Abstract
The efficiency of triple-plasmid transfection in recombinant Adeno-Associated Virus (rAAV) production was analyzed by examining two distinct HEK-293 cells lines. These were categorized as high producer (HP) and low producer (LP) based on their differing levels of productivity under identical conditions. Analysis of RNA expression levels of viral genes revealed disparities in plasmid derived gene expression between the cell lines. Further assessment of transfection efficiency utilizing labeled plasmids revealed lower plasmid uptake and less efficient nuclear transport in LP cell line. Additionally, we observed inferior translation activity in LP, contributing to its shortcomings in overall productivity. In our attempt to optimize plasmid ratios to enhance fully packaged rAAV particle yield, we discovered cell-line-specific optimization potential. The findings highlight the transfection's complexity, urging tailored strategies for improved rAAV production based on each cell line's characteristics, enhancing understanding and guiding further efficiency optimization in rAAV production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of viral vectors for gene therapy applications have evidently been shown for the past decades (Samulski and Shenk 1988, Rolling and Samulski 1995, Bulcha et al. 2021). One of them is the adeno-associated virus (AAV), a non-enveloped single-stranded DNA virus of the parvoviridae family, which requires the presence of adenovirus derived helper genes, to allow for replication (Samulski and Shenk 1988; Rolling and Samulski 1995). Mammalian cell lines became well-established cell factories to produce complex biomolecules (Durocher and Butler 2009; Khan 2013; Estes and Melville 2014; Akita et al. 2015). As an example of such cell lines, the human embryonic kidney cell 293 (HEK-293) line is widely utilized for various approaches in biotechnology in general, including recombinant protein or viral vector production due to its safety, efficiency, and secretory properties (Ooi et al. 2016, Tan et al. 2021).
To transfer research-based approaches into successful clinical applications, the manufacturing of sufficient quantities of rAAV is still one major challenge (Srivastava et al. 2021). Transient transfection of producer cells for viral vector production can be obtained by different methods, such as lipofection, electroporation or with the help of chemical transfection agents, and is an established standard in the industry today (Wright 2009). This first step in viral vector production is not only crucial for obtaining high overall yield, but additionally, for attaining suitable vector genome-to-capsid ratio. These are important quality and safety attributes and essential for maximizing yield, thus, enhancing the sustainability of gene therapy products in clinical applications (Li et al. 2020, Fus-Kujawa et al. 2021). However, the full process of transfection, including cellular uptake, intracellular trafficking, endosomal escape, and nuclear entry is still not fully understood. The complexity of transient transfection escalates even more with increasing numbers of plasmids being transfected, affecting not only yield and quality of the product, but also influences scalability and reproducibility of the manufacturing process (Wright, 2009). Multiple studies have been conducted to improve the transfection conditions with success (Grieger et al. 2016; Srivastava et al. 2021; Torabfam et al. 2022), measuring marker genes or immunofluorescence labeled proteins only as final outcome, but there is little known about the efficiency of the steps in between, especially for triple transfections in HEK-293 cells as used in gene therapy manufacture.
Delivering genetic material to the cells therefore remains a bottleneck in the production of recombinant viral particles, and its success not only depends on plasmid design and size, but also on the utilized reagents and the physiological status of the cells (Godbey et al. 1999, Hornstein et al. 2016, Rosazza et al. 2016). The prevailing method applied in the industry is transient triple transfection via cationic chemicals such as polyethyleneimine (PEI), due to its flexibility and simplicity (Nguyen et al. 2021).
In this study, we aimed to compare the transfection efficiency of two distinct HEK-293 cell lines, as previously characterized through transcriptome analysis (Pistek et al. 2023). Based on their capacity to produce viral vector particles, they were termed high (HP) and low (LP) producers. Our earlier published data indicated variations in their regulation of intracellular pathways before and during AAV production, as determined by gene set enrichment analysis. Previous findings showed that both cell line differ in various cellular pathways, such as ATP synthesis, electron transport, ER stress, unfolded protein response, and sterol biosynthesis, and further investigation of their viral gene expression suggested additional potential differences in their general transfectability. Hence, in this study we employed labeled plasmids via transient transfection using cationic chemicals to examine the cell-line-specific efficiency of plasmid uptake and transport to the nucleus. Numerous differences emerged throughout the transfection process, aligning with observed disparities in overall productivity.
Materials and methods
Cell culture, fermentation and transfection
HEK-293 cells were thawed and expanded in suspension as described in (Pistek et al. 2023). After 12 to 17 days, cells were inoculated into 125 ml baffled Erlenmeyer Flasks (Corning, Germany) or Nalgene™ PETG Erlenmeyer Flasks with plain bottom (ThermoScientific, US, MA) at a density of 4.0 × 106 cells/mL and further incubated at 150 rpm (2.5 cm throw) at + 37 °C and with 5% CO2 gassing. A triple-plasmid transfection containing Adenovirus 5 Helper genes, Rep from AAV2, Cap from AAV8 and a Factor IX transgene sequence, respectively, was performed using Polyethylenimine (Merck, Germany) at a 1:2.3 DNA to PEI ratio following the manufacturer’s instructions. For our standard condition (T), a total of 1.2 µg/ml DNA was used in a molar ratio of RepCap:Helper:Transgene = 1.00:0.47:0.73. Samples for further analyses were taken at 0 h, 4 h, 24 h, 48 h, and 72 h post transfection.
