that epinastine (0.05%) eye drops are well tolerated in patients with allergic conjunctivitis, and can improve ocular itching and conjunctival hyperaemia symptoms with a duration of action of ≥ 4–8 h [18,19,20,21]. Based on the proven effectiveness of epinastine drops in the eye, we postulated their effectiveness as a topical application to the outer skin of the eyelids. Since there are no topical cream formulations developed to employ transdermal absorption through the eyelids for intended action on the conjunctiva, a 0.5% epinastine topical eyelid cream (development code STN1011402) was developed as a treatment option to be applied onto the upper and lower eyelids for the prevention of allergic conjunctivitis. This novel route of administration delivers epinastine through the skin of the eyelid to the conjunctiva, where it exerts its effect [22].
The aim of the present phase 3 study was to evaluate the safety and efficacy of 0.5% epinastine topical eyelid cream in Japanese patients with seasonal allergic conjunctivitis, recruited during non-seasonal periods.
Materials and methods
Study design
This was a phase 3, double-masked, intra-patient randomised, placebo-controlled trial of 0.5% epinastine topical eyelid cream in patients with a history of seasonal allergic conjunctivitis. The study was conducted at one clinical site (Kitasato University Kitasato Institute Hospital, Tokyo, Japan) between May 13 and July 12, 2022. The study was conducted in compliance with ethical principles based on the Declaration of Helsinki as revised in 2013, standards set forth in the Japanese Pharmaceuticals and Medical Devices Act and Ministerial Ordinance on Good Clinical Practice. The Institutional Review Board reviewed and approved the study protocol before trial commencement, and all patients provided written informed consent to participate. This study is registered with the Japan Registry of Clinical Trials (JRCT ID: jRCT2031220074, available at https://jrct.niph.go.jp/latest-detail/jRCT2031220074).
Study population
Eligible patients were asymptomatic adults (aged 20–65 years) with a history of seasonal allergic conjunctivitis. Key exclusion criteria included comorbid inflammatory eye conditions or dry eye disease on the ocular surface or anterior segment, recent intraocular surgery (≤ 90 days before screening) or recent treatment for lacrimal punctum occlusion (≤ 30 days before screening). Patients treated with oral corticosteroids ≤ 28 days before screening, or treated with anti-allergic drugs, H1-receptor antagonists or non-steroidal anti-inflammatory drugs ≤ 7 days before screening were also excluded. Additional eligibility criteria for this study are provided in Table 1.
Study procedures
Screening period
The study comprised a screening period of ≥ 22 days followed by a treatment period of 2 days (Fig. 1). Eligible patients entered the screening period ≤ 30 days after providing written informed consent; if the screening period began > 30 days after the date of consent, written informed consent was re-collected.
At the start of the screening period (visit 1), eligible patients were tested for Japanese cedar pollen allergy using a serum antigen-specific immunoglobulin E (IgE) antibody test; those who tested negative were excluded from the study. At visit 2 (21 days before the start of the treatment period), conjunctival allergen challenge (CAC) tests were performed to determine the optimal concentration of allergen solution required to trigger allergic conjunctivitis symptoms.
Before the CAC tests, allergen solutions containing Japanese cedar pollen extract (25-fold, 50-fold, 100-fold and 200-fold dilutions) and negative control solutions containing glycerine (25-fold dilution) were prepared (Torii Pharmaceutical Co., Ltd.). All diluted allergen solutions contained glycerine. At visit 2, one drop of negative control solution (without allergen) was instilled in each eye, and patients with a conjunctival inflammatory response were excluded from the study.
At visit 3 (14 days before the start of the treatment period), a confirmatory CAC test was performed using the optimal allergen concentration determined at visit 2. After antigen challenge in both eyes, the severity of ocular itching was assessed at 3, 5 and 10 min, and the severity of bulbar conjunctival hyperaemia was assessed at 5, 10 and 20 min. The optimal allergen concentration was confirmed if ocular itching and bulbar conjunctival hyperaemia severity scores were ≥ 2 in each eye. Throughout the screening period, patients were required to show no signs or symptoms of ocular itching or conjunctival hyperaemia before the CAC tests, and those unable to achieve ocular itching and bulbar conjunctival hyperaemia severity scores ≥ 2 in both eyes after antigen challenge were excluded from the study.