Plasmid labeling
Helper, Transgene and RepCap plasmids, each were backbone-labeled with an individual fluorescent dye, MFP488, Cy3® or Cy5® (Label IT® Nucleic Acid Labeling Kit, Mirius Bio, WI, USA), respectively, according to the manufacturer’s instruction. After 1 h of incubation at + 37 °C, plasmids were purified and recovered using ethanol precipitation: to the respective reaction volume, 0.1 volume 5 M sodium chloride was added, following precipitation with 2.5 volumes 96% ethanol (Merck, Germany) at − 20 °C for 20 min. After centrifugation (25 min, 14,500 × g, + 4 °C), the supernatant was removed and the pellet was resuspended in sterile nucleic acid-free water (Invitrogen™, MA, USA). Concentration was determined via spectrophotometric analysis (Nanodrop, ThermoFisher, NY, USA) and diluted to a final concentration of 1 mg/ml for subsequent use or storage at − 20 °C.
Cell fixation
For each sample time point, approximately 7.0 x 106 cells were harvested and centrifuged at 500 × g for 5 min. Supernatant was discarded and the pellet was washed once with PBS. Cells were then fixed for 20 min at room temperature using Intracellular (IC) Fixation Buffer (Invitrogen™, MA, USA) and centrifuged for 5 min at 500 × g. After resuspension in 1 ml PBS, cells were stored at + 4 °C for further analyses.
Nucleus isolation
Approximately 7.0 x 106 cells were harvested and centrifuged at 500 × g for 5 min. Pellet was resuspended carefully in 200 µl of Lysis Buffer, containing 10 mM Tris–HCl adjusted to pH 7.4, 10 mM NaCl, 3 mM MgCl2 and 0.025% NP-40 Alternative (Merck, Germany) in PBS and incubated for 20 min on ice. After centrifugation at 650 × g for 5 min, the nuclei pellet was fixed for 15 min, using IC Fixation Buffer followed by resuspension in PBS containing 1% BSA. Samples were stored at + 4 °C.
Analytics
Flow cytometry analysis
Flow cytometry was performed with the CytoFLEX Flow Cytometer (Beckman Coulter, IN, USA) and evaluated with the according Acquisition and Analysis Software CytExpert 2.5. Fluorescence intensity was measured using the following lasers and filter settings: 4',6-diamidino-2-phenylindole (DAPI) (Ex: 405 nm, Em: 780/60 BP); GFP (Ex: 488 nm, Em: 525/40 BP); Cy3® (Ex: 488 nm, Em: 585/42 BP); Cy5® (Ex: 638 nm, Em: 660/10 BP). A compensation matrix for GFP and Cy3® channels was established and applied for all measurements, and a negative selection on DAPI positive cells in unfixed samples was used to determine viable cells. Analysis was performed according to the following parameters: Forward Scatter-Area (FSC-A) for particle size and Side Scatter-Area (SSC-A) for the granularity or complexity of the cells. A total number of 10,000 events were selected using the ratio FSC-A to Forward Scatter-Height (FSC-H) to identify and exclude cell aggregates, thereby selecting for singlets. For nucleus analysis, nuclei presence was confirmed through positive DAPI staining on fixed isolates, and 5,000 events were chosen for subsequent analysis. In all experiments, mock transfected cells were used as negative control.
Multiple plasmid copy transfection rate per cell was calculated using Poisson distribution and used for normalization and subsequent calculation of ratios between cellular and nuclear transfection rates.
Digital droplet PCR (ddPCR)
For vector genome quantification, a Bio-Rad based droplet digital PCR method was used, applying a fully automated QX One System or automated QX 200 AutoDG system. For supernatant (SN) probes, cells were centrifuged at 5,000 × g for 5 min and supernatant was stored at − 80 °C until further analysis. Thawed rAAV8 capsids were treated with DNase I (NEB) to remove extraneous DNA sequences. Encapsulated DNA was then released via induced heat shock during the initial PCR cycle. After the droplet generation, PCR was performed with Bio-Rad ddPCR Supermix (no dUTP) and transgene-specific primers and probe:—Fwd 5’-GGC ATC TAC ACC AAA GTC TCC AG -3’, Rev 5’-CAG CGA GCT CTA GGC ATG CT -3’, probe 5’-6FAM-AGA CCA AGC TGA CCT GAT-MGBNFQ -3’. Vector genome concentration was calculated by the QX Manager.
Enzyme-linked immunosorbent assay (ELISA)
A monoclonal antibody (ADK8) specific for a conformational epitope on assembled AAV8 capsids was utilized in the Progen AAV8 Titration ELISA Kit (Catalog No. PRAAV8), according to the manufacturer’s instructions. The streptavidin peroxidase-induced color reaction was measured photometrically at 450 nm. A provided internal control of empty rAAV8 particles was used as standard to determine particle concentration.
Transcriptome analysis
Transcriptome data used to support this study was retrieved from our previous study (Pistek et al. 2023).
Statistics
Figures were analyzed with GraphPad Prism® v9.2.0. For sequencing data analyses comparing the two cell lines, a 2-way ANOVA (Šídák’s multiple comparison) test was performed from 4 independent fermentation batches (n = 4). p ≤ 0.05 were considered as statistically significant and described in figures as follows: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. This approach allowed us to control the familywise error rate when performing multiple tests.