Treatment period
On day 1 of the treatment period (visit 4), the left and right eyes of each patient were randomised to receive either 0.5% epinastine topical eyelid cream (approximately 30 mg) [23] or placebo (base cream without epinastine hydrochloride). Eyes were randomly assigned to study cream or placebo using a permuted block method with a block size of 4. Because this was a double-masked study, the study cream and placebo were packaged in identical tubes, and patients and investigators were masked to the treatment allocations for each eye.
At visit 4, study investigators instructed patients to draw a single dose (approximately 1.3 cm of cream as a guide) of 0.5% epinastine topical eyelid cream/placebo cream onto the fingertip to apply onto the periocular area (outer skin of the upper and lower eyelids) of the assigned eye. On day 2 of the treatment period (visit 5; 24 h after application of the study cream and placebo), a CAC test was performed in each eye using the optimal allergen concentration that was determined during the screening period. The severity of ocular itching and bulbar and palpebral conjunctival hyperaemia were assessed after antigen challenge as performed at Visit 3 (Table 2).
Outcome measures
The primary efficacy endpoints were a 3-time point mean ocular itching score (the average of scores obtained at 3, 5 and 10 min after the antigen challenge) and a 3-time point mean conjunctival hyperaemia score (sum of the bulbar and palpebral conjunctival hyperaemia scores; averaged over 5, 10 and 20 min after the antigen challenge) in each eye on day 2 of the treatment period (24 h after treatment application, in line with the expected once daily administration). Secondary efficacy endpoints included the mean ocular itching, conjunctival hyperaemia, palpebral conjunctival hyperaemia and bulbar conjunctival hyperaemia scores over time during day 2. Treatment effects were assessed by performing CAC tests 24 h after the single-dose application of the study cream and placebo. Safety endpoints included the incidence and severity of adverse events (AEs) and adverse drug reactions (ADRs) during the treatment period, and intraocular pressure (IOP) and funduscopy assessments throughout the screening and treatment periods.
Statistical analysis
A sample size of 30 patients (60 eyes) was estimated to provide 90% power to detect the superiority of 0.5% epinastine topical eyelid cream versus placebo for the primary endpoints. This was calculated assuming mean ± standard deviation (SD) differences in ocular itching and conjunctival hyperaemia scores of 1.0 ± 0.5 and 1.0 ± 1.6, respectively, paired t-tests, type I error rate of 5% and power of 90%.
Baseline demographics and clinical characteristics (measured at visit 1) were based on the intention to treat (ITT) population and summarised using descriptive statistics (means, SDs, medians, ranges, number of patients and/or proportions as appropriate). Efficacy analyses were based on the full analysis set (FAS), defined as all randomised patients who had received ≥ 1 application of the study cream or placebo and for whom efficacy data were available.
Primary efficacy endpoints were summarised using least squares (LS) means, standard errors (SE), mean differences and 95% confidence intervals (CIs). Secondary efficacy endpoints were summarised using means and SDs. The analysis of the primary endpoints was performed on data from the FAS using a linear mixed-effect model, with treatment as the fixed effect and patient as the random effect.
The safety analysis population included all patients who had received ≥ 1 application of study cream or placebo and had available safety data. Safety was assessed through descriptive summaries of AEs, ADRs, IOP and fundoscopy findings by treatment group. AEs and ADRs were coded using the Medical Dictionary for Regulatory Activities’ thesaurus terms and summarised by Preferred Term and System Organ Class. Statistical analyses were performed using SAS version 9.4 or later, and efficacy analyses used a two-tailed significance level of α = 0.05.
Results
Study population
In total, 30 patients with a history of seasonal allergic conjunctivitis provided written consent, completed the screening period and were randomised to receive study cream or placebo in each eye (ITT population). All 30 patients received ≥ 1 application of study cream or placebo (Fig. 2). At the start of the screening period, the mean ± SD age of the patients was 43.7 ± 9.9 years, 17/30 (57%) patients were men and by design, all 30 patients tested positive for Japanese cedar pollen allergy (Table 3).
Efficacy of 0.5% epinastine topical eyelid cream
On day 2 of the treatment period (24 h after application of study cream and placebo), CAC tests showed that 0.5% epinastine topical eyelid cream was superior versus placebo in reducing mean ocular itching and conjunctival hyperaemia severity scores after antigen challenge (Table 4). The LS mean ± SE ocular itching score (averaged over 3, 5 and 10 min) was significantly reduced in 0.5% epinastine-treated eyes compared with placebo-treated eyes (0.71 ± 0.160 vs. 1.83 ± 0.160; difference − 1.12 ± 0.214; p < 0.0001). Similarly, the LS mean ± SE conjunctival hyperaemia score (averaged over 5, 10 and 20 min) was significantly lower in eyes treated with 0.5% epinastine topical eyelid cream versus placebo (2.34 ± 0.278 vs. 2.89 ± 0.278; difference − 0.54 ± 0.197; p = 0.0097).
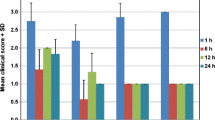
In the secondary endpoint analyses, the mean difference in ocular itching scores (Fig. 3a) at 3, 5 and 10 min after antigen challenge on day 2 were − 0.83 (95% CI − 1.315, − 0.352), − 1.33 (95% CI − 1.855, − 0.811) and − 1.20 (95% CI − 1.704, − 0.696), respectively, and the mean difference in conjunctival hyperaemia scores (Fig. 3b) at 5, 10 and 20 min after antigen challenge were − 0.57 (95% CI − 0.929, − 0.204), − 0.70 (95% CI − 1.172, − 0.228) and − 0.37 (95% CI − 0.943, − 0.209), respectively. The mean difference in bulbar conjunctival hyperaemia scores at 5, 10 and 20 min (Fig. 4a) were − 0.47 (95% CI − 0.701, − 0.232), − 0.50 (95% CI − 0.806, − 0.194) and − 0.30 (95% CI − 0.656, 0.056), respectively, and the mean difference in palpebral conjunctival hyperaemia scores at 5, 10 and 20 min (Fig. 4b) were − 0.10 (95% CI − 0.279, 0.079), − 0.20 (95% CI − 0.428, 0.028) and − 0.07 (95% CI − 0.343, 0.210), respectively. When these scores were considered separately, the effect of 0.5% epinastine topical eyelid cream on reducing conjunctival hyperaemia severity was mostly driven by lower bulbar conjunctival hyperaemia scores among 0.5% epinastine-treated versus placebo-treated eyes.
Antigen challenge data showing mean (a) ocular itching scores and (b) conjunctival hyperaemia scores of epinastine-treated eyes and placebo-treated eyes. The filled circles and empty circles represent the scores measured at each time point for epinastine-treated eyes and placebo-treated eyes, respectively. The graphs to the right in panels (a) and (b) with the filled squares represent the mean difference between the scores measured at each time point for epinastine-treated eyes and placebo-treated eyes. CI, confidence interval; SD, standard deviation
Antigen challenge data showing mean (a) bulbar conjunctival hyperaemia scores and (b) palpebral conjunctival hyperaemia scores of epinastine-treated eyes and placebo-treated eyes. The filled circles and empty circles represent the scores measured at each time point for epinastine-treated eyes and placebo-treated eyes, respectively. The graphs to the right in panels (a) and (b) with filled squares represent the mean difference between the scores measured at each time point for epinastine-treated eyes and placebo-treated eyes. CI, confidence interval; SD, standard deviation
Safety of 0.5% epinastine topical eyelid cream
In this study, 0.5% epinastine topical eyelid cream was well tolerated by all patients. No AEs or ADRs of any severity were observed during the treatment period. IOP and fundoscopy assessments throughout the study found no clinically relevant changes after application of either 0.5% epinastine topical eyelid cream or placebo.
Discussion
The benefits demonstrated in this phase 3 study of epinastine 0.5% formulated as a cream for topical application to the outer eyelids are consistent with other published trials of epinastine 0.05% or 0.1% eye drops in patients with allergic conjunctivitis, which show that treatment was well tolerated and significantly reduced ocular itching and conjunctival hyperaemia severity after allergen exposure [18,19,20,21, 24]. In an environmental trial, epinastine eye drops were instilled twice daily for 8 weeks [18], while in CAC studies, epinastine was instilled 15 min, 4–8 h before antigen challenge [19,20,21]. Collectively, these studies found that epinastine eye drops demonstrated sustained treatment effect of ≥ 4–8 h (equivalent to 2–4 times daily dosing) [18,19,20,21]. In comparison, 0.5% epinastine topical eyelid cream demonstrated sustained treatment effects 24 h after application to the outer skin of the upper and lower eyelids. These data suggest that the study cream exhibits an extended duration of action to alleviate allergic conjunctivitis symptoms. In addition to the convenience of a once-daily dosage frequency, 0.5% epinastine topical eyelid cream is suitable because of its relative ease of application to the skin of the outer eyelids (rather than the challenge of being instilled into the eye) and can be used proactively as a prophylactic for allergic conjunctivitis.