Results
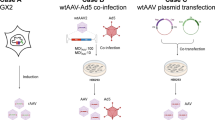
In this study, we compared the efficiency of a triple plasmid transfection (Fig. 1A) during rAAV production in two different HEK-293 cell lines. We selected the Factor IX transgene for our studies due to its small size and, consequently, its rapid transcription and efficient packaging into pre-formed AAV particles. In our prior research, we demonstrated that even when transfected with equal amounts of plasmid DNA and cultivated under the same conditions, different cell lines exhibited varying levels of productivity (Pistek & Kahlig et al. 2023). The previous study showed that the high producer cell line (HP) not only secreted 2.8 times more capsids, but also had 2.2 times more transgene DNA encapsulated as compared to the low producer cell line (LP). Since transient DNA transfection is a multistep process as shown in Fig. 1B, we aimed to identify bottlenecks and eventually shortcomings for the low producer cell line. Our goal was to separately track each of the three plasmids to provide insight into how to optimize plasmid ratios for future work. By tailoring these ratios according to their respective transfectability, we aimed to potentially improve overall yield and encapsulation of transgenes.
Schematic illustration of the transfection process used for rAAV production in Gene Therapy. Created with BioRender.com. A Triple plasmid transfection containing AdV5-derived helper (pink), ITR-flanked transgene (blue) and AAV RepCap (yellow) plasmids for rAAV production. Additionally, utilized producer cells carry two stably integrated AdV 5 genes. B Transfection reagent forms complex with plasmid DNA, attaches to the cell surface and enters the cell via endocytosis. After endosomal escape, nuclear entry is mediated to initiate transcription and subsequent protein translation
Viral plasmid-derived genes are higher expressed in high producer (HP) compared to low producer (LP) cell line
We investigated the RNA expression levels of viral genes using 3’MACE sequencing analysis as described by (Pistek & Kahlig et al. 2023), and compared the Rep (AAV2) and Cap (AAV8) associated viral genes and in addition, both, cell-line integrated and plasmid-derived adenoviral helper genes at different timepoints post-transfection (0 h, 4 h, 24 h, 48 h, 72 h). For quantitative comparison, we normalized log2 transformed data on counts per million (CPM). Expression data of viral rep and cap RNA reflect pre-mRNA transcripts in this analysis, including all splicing variants (used construct harbors Rep-AAV2 and Cap-AAV8). As depicted in Fig. 2A, we observed that plasmid-derived genes were significantly higher expressed in HP as in LP, whereas the viral genes which are stably integrated into the HEK-293 cells showed comparable levels in both cell lines. While the expression levels of AAV2- and AAV8-derived RepCap genes were apparent from 24 to 72 h post-transfection, the most significant variation between HP and LP occurred at 48 h for both, AAV2 (mean difference 4869 CPM) and AAV8 (mean difference 10,910 CPM) transcripts. Similar, plasmid-derived helper genes were significantly higher expressed in HP, showing highest divergence already at 24 h post transfection. A representative subset of these genes can be seen in Fig. 2B (DBP mean diff. 3.321, p < 0.0001; E4 ORF1 mean diff. 1.870, p = 0.0179; E4 ORF4 mean diff. 1.570, p = 0.0023).
Comparison of viral gene expression in two HEK-293 cell lines based on transcriptome sequencing data. A Plasmid-derived genes of the Rep and Cap reading frames are significantly differentially expressed in high (HP) and low producer (LP) cells, as shown in normalized sequencing counts per million (CPM). Significance according to 2-way ANOVA test (n = 4), 95% CI of diff., **** p ≤ 0.0001; *** p ≤ 0.0005. B Comparison of plasmid-derived and cell line-integrated helper genes, y-axis shows normalized log2 transformed CPM
In contrast, for the cell line-integrated E1B and E1A HAdV-5 helper genes, no significant differences of expression levels were observed between the two HEK-293 lines, indicating that the differences in their productivity to produce AAV particles are highly influenced by their ability to express plasmid-derived genes. Possible explanations for those findings include reduced transfectability of the LP cell line, impairments in the cellular machinery to transport foreign DNA constructs to the nucleus and its subsequent transcription, or a combination of these factors.
To summarize, our findings indicate a consistent under-representation of all viral transcripts originating from plasmids in LP when compared to HP. This observation held true for all transiently expressed viral genes, irrespective of the originating plasmid. Conversely, the expression of viral genes stably integrated into the host cell genome exhibited no significant difference between the two cell lines. To elucidate the lower expression of transfected viral genes, we aimed to determine whether LP exhibits only lower transcription rates or encounters additional limitations in plasmid uptake.
LP cell line takes up fewer plasmids in comparison to HP and has less transfected DNA available in the nucleus
To investigate transfection efficiency, we transfected our two cell lines with our standard transfection condition T as previously described, and used labeled plasmids, which can be traced individually based on their respective dye. Since the three dyes have different intensities, total amount of positive cells may vary when we change the dye for each plasmid. We would like to emphasize that with this method, we cannot draw any conclusions about the absolute transfection efficiency when comparing the three plasmids to each other. This analysis only allows to investigate differences between cell lines or changes for each plasmid individually under different conditions.
Cells were harvested 48 h after transfection and fixed for flow cytometry analysis. A summary box plot of three independent experiments is shown in Fig. 3A. Notable data variability regarding the helper and transgene plasmids was discerned, which was associated with the use of different shaker flasks due to long-term supply shortages from the manufacturer. Two experiments utilized baffled shakers, while one employed flat-bottom flasks and this variation suggested additional influences on the culture conditions. Both cell lines exhibited slower growth and, consequently, a reduced rAAV yield overall in flat-bottom shakers. However, HP cell line benefited more from the culture conditions in baffled flasks compared to flat-bottom flasks. While optimization of culture conditions warrants further investigation, differences between HP and LP were still observed even under inferior culture conditions, with a general trend in LP for lower transfection efficiency: Greatest variability between the two cell lines was detected between helper plasmid (min. diff. = 7.12% in flat bottom flasks, max. diff. 27.32% in baffled flasks), followed by transgene plasmid (min. diff. = 7.73%, max. diff. = 22.39%). However, since RepCap plasmid was associated with almost all viable singlet cells (> 99%) in both, HP and LP cell lines, there was no difference observed. When relative transfection efficiency was examined over a time course for each plasmid (immediately after and 4 h, 24 h, 48 h post transfection), highest signals were detected 4 h after transfection for all plasmids in HP. For LP, however, the uptake patterns between the individual plasmids varied (Fig. 3B).
Transfection efficiency of three distinct plasmids in a triple transfection strategy across two distinct HEK-293 cell line variants. A Box plot of plasmid positive cells, analyzed with flow cytometry. Mean, and the range between min and max from three individual experiments are shown (n = 3). B A time course between 0 and 48 h is shown for two different experiments including highest (baffled shaker) and lowest (flat bottom shaker) variances between HP and LP cell lines. C Flow cytometry analysis of isolated fixed nuclei (n = 1, flat bottom shaker). D Transfection rate at 48 h post transfection (n = 1, flat bottom shaker) was normalized considering multiple plasmid copy transfection for cells and nuclei. A ratio between these two was calculated to indicate the relative number of plasmids that successfully reach the nucleus. Higher ratios indicate greater difference between cellular and nuclear multiple copy transfection, thus, less efficient nuclear localization of plasmids
Given that successful transient gene delivery depends not only on cell entry but also on overcoming various intracellular challenges like endosomal escape, cytoplasmic transport, and nucleus entry, those natural barriers may limit the number of transfected cells capable of expressing the delivered genes. Nuclear localization is a prerequisite for DNA transcription. Consequently, our interest further lied in assessing the effectiveness of our plasmids in reaching the nucleus and determining if any distinctions can be observed between HP and LP.
Nuclei isolation and fixation was performed as described previously, and nucleus identity was confirmed using DAPI staining. Data in Fig. 3C show the relative amount of plasmid-dye positive nuclei obtained after isolation and came from a single experiment in flat bottom flasks, thus verification requires further evidence. Nevertheless, data is coherent with the transfection pattern observed on cellular level for both HP and LP. The relative amount of plasmid that reaches the nucleus, however, is lower in LP, as shown in Fig. 3D. This data suggests that LP not only has lower plasmid uptake rates in general, but in addition, nuclear transport might be less efficient.
Taken together, we demonstrated a superior plasmid uptake for HP cell line in comparison to LP. This was shown for all timepoints on cellular level and for isolated nuclei. To produce transgene loaded rAAV particles, availability of all plasmids in the nucleus in sufficient quantities is crucial. We were therefore interested to find out if we can improve transgene loaded rAAV yield by increasing nuclear accumulation of plasmids.
Plasmid ratio plays a crucial role in enhancing yield over total transfected DNA Quantity
Based on preliminary experiments, we identified a potential for plasmid ratio optimization by increasing helper and transgene DNA. Based on the findings of Nguyen et al. (2021), we were interested in how a decrease in RepCap plasmid will alter the equilibrium between the transgene available in the nucleus primed for packaging and capsids, potentially resulting in an increased ratio of fully packaged rAAV capsids.
We, therefore, increased helper and transgene DNA by 3.8-fold while RepCap DNA was reduced to 69% of the original transfection mix, resulting in a 2.47-fold elevation of total input DNA (= 3 µg/ml). Consequently, the standard molar plasmid DNA ratio T (RepCap:helper:transgene = 1.00:0.47:0.73) was altered to the relative molar ratio of 1.00:2.58:3.95. For a second condition, the ratio of T-100 was kept, but total DNA amount was decreased to 75% (T-75), to a total amount of input DNA of 2.2 µg/ml. In a third condition we additionally changed the relative ratio between the plasmids (1.00:1.17:2.96), using an equal DNA amount of RepCap as in T-100 (69% of initial transfection mix), same amount of transgene DNA as in T-75 (2.82-fold increase of standard transfection T), but only 1.7-fold higher concentration of helper DNA, resulting in total DNA input of 1.9 µg/ml. A summary of used DNA amounts across all conditions is shown in Table 1.
A comparison of transfection efficacies between all three conditions and the original plasmid combination is shown in Fig. 4A. In general, the number of transfected cells could be improved for both, HP and LP in all conditions, with only one exception for LP: although highest DNA amounts used for transfection, T-100 displayed lowest numbers of positive cells, which were even below our standard condition T. With our special interest in improving transfection rates for LP cells, we considered T-LO as the superior condition as it showed highest transfection rates for each plasmid. The cellular accumulation was further reflected on nuclear level, with the exception of helper genes, which showed highest expression in T-75 condition.