In this study, the efficacy of 0.5% epinastine topical eyelid cream was assessed using CAC tests, developed as an alternative to, but not a complete substitute for, environmental studies of allergic conjunctivitis [13, 25]. Advantages of CAC tests over environmental studies include the ability to reproduce the signs and symptoms of allergic conjunctivitis in a controlled setting, the transient nature of symptoms after antigen challenge and the level of internal control provided by bilateral administration of study drugs and comparator/placebo [13, 25]. On the other hand, environmental trials allow the effectiveness of ocular anti-allergy agents to be studied in a setting that most accurately reflects real-world clinical practice [13, 25]. In support of the results of the present study, once-daily 0.5% epinastine topical eyelid cream has shown sustained efficacy when administered for 8 weeks in patients with allergic conjunctivitis, with significant reductions in ocular pruritus and conjunctival hyperaemia scores observed from Week 1 of administration and no safety concerns identified. A 0.1% epinastine eye drops formulation (instilled twice daily) was used as a reference; similar efficacy and safety were observed with both formulations, and patients considered 0.5% epinastine topical eyelid cream easy to use and apply and less burdensome than 0.1% epinastine eye drops [24, 26]. Epinastine 0.5% eyelid cream has received approval in Japan to treat and prevent allergic conjunctivitis; the easy-to-use topical formulation may be particularly beneficial in people who find eye drop administration burdensome, and for those with dexterity difficulties [24, 27].
Study limitations included assessment of safety and efficacy only after a single application (i.e. consecutive daily dosing was not evaluated), subjective outcomes (e.g. ease of application, cosmetic acceptability) were not considered, and 0.5% epinastine topical eyelid cream was not directly compared with anti-allergic eye drops that currently represent the standard of care [1, 2, 8,9,10]. These limitations may be addressed by future studies including the ongoing phase 3 environmental trial, which will evaluate 0.5% epinastine topical eyelid cream, applied once daily for 8 weeks, in approximately 180 patients with allergic conjunctivitis [26].
A key strength of this study is that it was the first to evaluate a once-daily, topical ophthalmic cream (containing a known anti-allergy agent, epinastine) in a population that represents the most prevalent form of allergic conjunctivitis in Japan [5]. Although previous studies have assessed the efficacy of once-daily olopatadine [28], alcaftadine [29] and bilastine [30] eye drops in patients with allergic conjunctivitis, these treatment options are not currently available in Japan. In addition, some of these studies evaluated effectiveness using 16-hour CAC tests [28,29,30,31,32,33], suggesting that such agents may not provide full 24-hour symptom control (e.g. during sleep). Regardless, the difficulty and discomfort of administering eye drops may lead to poor instillation techniques, suboptimal or missed doses, increased risk of infection and reduced therapeutic benefit [14, 15, 34]; all of which could be avoided by a topically-administered ophthalmic cream that can be easily applied to the eyelids. Thus, in conjunction with its relative ease of application, the results of the current study suggest that 0.5% epinastine topical eyelid cream is a convenient, safe, and effective treatment option for patients with simulated allergic conjunctivitis.
In conclusion, the primary endpoint of reduced ocular itching and conjunctival hyperaemia severity was achieved in this phase 3 study. Superiority of 0.5% epinastine topical eyelid cream was demonstrated over the placebo cream up to 24 h after applying 0.5% epinastine topical eyelid cream to the outer skin of the upper and lower eyelids, suggesting that it is suitable for once-daily use in patients with seasonal allergic conjunctivitis. Moreover, 0.5% epinastine topical eyelid cream displayed an acceptable safety profile in the context of this study, with no AEs, ADRs or clinically relevant changes in ocular assessments reported. Together, these data suggest that 0.5% epinastine topical eyelid cream, via its novel route of administration, may address an unmet clinical need for a convenient, easy-to-use, once-daily anti-allergy medication for the long-term prevention of allergic conjunctivitis. Future studies are recommended to develop epinastine 0.5% ophthalmic cream further.
References
Miyazaki D, Fukushima A, Uchio E, Shoji J, Namba K, Ebihara N, et al. Executive summary: Japanese guidelines for allergic conjunctival diseases 2021. Allergol Int. 2022;71:459–71.