Transfection efficiency and yield comparison with adapted plasmid ratios. Flow cytometry results depicting whole-cell analysis A and isolated fixed nuclei B following triple plasmid transfection, with distinct detection of each plasmid. C Yield comparison from supernatant harvest of capsids, vector genome and their relative ratio (vg/cp) to each other
In the next step, we investigated whether our improved transfection efficiency consequently resulted in higher productivity of the cells. Therefore, we analyzed our samples 72 h post transfection, and used ddPCR and ELISA to detect transgene copies and assembled capsids in secreted particles obtained from supernatant (Fig. 4B). The reduction in RepCap plasmid initially seemed inconspicuous, with all cells still showing comparable outcome in FACS analysis; however, the yield significantly declined down to 57% (T-LO) or 35% (T-75), respectively. This decrease was unproportional to the reduced input DNA, underscoring the crucial role of multiple plasmid copies transfected per cell in maintaining high levels of particle production.
While the RepCap DNA quantity plays a prominent role in rAAV production, the overall particle yield also seems to be influenced by the numbers of both transgene and helper plasmids. Despite utilizing an identical amount of RepCap plasmid for T-100 and T-LO, the variation in helper and transgene DNA ratios resulted in a significantly higher particle yield under the latter condition.
Upon comparing the vector genome-to-capsid ratio, it became evident that, under standard conditions, LP excels in transgene encapsulation compared to HP. This ratio exhibited potential improvement by up to 11% for LP and 31% for HP in T-75 and T-100 (Fig. 4C). Remarkably, these conditions promoted equivalent Transgene:Helper ratios as in T (1.00:0.65), with a lower relative ratio between Transgene:RepCap (1.00:0.25) and Helper:RepCap (1.00:0.39). Despite the variance in the total transfected DNA amounts for both conditions, T-75 and T-100, the consistent plasmid ratio, distinguished by a substantial increase in both, helper and transgene plasmids from the standard condition, suggests that the observed enhancement may be linked to the altered plasmid ratio rather than the total DNA amount transfected.
Up to this point, we have illustrated that the LP cell line exhibits shortcomings in both plasmid uptake and nucleus targeting, leading to limited rAAV production capabilities. However, disparities in transfection efficiency alone may not completely account for the differences in capsid yield between the two cell lines. Potential additional variations along transcriptional and translational pathways could play a role, further expanding the gap in their respective productivity.
LP showed delayed protein expression in comparison to HP
To discern potential differences in the protein production process, we introduced a GFP-labeled plasmid and evaluated both, the transfection efficiency of the plasmid and the expression of the protein. In this study, a commercially available GFP plasmid was used, and the plasmid backbone was labeled with Cy5® dye using the previously described method for plasmid tracing. The labeled GFP plasmid was uniformly transfected into the two cell lines, and the assessment encompassed both, the quantification of cells positive for the plasmid backbone and the measurement of produced GFP protein through flow cytometry analysis. Figure 5A presents data from three independent experiments conducted in baffled and flat-bottom shaker flasks.
Protein expression compared for two different HEK-293 cell lines. A Flow cytometry analysis of GFP transfected cells using backbone labeled plasmids to compare transfection efficiency and corresponding protein expression. B Gene set enrichment analysis performed with mRNA sequencing data. Positive NES indicates higher expression in HP
Consistent with our prior observations from triple-plasmid transfections, the GFP plasmid exhibited a higher transfection efficiency in HP compared to LP in general. Subsequently, robust GFP expression was evident in HP at the anticipated 24-h mark. In contrast, only a weak GFP expression was observed in less than 5.5% of LP cells until 48 h, despite the plasmid itself being detected in over 99% of the cells. One of the three experiments was extended to 72 h, to capture a potential delayed initiation of protein translation in LP. Indeed, at 72 h, the GFP signal began to increase and was detectable in 20.5% of the LP cells.
Taking our previous findings into consideration, we hypothesized that an accumulative effect contributes to LP’s decreased manufacturing performance, including weaker transfectability, transcription and translation. To further corroborate our hypothesis, we went back to our transcriptome sequencing data to find evidence for inferior translation activity in LP, since pathways associated with transcriptional activity were already shown to be more active in HP. Gene set enrichment analysis (GSEA) was performed as described (Pistek & Kahlig et al. 2023) comparing HP and LP mRNA expression profiles of transfected cells (n = 4). Five timepoints were investigated as seen in Fig. 5B, starting with an initial comparison of naïve cells before transfection (t = 0 h). Positive normalized enrichment scores (NES) indicate elevated expression levels in HP, while negative NES is associated with increased transcriptional activity in LP. In naïve cells, HP already shows higher activity in GO terms considering translation initiation, modification and membrane targeting. Although certain GO terms maintain superiority in HP across all sample timepoints (GO COTRANSLATIONAL PROTEIN TARGETING TO MEMBRANE, GO CYTOPLASMIC TRANSLATION, GO POST TRANSLATION PROTEIN MODIFICATION, etc.), others gained importance in LP cells only after 24 h, even surpassing expression levels of HP (GO REGULATION OF TRANSLATION INITIATION, GO POSITIVE REGULATION OF TRANSLATION). This underscores the hypothesis that initially, translation activity in LP is lower in the steady state before transfection when compared to HP and consequently, requires more time to adapt to plasmid transfection by upregulating its translation machinery. This results in a later onset of protein expression, which contributes further to the already unraveled bottlenecks of LP such as weaker transfectability and a lower transcription rate.
Discussion
Our two investigated cell lines, HP and LP, although derived from the same ancestral HEK-293 cell, have already been shown to exhibit a highly versatile transcriptome. Accordingly, their response to transient plasmid transfection is unique, reflecting their strength and weaknesses as a producer cell line (Grieger et al. 2016, Srivastava et al. 2021, Torabfam et al. 2022, Pistek et al. 2023). In this study, we identified multiple bottlenecks for LP cell line along the whole transfection process, including reduced plasmid uptake and nucleus entry in addition to disadvantages in protein translation. The data shown here indicates that the ratio of vector genome-to-capsid can be improved for both cell lines to an individual extent by optimizing relative plasmid ratios. However, further optimization of the transfected DNA composition is required, and must be tailored to each production cell line.
Cell line-specific transfectability
The efficiency of nuclear transport of plasmids is a multifaceted issue in transient gene delivery, influenced by both, their intrinsic design and varying cellular environments (Vacik et al. 1999; Le Guen et al. 2021; Haraguchi et al. 2022). For example, plasmid size and supercoiled structures impact their ability for cellular and nuclear entry (Sousa et al. 2009; Ribeiro et al. 2012), methylation patterns can affect their stability and susceptibility to enzymatic degradation (Hong et al. 2001), and the choice of codon usage determines translational efficiency within the host cell system (Powell and Dion 2015).
Nevertheless, identical plasmids may show different transfection efficiency across different cell lines, indicating a strong interplay with cellular factors (Vacik et al. 1999, Maurisse et al. 2010). For instance, elevated expression of cytosolic nucleases may lead to the degradation of higher amounts of plasmid DNA; to allow cytosolic movement along the cytoskeleton, an intricate interplay of numerous cellular genes is required; and the presence of importins has shown to be crucial for successful nuclear entry (James and Giorgio 2000; Pollard et al. 2001; Vaughan and Dean 2006; Yang and Musser 2006).
In our comprehensive study, we demonstrated that transfection efficiency varies in our two investigated HEK-293 producer cell lines. Cellular uptake, nuclear accumulation, and the ability to express the genetic information has been shown to differ between HP and LP. Underlying mechanisms may be found in the cells’ distinct transcriptome, and thus, diverse responses to transfection stimuli (Pistek & Kahlig et al. 2023). Detailed investigation presented by Warga and colleagues (Warga et al. 2023), sheds further light on molecular factors influencing gene delivery outcomes and provide a foundation to our current findings. However, deeper analysis of our transcriptome data will be required to unravel potential influencing host cell genes for future cell line optimization.
The importance of balancing the amount of exogenous viral genes during rAAV particle production
Numerous efforts have been made to refine the production of recombinant adeno-associated virus particles, focusing on improving culture conditions and transfection techniques, such as plasmid ratio optimization (Durocher et al. 2007; Zhao et al. 2020). We found that the success of those approaches varies across literature, and that the effectiveness of these optimizations is influenced by variables like the specific producer cell lines used, the reagents and methods applied, and the produced rAAV serotype. Consequently, the ideal conditions including the plasmid ratios, may vary depending on each unique experimental setup.
In our study, we reduced the amount of RepCap plasmid, which expectedly resulted in a decrease in the number of produced particles. However, this reduction in virus capsid yield was not proportional to the plasmid input reduction, instead substantially less particles were produced. The multiple plasmid copies transfected per cell therefore emerged as a critical factor for RepCap plasmids, determining overall yield. Concurrently, we significantly elevated the quantity of transgene plasmid with the expectation of remarkably enhancing transgene integration into our pre-formed empty capsids. Nevertheless, this was not observed to the expected extent, indicating that the availability of the transgene may not be the limiting factor. Instead, we found upon closer examination, the packaging mechanism might entail a more complex interplay among the gene products of all three plasmids: we discovered that the quantity of RepCap plasmid alone may not solely determine the number of produced particles. Reducing the amount of transgene and helper plasmids while keeping the RepCap plasmid constant resulted in increased particle production. Potential explanations consider the impact of varying amounts of total input DNA used or that the ratio between RepCap and helper plasmid plays a significant role in the outcome of overall production (Grieger et al. 2016, Guan et al. 2022). Support for the latter hypothesis is evident in our comparison of the vector genome-to-particle ratio: a superior full-empty ratio of particles was observed under conditions with a higher relative ratio of helper to RepCap plasmid. This aligns with the findings of Nguyen and colleagues (Nguyen et al. 2021), suggesting that increasing the relative amount of helper to RepCap plasmids to a certain extent might yield beneficial outcomes in generating full particles. Nevertheless, helper genes have been shown to not only have beneficial, but also negative effects on rAAV production: controversial to their promoting function in transcription and second strand synthesis, E4orf6 together with E1b55k can also induce proteasomal degradation of preassembled rAAV capsids and Rep52, a helicase important for transgene packaging (Smith and Kotin 1998; Nayak and Pintel 2007). Thus, balancing the gene products in a triple plasmid transfection is crucial for resulting yield. We therefore propose for subsequent experiments to increase the helper:RepCap ratio of our standard condition T without significantly raising the overall DNA amount to obtain a better vector genome-to-capsid ratio in secreted particles.