Japanese society of ophthalmic allergy clinical practice guideline development committee. Clinical practice guidelines for allergic conjunctival diseases (3rd edition). Nippon Ganka Gakkai Zasshi. 2021;125:741–85. (in Japanese).
Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5.
Miyazaki D, Fukagawa K, Okamoto S, Fukushima A, Uchio E, Ebihara N, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. 2020;69:487–95.
Miyazaki D, Fukagawa K, Fukushima A, Fujishima H, Uchio E, Ebihara N, et al. Air pollution significantly associated with severe ocular allergic inflammatory diseases. Sci Rep. 2019;9:18205.
Zhang SY, Li J, Liu R, Lao HY, Fan Z, Jin L, et al. Association of allergic conjunctivitis with health-related quality of life in children and their parents. JAMA Ophthalmol. 2021;139:830–7.
Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Annals of Allergy, Asthma & Immunology. 2020;124:118 – 34.
Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia Ferrer FJ, et al. Conjunctivitis preferred practice pattern. Ophthalmology. 2019;126:94–169.
Leonardi A, Silva D, Perez Formigo D, Bozkurt B, Sharma V, Allegri P, et al. Management of ocular allergy. Allergy. 2019;74:1611–30.
Chan VF, Yong AC, Azuara-Blanco A, Gordon I, Safi S, Lingham G, et al. A systematic review of clinical practice guidelines for infectious and non-infectious conjunctivitis. Ophthalmic Epidemiol. 2022;29:473–82.
Fukagawa K, Kishimoto H, Shoji J, Fukushima A, Hori Y. Effects of the compliance of anti-histamine ophthalmic solutions on quality of life in patients with seasonal allergic conjunctivitis using web questionnaire. Allergy Pract. 2019;39:825–37. (in Japanese).
Fukushima A, Miyazaki D, Kishimoto H, Ebihara N, Proactive Study Group. Efficacy of proactive topical antihistamine use in patients with seasonal allergic conjunctivitis. Adv Ther. 2022;39:5568–81.
Abelson MB, Loeffler O. Conjunctival allergen challenge: models in the investigation of ocular allergy. Curr Allergy Asthma Rep. 2003;3:363–8.
Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127:732–6.
Tatham AJ, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye (Lond). 2013;27:1293–8.
Juniper EF, Guyatt GH, Ferrie PJ, King DR. Sodium cromoglycate eye drops: regular versus as needed use in the treatment of seasonal allergic conjunctivitis. J Allergy Clin Immunol. 1994;94:36–43.
Carr W, Schaeffer J, Donnenfeld E. Treating allergic conjunctivitis: a once-daily medication that provides 24-hour symptom relief. Allergy Rhinol (Providence). 2016;7:107–14.
Whitcup SM, Bradford R, Lue J, Schiffman RM, Abelson MB. Efficacy and tolerability of ophthalmic epinastine: a randomized, double-masked, parallel-group, active- and vehicle-controlled environmental trial in patients with seasonal allergic conjunctivitis. Clin Ther. 2004;26:29–34.
Abelson MB, Gomes P, Crampton HJ, Schiffman RM, Bradford RR, Whitcup SM. Efficacy and tolerability of ophthalmic epinastine assessed using the conjunctival antigen challenge model in patients with a history of allergic conjunctivitis. Clin Ther. 2004;26:35–47.
Fujishima H, Ohashi Y, Takamura E. Efficacy of epinastine hydrochloride ophthalmic solution in allergic conjunctivitis by conjunctival cedar pollen allergen challenge. Ann Allergy Asthma Immunol. 2014;113:476–81.
Tagawa Y, Namba K, Nakazono Y, Iwata D, Ishida S. Evaluating the efficacy of epinastine ophthalmic solution using a conjunctivitis allergen challenge model in patients with birch pollen allergic conjunctivitis. Allergol Int. 2017;66:338–43.
Mochizuki T, Hata T, Mori N, Yamazaki T, Noto T, Mano H. Trans-eyelid distribution of epinastine to the conjunctiva following eyelid application in rabbits. Jpn J Ophthalmol Published Online May. 2024;25. https://doi.org/10.1007/s10384-024-01070-6.