Introducing another layer of complexity involves considering the individual transcriptional and translational capacities of the respective cell lines. It is established that rAAV production follows a sequential process, commencing with the generation of empty capsids and subsequently involving the replication of the transgene and its encapsulation into pre-formed capsids. Consequently, swift gene expression may lead to the predominant production of empty capsids, as they are secreted before the incorporation of the desired transgene can occur (Nguyen et al. 2021). Thus, maintaining a moderate level of protein expression may be advantageous, facilitating both concurrently, protein translation and transgene transcription. This could potentially lead to higher quantities of transgene encapsulation, and consequently may result in a higher yield of full particles. The difference in protein expression dynamics potentially explains why our high producer (HP) cell line secretes a substantial number of particles, yet the low producer (LP) cell line exhibits superior vector genome-to-capsid ratios despite an overall lower yield: in addition to the previously mentioned potential interference of viral helper genes, Ohba et al. (2023) suggested that the timely coordination of viral gene expression may contribute not only to overall particle yield but, more importantly, to packaging quality. Our study demonstrated that protein expression is delayed in LP, potentially allowing the cell line to evade the accumulation of interfering viral proteins, thus resulting in a better vector genome-to-capsid ratio. However, the overall yield of fully packaged particles was still higher in HP. Given the significant differences in the cellular transcriptome and its response to plasmid-induced rAAV production between HP and LP (Pistek & Kahlig et al. 2023), we cannot exclude the possibility that cellular factors also contribute to the observed differences in the vector genome-to-capsid ratio, which will require further examination.
Taken together, rAAV production is a delicate interplay between cellular pathways and induced viral gene expression. Cellular burden stimulated by exogenous DNA may differ between cell lines and need to be considered when designing future gene therapy approaches. Further dissection of factors that influence viral vector production will allow to increase productivity, and moreover, patient safety. Therefore, understanding the unique capabilities of the utilized producer cell line and their reaction on viral vector production is crucial for the development of efficient production processes in biopharmaceutical manufacturing and will contribute to future advances in rAAV gene therapy applications.
References
Akita H, Kurihara D, Schmeer M, Schleef M, Harashima H (2015) Effect of the compaction and the size of DNA on the nuclear transfer efficiency after microinjection in synchronized cells. Pharmaceutics 7(2):64–73
Bulcha JT, Wang Y, Ma H, Tai PWL, Gao GP (2021) Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. https://doi.org/10.1038/s41392-021-00487-6
Durocher Y, Butler M (2009) Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol 20(6):700–707
Durocher Y, Pham PL, St-Laurent G, Jacob D, Cass B, Chahal P, Lau CJ, Nalbantoglu J, Kamen A (2007) Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J Virol Methods 144(1–2):32–40
Estes S, Melville M (2014) Mammalian cell line developments in speed and efficiency. Mammal Cell Cult Biol Manuf 139:11–33
Fus-Kujawa A, Prus P, Bajdak-Rusinek K, Teper P, Gawron K, Kowalczuk A, Sieron AL (2021) An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.701031
Godbey WT, Wu KK, Mikos AG (1999) Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res 45(3):268–275
Grieger JC, Soltys SM, Samulski RJ (2016) Production of recombinant adeno-associated virus vectors using suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol Ther 24(2):287–297
Guan JS, Chen K, Si Y, Kim T, Zhou Z, Kim S, Zhou L, Liu XM (2022) Process improvement of adeno-associated virus (AAV) production. Front Chem Eng. https://doi.org/10.3389/fceng.2022.830421
Haraguchi T, Koujin T, Shindo T, Bilir S, Osakada H, Nishimura K, Hirano Y, Asakawa H, Mori C, Kobayashi S, Okada Y, Chikashige Y, Fukagawa T, Shibata S, Hiraoka Y (2022) Transfected plasmid DNA is incorporated into the nucleus via nuclear envelope reformation at telophase. Commun Biol 5(1):78
Hong K, Sherley J, Lauffenburger DA (2001) Methylation of episomal plasmids as a barrier to transient gene expression via a synthetic delivery vector. Biomol Eng 18(4):185–192
Hornstein BD, Roman D, Arevalo-Soliz LM, Engevik MA, Zechiedrich L (2016) Effects of Circular DNA Length on Transfection Efficiency by Electroporation into HeLa Cells. PloS One 11(12):e0167537
James MB, Giorgio TD (2000) Nuclear-associated plasmid, but not cell-associated plasmid, is correlated with transgene expression in cultured mammalian cells. Mol Ther 1(4):339–346
Khan KH (2013) Gene expression in Mammalian cells and its applications. Adv Pharm Bull 3(2):257–263
Le Guen YT, Pichon C, Guegan P, Pluchon K, Haute T, Quemener S, Ropars J, Midoux P, Le Gall T, Montier T (2021) DNA nuclear targeting sequences for enhanced non-viral gene transfer: an in vitro and in vivo study. Mol Ther Nucleic Acids 24:477–486
Li TT, Gao T, Chen HX, Pekker P, Menyhart A, Guttman A (2020) Rapid determination of full and empty adeno-associated virus capsid ratio by capillary isoelectric focusing. Curr Mol Med 20(10):814–820
Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, Gruenert DC (2010) Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. Bmc Biotechnol. https://doi.org/10.1186/1472-6750-10-9
Nayak R, Pintel DJ (2007) Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production. J Virol 81(5):2205–2212
Nguyen TNT, Sha S, Hong MS, Maloney AJ, Barone PW, Neufeld C, Wolfrum J, Springs SL, Sinskey AJ, Braatz RD (2021) Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells. Mol Ther-Methods Clin Dev 21:642–655
Ohba K, Sehara Y, Enoki T, Mineno J, Ozawa K, Mizukami H (2023) Adeno-associated virus vector system controlling capsid expression improves viral quantity and quality. iScience 26(4):106487
Ooi A, Wong A, Esau L, Lemtiri-Chlieh F, Gehring C (2016) A guide to transient expression of membrane proteins in HEK-293 cells for functional characterization. Front Physiol. https://doi.org/10.3389/fphys.2016.00300
Pistek M, Kahlig CI, Hackl M, Unterthurner S, Kraus B, Grabherr R, Grillari J, Bort JAH (2023) Comprehensive mRNA-sequencing-based characterization of three HEK-293 cell lines during an rAAV production process for gene therapy applications. Biotechnol J. https://doi.org/10.1002/biot.202200513
Pollard H, Toumaniantz G, Amos JL, Avet-Loiseau H, Guihard G, Behr JP, Escande D (2001) Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J Gene Med 3(2):153–164
Powell J, Dion K (2015) Effects of codon usage on gene expression: empirical studies on drosophila. J Mol Evol 80(3–4):219–226
Ribeiro S, Mairhofer J, Madeira C, Diogo MM, da Silva CL, Monteiro G, Grabherr R, Cabral JM (2012) Plasmid DNA size does affect nonviral gene delivery efficiency in stem cells. Cell Reprogram 14(2):130–137
Rolling F, Samulski RJ (1995) Aav as a viral vector for human gene-therapy - generation of recombinant virus. Mol Biotechnol 3(1):9–15
Rosazza C, Deschout H, Buntz A, Braeckmans K, Rols MP, Zumbusch A (2016) Endocytosis and endosomal trafficking of DNA after gene electrotransfer In Vitro. Molecular Therapy-Nucleic Acids 5:1–11
Samulski RJ, Shenk T (1988) Adenovirus E1B 55-Mr polypeptide facilitates timely cytoplasmic accumulation of adeno-associated virus mRNAs. J Virol 62(1):206–210
Smith RH, Kotin RM (1998) The Rep52 gene product of adeno-associated virus is a DNA helicase with 3 ’-to-5 ’ polarity. J Virol 72(6):4874–4881
Sousa F, Prazeres DMF, Queiroz JA (2009) Improvement of transfection efficiency by using supercoiled plasmid DNA purified with arginine affinity chromatography. Journal of Gene Medicine 11(1):79–88
Srivastava A, Mallela KMG, Deorkar N, Brophy G (2021) Manufacturing challenges and rational formulation development for AAV Viral Vectors (vol 110, pg 2609, 2021). J Pharm Sci 110(9):3324–3324
Tan EV, Chin CSH, Lim ZFS, Ng SK (2021) HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front Bioeng Biotech. https://doi.org/10.3389/fbioe.2021.796991
Torabfam GC, Yetisgin AA, Erdem C, Cayli A, Kutlu O, Cetinel S (2022) A feasibility study of different commercially available serum-free mediums to enhance lentivirus and adeno-associated virus production in HEK 293 suspension cells. Cytotechnology 74(6):635–655
Vacik J, Dean BS, Zimmer WE, Dean DA (1999) Cell-specific nuclear import of plasmid DNA. Gene Ther 6(6):1006–1014
Vaughan EE, Dean DA (2006) Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther 13(2):422–428
Warga E, Anderson J, Tucker M, Harris E, Elmer J (2023) Transcriptomic analysis of the innate immune response to in vitro transfection of plasmid DNA. Mol Ther Nucleic Acids 31:43–56
Wright JF (2009) Transient transfection methods for clinical adeno-associated viral vector production. Hum Gene Ther 20(7):698–706
Yang W, Musser SM (2006) Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol 174(7):951–961
Zhao H, Lee KJ, Daris M, Lin Y, Wolfe T, Sheng J, Plewa C, Wang S, Meisen WH (2020) Creation of a high-yield AAV vector production platform in suspension cells using a design-of-experiment approach. Mol Ther Methods Clin Dev 18:312–320
Acknowledgements
The presented work was supported by Baxalta Innovations GmbH, a part of Takeda companies.
Funding
The authors declare that no funds or grants, were received during the preparation of this manuscript. This study was supported by Baxalta Innovations GmbH, a part of Takeda companies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
Martina Pistek, Peter Andorfer, Barbara Kraus and Juan A. Hernandez Bort are all employees of Baxalta Innovations GmbH, a part of Takeda companies, which are involved in the development of gene therapy products and may be owners of stock options.
Juan A. Hernandez Bort conducted the research presented in this manuscript while employed at Baxalta Innovations GmbH, a part of Takeda companies. Between the completion of the research and the publication of this manuscript, he has transitioned to an additional affiliation with the University of Vienna, which is now listed as his correspondence address.
Reingard Grabherr is an employee of the University of Natural Resources and Applied Life Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pistek, M., Andorfer, P., Grabherr, R. et al. Factors affecting rAAV titers during triple-plasmid transient transfection in HEK-293 cells. Biotechnol Lett (2024). https://doi.org/10.1007/s10529-024-03520-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10529-024-03520-0