Alesion eyelid cream. Package insert. Santen pharmaceutical Co., Ltd. 2024. (in Japanese) https://www.info.pmda.go.jp/go/pack/1319762N1021_1_01
Shoji J, Fujishima H. Open-label long-term administration study of epinastine hydrochloride eyelid cream 0.5% in patients with allergic conjunctivitis: Phase III. Ganka. 2024;66:267–78. (in Japanese).
Abelson MB. Comparison of the conjunctival allergen challenge model with the environmental model of allergic conjunctivitis. Acta Ophthalmol Scand. 1999;77:38–42.
National Institute of Public Health. Phase 3 study of STN1011402 ophthalmic cream in patients with allergic conjunctivitis. 2022. https://rctportal.niph.go.jp/en/detail?trial_id=jRCT2031210639. Accessed March 21, 2023.
Santen pharmaceutical Co., Ltd. Santen obtains manufacturing and marketing approval in Japan for Alesion® eyelid cream (epinastine hydrochloride), a new treatment for allergic conjunctivitis. 2024. https://www.santen.com/en/news/2024/2024_1/20240326. Accessed April 15, 2024.
Torkildsen G, Narvekar A, Bergmann M. Efficacy and safety of olopatadine hydrochloride 0.77% in patients with allergic conjunctivitis using a conjunctival allergen-challenge model. Clin Ophthalmol. 2015;9:1703–13.
Greiner JV, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%. Clin Ophthalmol. 2011;5:87–93.
Gomes PJ, Ciolino JB, Arranz P, Hernández G, Fernández N. Bilastine 0.6% preservative-free eye drops, a once-daily treatment for allergic conjunctivitis. J Investig Allergol Clin Immunol. 2023;34.
Gomes PJ, Ciolino JB, Arranz P, Hernández G, Fernández N. Efficacy of once-daily ophthalmic bilastine for the treatment of allergic conjunctivitis: a dose-finding study. J Investig Allergol Clin Immunol. 2023;33:271–80.
Ciolino JB, McLaurin EB, Marsico NP, et al. Effect of alcaftadine 0.25% on ocular itch associated with seasonal or perennial allergic conjunctivitis: a pooled analysis of two multicenter randomized clinical trials. Clin Ophthalmol. 2015;9:765–72.
McLaurin EB, Marsico NP, Ackerman SL, et al. Ocular itch relief with alcaftadine 0.25% versus olopatadine 0.2% in allergic conjunctivitis: pooled analysis of two multicenter randomized clinical trials. Adv Ther. 2014;31:1059–71.
Spencer SKR, Shulruf B, McPherson ZE, Zhang H, Lee MB, Francis IC, et al. Factors affecting adherence to topical glaucoma therapy: a quantitative and qualitative pilot study analysis in Sydney, Australia. Ophthalmol Glaucoma. 2019;2:86–93.
Acknowledgements
The study sponsor (Santen Pharmaceutical Co., Ltd.) participated in the study design; the collection, analysis and interpretation of data; and writing the report. The authors would like to thank Yuko Tone, Rie Ishii and Toshihiro Ikeda, of Santen Pharmaceutical Co., Ltd., for their contributions to the study design and conduct, statistical analysis and interpretation of results, and the members of the Japan Medical Affairs at Santen Pharmaceutical Co., Ltd. for their contribution to the content development for the manuscript, including the discussion section. The authors also thank Karina Hamilton-Peel, PhD, CMPP, of inScience Communications, Springer Healthcare, who wrote the outline and subsequent drafts of this manuscript and Nireshnee Ramchundar, PhD, of inScience Communications, Springer Healthcare who assisted with post-submission revisions. This medical writing assistance was funded by Santen Pharmaceutical Co., Ltd.
Funding
This work and editorial assistance for the preparation of this article were supported by Santen Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Fujishima, Grants to the author’s institution (Santen), Research grant (White Medical, Kobayashi), Consulting fees (Santen), Lecture fees (Santen, Senju, Otsuka), Payment for expert testimony (Santen, Kobayashi); J. Shoji, Grants to the author’s institution (Santen), Consulting fees (Santen); Lecture fees (Santen, Senju, AbbVie, Rohto Nitten).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Hiroshi Fujishima
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fujishima, H., Shoji, J. Safety and efficacy of a novel 0.5% epinastine topical eyelid cream in allergic conjunctivitis: a phase 3 trial. Jpn J Ophthalmol (2024). https://doi.org/10.1007/s10384-024-01108-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10384-024-01108-9